Under-Reported Aspects of Platinum Drug Pharmacology
Abstract
:1. Introduction
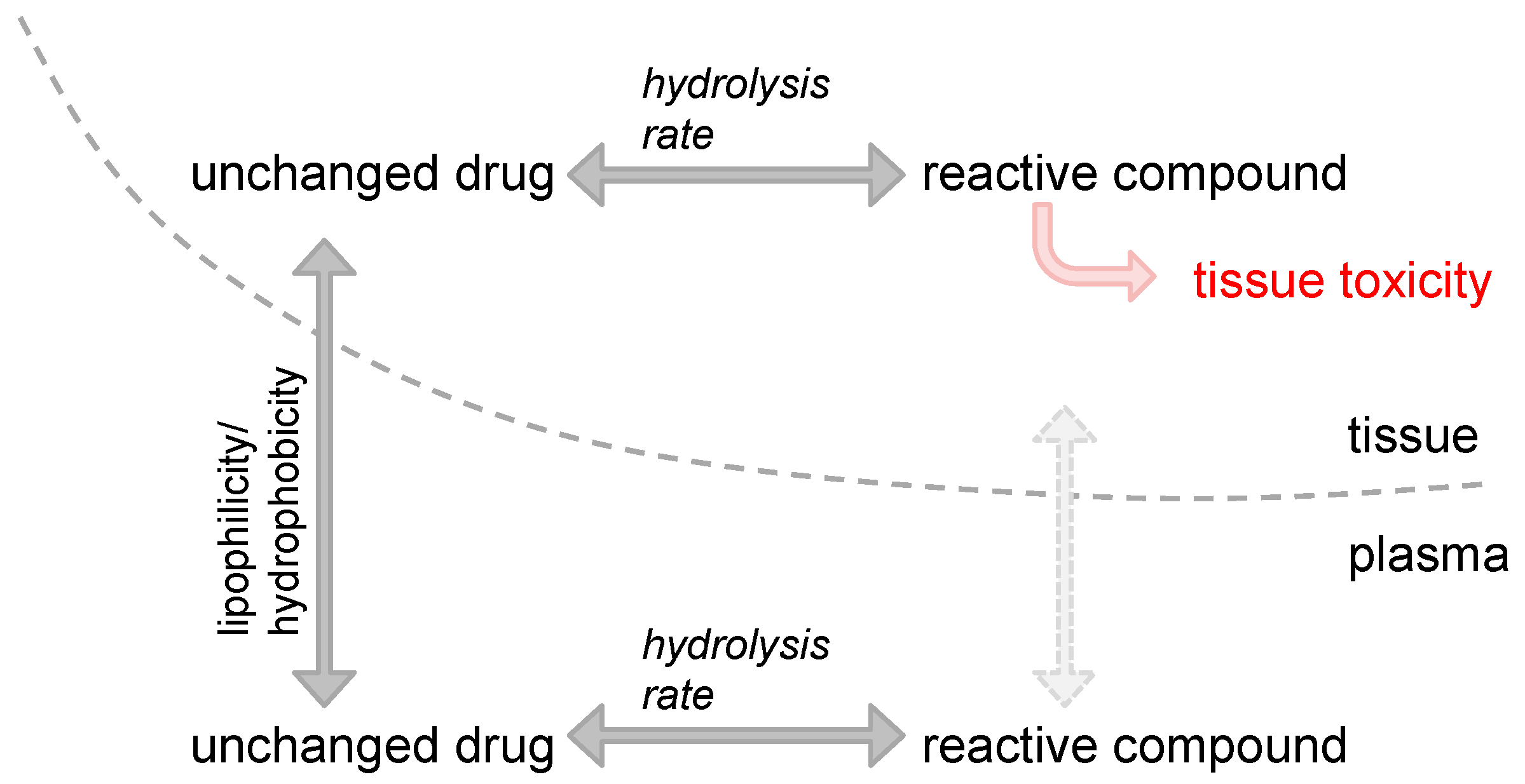
2. Impact of Platinum Drugs’ Physico-Chemical Properties on Site-Specific Toxicities
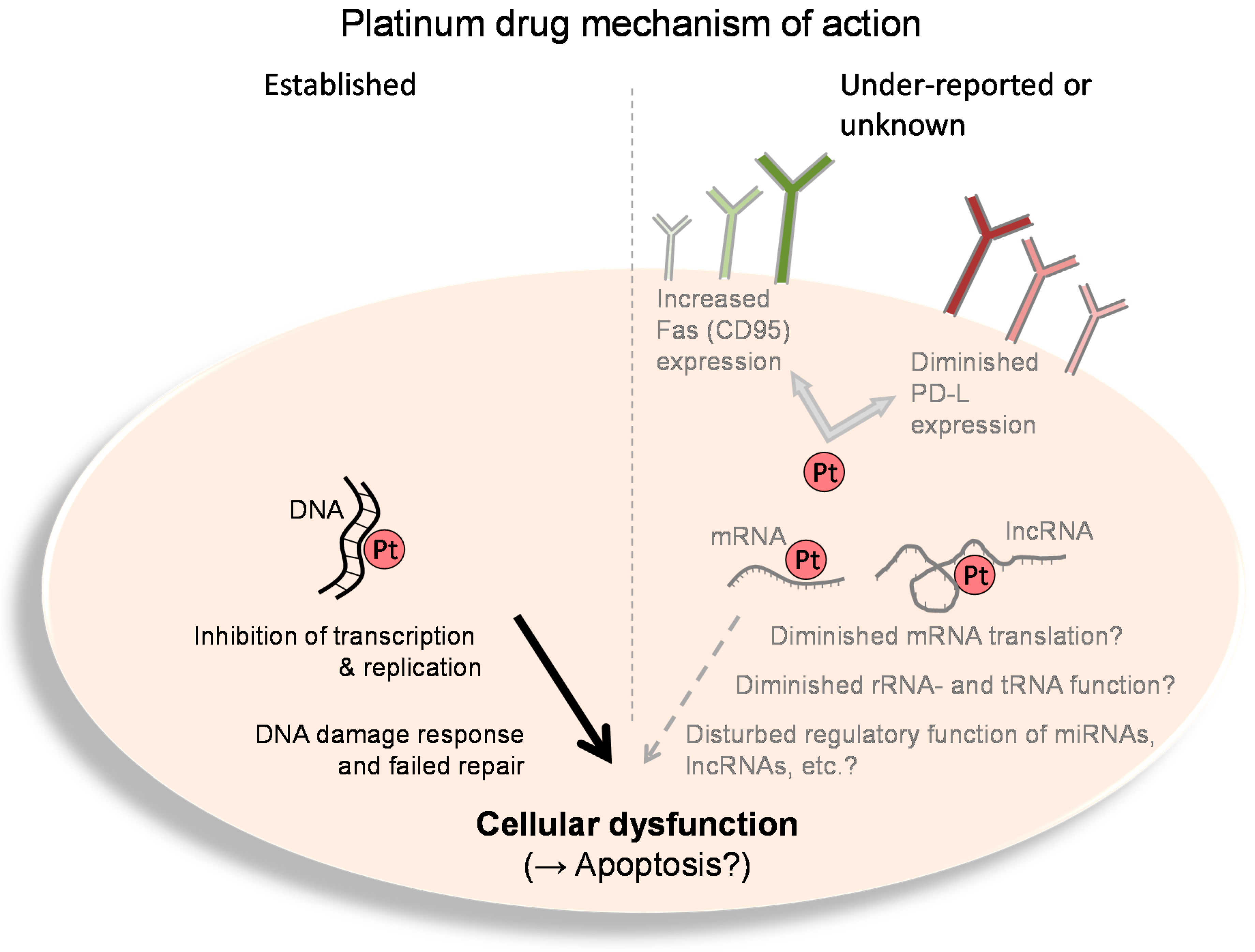
3. Interaction of Platinum Drugs with RNA
4. Immunogenic Effects of Platinum Drugs
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef] [PubMed]
- O’Dwyer, P.J.; Stevenson, J.P.; Johnson, S.W. Clinical pharmacokinetics and administration of established platinum drugs. Drugs 2000, 59 (Suppl. 4), 19–27. [Google Scholar] [CrossRef] [PubMed]
- Theile, D.; Detering, J.C.; Herold-Mende, C.; Dyckhoff, G.; Haefeli, W.E.; Weiss, J.; Burhenne, J. Cellular pharmacokinetic/pharmacodynamic relationship of platinum cytostatics in head and neck squamous cell carcinoma evaluated by liquid chromatography coupled to tandem mass spectrometry. J. Pharmacol. Exp. Ther. 2012, 341, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Rabik, C.A.; Dolan, M.E. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat. Rev. 2007, 33, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Owens, S.E.; Thatcher, N.; Sharma, H.; Adam, N.; Harrison, R.; Smith, A.; Zaki, A.; Baer, J.C.; McAuliffe, C.A.; Crowther, D.; Fox, B.W. In vivo distribution studies of radioactively labelled platinum complexes; cis-dichlorodiammine platinum(II), cis-trans-dichlorodihydroxy-bis-(isopropylamine) platinum(IV), cis-dichloro-bis-cyclopropylamine platinum(II), and cis-diammine 1,1-cyclobutanedicarboxylate platinum(II) in patients with malignant disease, using a gamma camera. Cancer Chemother. Pharmacol. 1985, 14, 253–257. [Google Scholar] [PubMed]
- Hanada, K.; Asano, K.; Nishimura, T.; Chimata, T.; Matsuo, Y.; Tsuchiya, M.; Ogata, H. Use of a toxicity factor to explain differences in nephrotoxicity and myelosuppression among the platinum antitumour derivatives cisplatin, carboplatin and nedaplatin in rats. J. Pharm. Pharmacol. 2008, 60, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, E.R.; Lippard, S.J. Structure, Recognition, and Processing of Cisplatin-DNA Adducts. Chem. Rev. 1999, 99, 2467–2498. [Google Scholar] [CrossRef] [PubMed]
- Arnould, S.; Hennebelle, I.; Canal, P.; Bugat, R.; Guichard, S. Cellular determinants of oxaliplatin sensitivity in colon cancer cell lines. Eur. J. Cancer 2003, 39, 112–119. [Google Scholar] [CrossRef]
- Brauckmann, C.; Wehe, C.A.; Kieshauer, M.; Lanvers-Kaminsky, C.; Sperling, M.; Karst, U. The interaction of platinum-based drugs with native biologically relevant proteins. Anal. Bioanal. Chem. 2013, 405, 1855–1864. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.P.; Phillips, H.I.; Campuzano, I.; Sadler, P.J. Shape changes induced by N-terminal platination of ubiquitin by cisplatin. J. Am. Soc. Mass Spectrom. 2010, 21, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Ishida, R.; Takaoka, Y.; Yamamoto, S.; Miyazaki, T.; Otaka, M.; Watanabe, S.; Komatsuda, A.; Wakui, H.; Sawada, K.; Kubota, H.; et al. Cisplatin differently affects amino terminal and carboxyl terminal domains of HSP90. FEBS Lett. 2008, 582, 3879–3883. [Google Scholar] [CrossRef] [PubMed]
- Köpf-Maier, P.; Mühlhausen, S.K. Changes in the cytoskeleton pattern of tumor cells by cisplatin in vitro. Chem. Biol. Interact. 1992, 82, 295–316. [Google Scholar] [CrossRef]
- Peleg-Shulman, T.; Najajreh, Y.; Gibson, D. Interactions of cisplatin and transplatin with proteins. Comparison of binding kinetics, binding sites and reactivity of the Pt-protein adducts of cisplatin and transplatin towards biological nucleophiles. J. Inorg. Biochem. 2002, 91, 306–311. [Google Scholar] [CrossRef]
- Rijal, K.; Chow, C.S. A new role for cisplatin: Probing ribosomal RNA structure. Chem. Commun. (Camb). 2009, 1, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Papsai, P.; Snygg, A.S.; Aldag, J.; Elmroth, S.K. Platination of full length tRNA(Ala) and truncated versions of the acceptor stem and anticodon loop. Dalton Trans. 2008, 38, 5225–5234. [Google Scholar] [CrossRef] [PubMed]
- Hostetter, A.A.; Osborn, M.F.; DeRose, V.J. RNA-Pt adducts following cisplatin treatment of Saccharomyces cerevisiae. ACS Chem. Biol. 2012, 7, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Hägerlöf, M.; Papsai, P.; Chow, C.S.; Elmroth, S.K. More pronounced salt dependence and higher reactivity for platination of the hairpin r(CGCGUUGUUCGCG) compared with d(CGCGTTGTTCGCG). J. Biol. Inorg. Chem. 2006, 11, 974–990. [Google Scholar] [CrossRef] [PubMed]
- Osborn, M.F.; White, J.D.; Haley, M.M.; DeRose, V.J. Platinum-RNA modifications following drug treatment in S. cerevisiae identified by click chemistry and enzymatic mapping. ACS Chem. Biol. 2014, 9, 2404–2411. [Google Scholar] [PubMed]
- Theile, D.; Kos, M. Structural and functional evaluation of interaction between mammalian ribosomal RNA with platinum-containing antineoplastic drugs. Toxicol. Lett. 2016, 242, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.P.; Weiss, J.; Theile, D. Cisplatin, oxaliplatin, and carboplatin unequally inhibit in vitro mRNA translation. Toxicol. Lett. 2014, 225, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Knox, R.J.; Friedlos, F.; Lydall, D.A.; Roberts, J.J. Mechanism of cytotoxicity of anticancer platinum drugs: Evidence that cis-diamminedichloroplatinum(II) and cis-diammine-(1,1-cyclobutanedicarboxylato)platinum(II) differ only in the kinetics of their interaction with DNA. Cancer Res. 1986, 46, 1972–1979. [Google Scholar] [PubMed]
- Los, G.; Verdegaal, E.; Noteborn, H.P.; Ruevekamp, M.; de Graeff, A.; Meesters, E.W.; ten Bokkel Huinink, D.; McVie, J.G. Cellular pharmacokinetics of carboplatin and cisplatin in relation to their cytotoxic action. Biochem. Pharmacol. 1991, 42, 357–363. [Google Scholar] [CrossRef]
- Bellacosa, A.; Moss, E.G. RNA repair: Damage control. Curr. Biol. 2003, 13, R482–R484. [Google Scholar] [CrossRef]
- Aas, P.A.; Otterlei, M.; Falnes, P.O.; Vågbø, C.B.; Skorpen, F.; Akbari, M.; Sundheim, O.; Bjørås, M.; Slupphaug, G.; Seeberg, E.; et al. Human and bacterial oxidative demethylases repair alkylation damage in both RNA and DNA. Nature 2003, 421, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Kuwano, M.; Uchiumi, T.; Hayakawa, H.; Ono, M.; Wada, M.; Izumi, H.; Kohno, K. The basic and clinical implications of ABC transporters, Y-box-binding protein-1 (YB-1) and angiogenesis-related factors in human malignancies. Cancer Sci. 2003, 94, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, H.; Uchiumi, T.; Fukuda, T.; Ashizuka, M.; Kohno, K.; Kuwano, M.; Sekiguchi, M. Binding capacity of human YB-1 protein for RNA containing 8-oxoguanine. Biochemistry 2002, 41, 12739–12744. [Google Scholar] [CrossRef] [PubMed]
- Shibahara, K.; Sugio, K.; Osaki, T.; Uchiumi, T.; Maehara, Y.; Kohno, K.; Yasumoto, K.; Sugimachi, K.; Kuwano, M. Nuclear expression of the Y-box binding protein, YB-1, as a novel marker of disease progression in non-small cell lung cancer. Clin. Cancer Res. 2001, 7, 3151–3155. [Google Scholar] [PubMed]
- Janz, M.; Harbeck, N.; Dettmar, P.; Berger, U.; Schmidt, A.; Jürchott, K.; Schmitt, M.; Royer, H.D. Y-box factor YB-1 predicts drug resistance and patient outcome in breast cancer independent of clinically relevant tumor biologic factors HER2, uPA and PAI-1. Int. J. Cancer 2002, 97, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Parasramka, M.A.; Maji, S.; Matsuda, A.; Yan, I.K.; Patel, T. Long non-coding RNAs as novel targets for therapy in hepatocellular carcinoma. Pharmacol. Ther. 2016, 161, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Hedman, H.K.; Kirpekar, F.; Elmroth, S.K. Platinum interference with siRNA non-seed regions fine-tunes silencing capacity. J. Am. Chem. Soc. 2011, 133, 11977–11984. [Google Scholar] [CrossRef] [PubMed]
- Bergmann-Leitner, E.S.; Abrams, S.I. Treatment of human colon carcinoma cell lines with anti-neoplastic agents enhances their lytic sensitivity to antigen-specific CD8 cytotoxic T lymphocytes. Cancer Immunol. Immunother. 2001, 50, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Lesterhuis, W.J.; Punt, C.J.; Hato, S.V.; Eleveld-Trancikova, D.; Jansen, B.J.; Nierkens, S.; Schreibelt, G.; de Boer, A.; Van Herpen, C.M.; Kaanders, J.H.; et al. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J. Clin. Investig. 2011, 121, 3100–3108. [Google Scholar]
- Hato, S.V.; de Vries, I.J.; Lesterhuis, W.J. Stating the importance of immune modulation by platinum chemotherapeutics. Oncoimmunology 2012, 1, 234–236. [Google Scholar] [CrossRef] [PubMed]
- Hato, S.V.; Khong, A.; de Vries, I.J.; Lesterhuis, W.J. Molecular pathways: The immunogenic effects of platinum-based chemotherapeutics. Clin. Cancer Res. 2014, 20, 2831–2837. [Google Scholar] [CrossRef] [PubMed]
- Tesniere, A.; Schlemmer, F.; Boige, V.; Kepp, O.; Martins, I.; Ghiringhelli, F.; Aymeric, L.; Michaud, M.; Apetoh, L.; Barault, L.; et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 2010, 29, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Vasaturo, A.; di Blasio, S.; Peeters, D.G.; de Koning, C.C.; de Vries, J.M.; Figdor, C.G.; Hato, S.V. Clinical Implications of Co-Inhibitory Molecule Expression in the Tumor Microenvironment for DC Vaccination: A Game of Stop and Go. Front. Immunol. 2013, 4, 417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Theile, D. Under-Reported Aspects of Platinum Drug Pharmacology. Molecules 2017, 22, 382. https://doi.org/10.3390/molecules22030382
Theile D. Under-Reported Aspects of Platinum Drug Pharmacology. Molecules. 2017; 22(3):382. https://doi.org/10.3390/molecules22030382
Chicago/Turabian StyleTheile, Dirk. 2017. "Under-Reported Aspects of Platinum Drug Pharmacology" Molecules 22, no. 3: 382. https://doi.org/10.3390/molecules22030382




