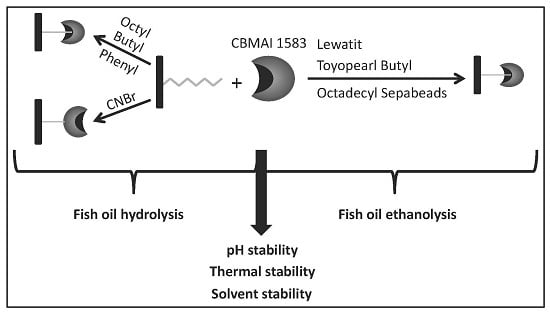
Immobilization of Lipase from Penicillium sp. Section Gracilenta (CBMAI 1583) on Different Hydrophobic Supports: Modulation of Functional Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Immobilization on Hydrophobic and Cyanogen Bromide Supports
2.2. Derivatives Characterization
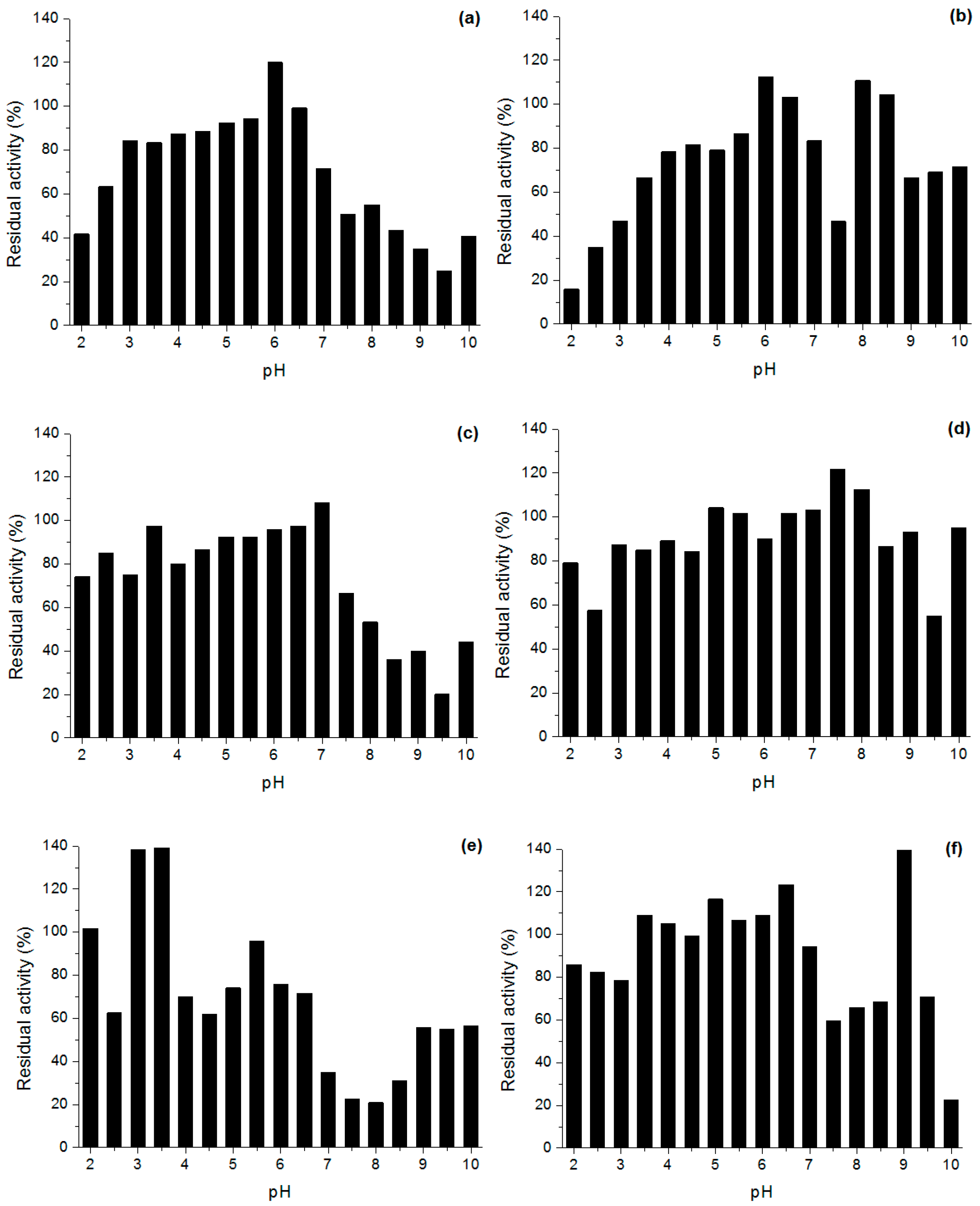
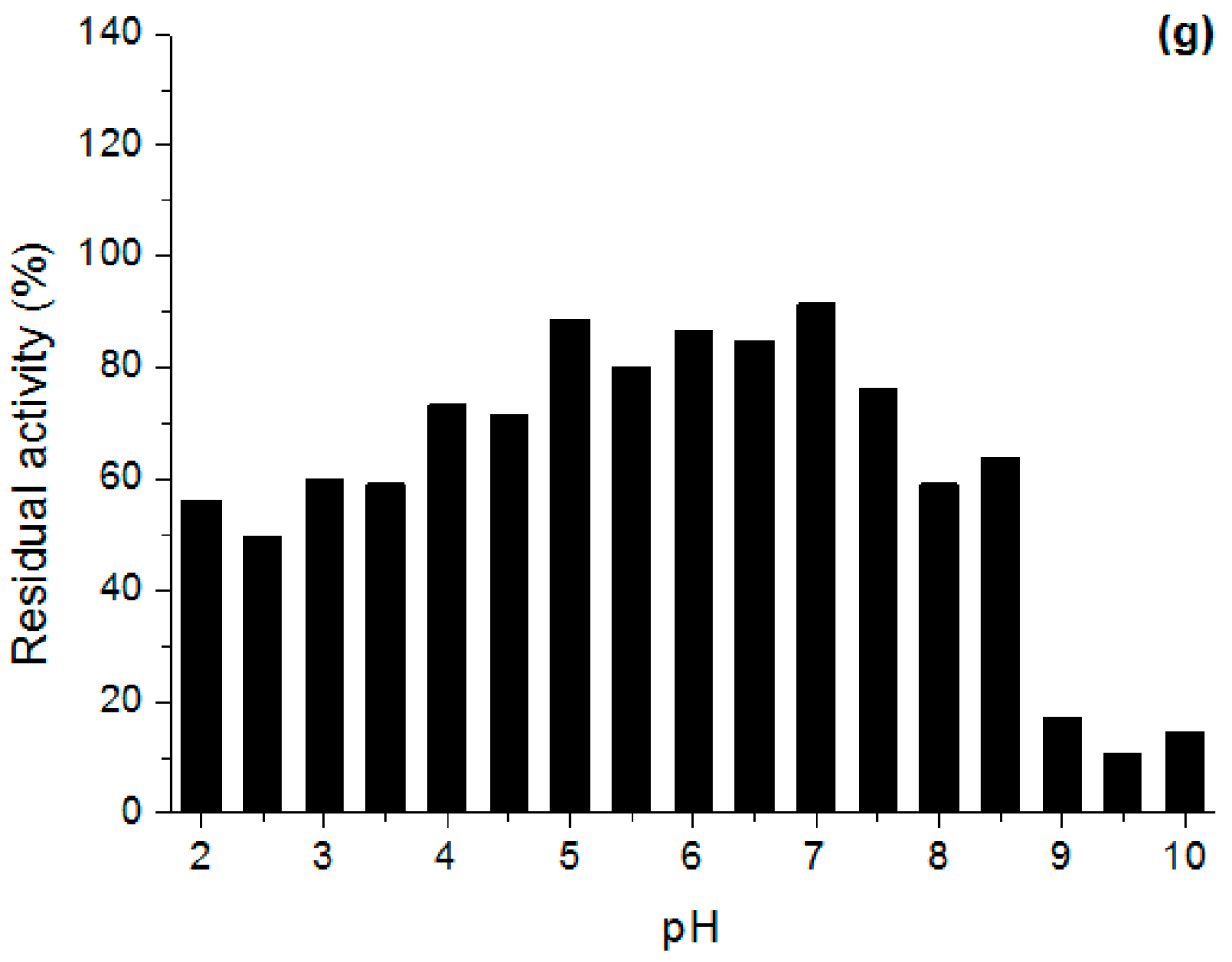
Effect of pH and Temperature on Lipases Activity
2.3. Hydrolysis of Fish Oil in Aqueous Media
2.4. Ethanolysis of Fish Oil in Organic Media
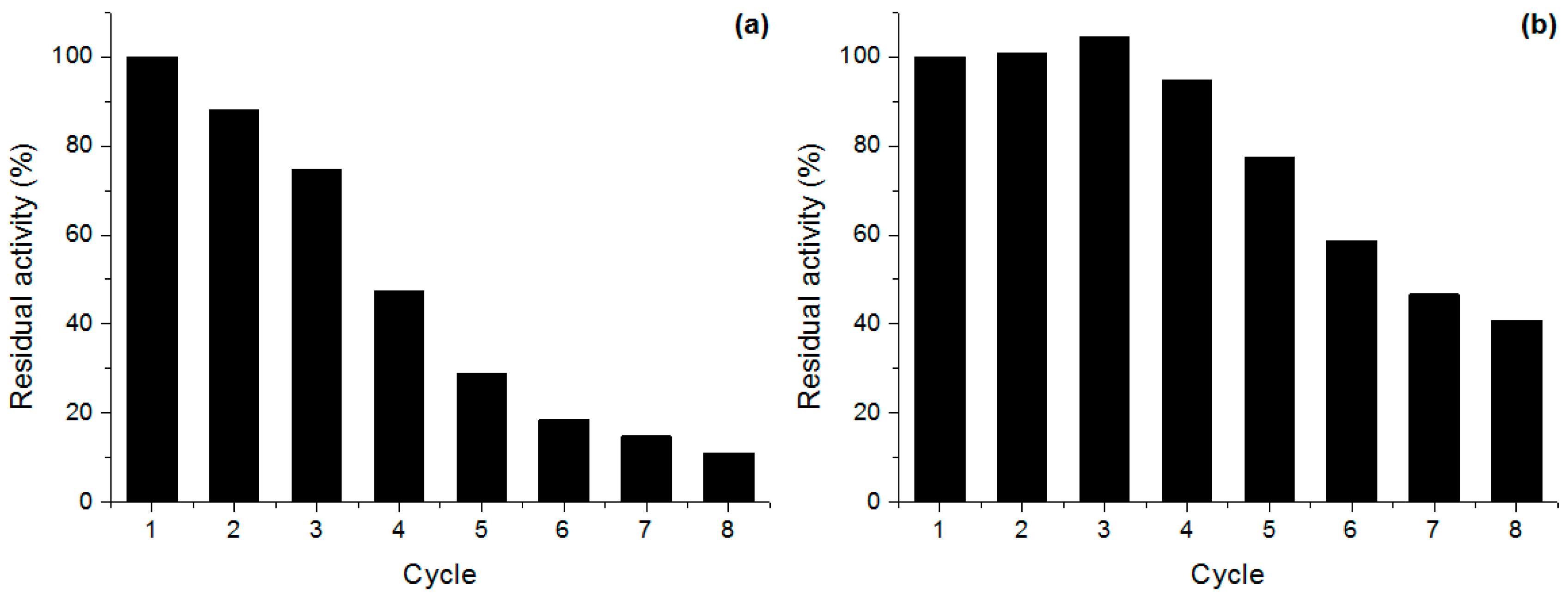
2.5. Derivative Reuse
3. Materials and Methods
3.1. Materials
3.2. Strain Maintenance and Lipase Production
3.3. Enzyme Activity and Protein Determination Assays
3.4. Supports Preparation
3.5. Enzyme Immobilization
3.6. Derivative Characterization
3.6.1. Thermal Stability
3.6.2. Stability in Different pH
3.6.3. Stability in Different Organic Media
3.7. Fish Oil Hydrolysis
3.8. Fish Oil Ethanolysis
3.9. Derivative Reuse
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gupta, R.; Rathi, P.; Bradoo, S. Lipase mediated upgradation of dietary fats and oils. Crit. Rev. Food Sci. Nutr. 2003, 43, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Gosh, P.K.; Saxena, R.K.; Gupta, R.; Yadav, R.P.; Davidson, S. Microbial lipases: Production and applications. Sci. Prog. 1996, 79, 119–157. [Google Scholar]
- Reetz, M.T. Lipases as practical biocatalysts. Curr. Opin. Chem. Biol. 2002, 6, 145–150. [Google Scholar] [CrossRef]
- Brady, D.; Jordaan, J. Advances in enzyme immobilization. Biotechnol. Lett. 2009, 31, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernández-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lafuente, R.; Armisén, P.; Sabuquillo, P.; Fernández-Lorente, G.; Guisán, J.M. Immobilization of lipases by selective adsorption on hydrophobic supports. Chem. Phys. Lipids 1998, 93, 185–197. [Google Scholar] [CrossRef]
- Manoel, E.A.; dos Santos, J.C.S.; Freire, D.M.G.; Rueda, N.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzyme Microb. Technol. 2015, 71, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Bastida, A.; Sabuquillo, P.; Armisen, P.; Fernandez-Lafuente, R.; Huguet, J.; Guisan, J.M. A single step purification, immobilization, and hyperactivation of lipases via interfacial adsorption on strongly hydrophobic supports. Biotechnol. Bioeng. 1998, 58, 486–493. [Google Scholar] [CrossRef]
- Palomo, J.M.; Muñoz, G.; Fernández-Lorente, G.; Mateo, C.; Fernández-Lafuente, R.; Guisan, J.M. Interfacial adsorption of lipases on very hydrophobic support (octadecyl–Sepabeads): Immobilization, hyperactivation and stabilization of the open form of lipases. J. Mol. Catal. B Enzyme 2002, 19–20, 279–286. [Google Scholar] [CrossRef]
- Fernández-Lorente, G.; Palomo, J.M.; Cabrera, Z.; Guisán, J.M.; Fernández-Lafuente, R. Specificity enhancement towards hydrophobic substrates by immobilization of lipases by interfacial activation on hydrophobic supports. Enzyme Microb. Technol. 2007, 41, 565–569. [Google Scholar] [CrossRef]
- Fernández-Lorente, G.; Cabrera, Z.; Godoy, C.; Fernandez-Lafuente, R.; Palomo, J.M.; Guisán, J.M. Interfacially activated lipases against hydrophobic supports: Effect of the support nature on the biocatalytic properties. Process Biochem. 2008, 43, 1061–1067. [Google Scholar]
- Fernández-Lorente, G.; Betancor, L.; Carrascosa, A.V.; Guisán, J.M. Release of Omega-3 Fatty Acids by the Hydrolysis of Fish Oil Catalyzed by Lipases Immobilized on Hydrophobic Supports. J. Am. Oil Chem. Soc. 2011, 88, 1173–1178. [Google Scholar] [CrossRef]
- Pizarro, C.; Brañes, M.C.; Markovits, A.; Fernández-Lorente, G.; Guisán, J.M.; Chamy, R.; Wilson, L. Influence of different immobilization techniques for Candida cylindracea lipase on its stability and fish oil hydrolysis. J. Mol. Catal. B Enzyme 2012, 78, 111–118. [Google Scholar] [CrossRef]
- Bassi, J.J.; Todero, L.M.; Lage, F.A.P.; Khedy, G.I.; Dell Ducas, J.; Custódio, A.P.; Pinto, M.A.; Mendes, A.A. Interfacial activation of lipases on hydrophobic support and application in the synthesis of a lubricant ester. Int. J. Biol. Macromol. 2016, 92, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Perez, S.; Turati, D.F.M.; Borges, J.P.; Luna, P.; Señorans, F.J.; Guisan, J.M.; Fernandez-Lorente, G. Critical role of different immobilized biocatalysts of a given lipase in the selective ethanolysis of sardine oil. J. Agric. Food Chem. 2017, 65, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Virgen-Ortíz, J.J.; Tacias-Pascacio, V.G.; Hirata, D.B.; Torrestiana-Sanchez, B.; Rosales-Quintero, A.; Fernandez-Lafuente, R. Relevance of substrates and products on the desorption of lipases physically adsorbed on hydrophobic supports. Enzyme Microb. Technol. 2017, 96, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Deckelbaum, R.J.; Torrejon, C. The Omega-3 Fatty Acid Nutritional Landscape: Health Benefits and Sources. J. Nutr. 2012, 142, 587S–591S. [Google Scholar] [CrossRef] [PubMed]
- Da Boit, M.; Sibson, R.; Sivasubramaniam, S.; Meakin, J.R.; Greig, C.A.; Aspden, R.M.; Thies, F.; Jeromson, S.; Hamilton, D.L.; Speakman, J.R.; et al. Sex differences in the effect of fish oil supplementation on the adaptive response to resistance exercise training in older people: A randomized control trial. Am. J. Clin. Nutr. 2017, 105, 151–158. [Google Scholar] [PubMed]
- Tauk-Tornisielo, S.M.; Garlipp, A.; Ruegger, M.; Attili, D.S.; Malagutti, E. Soilborne filamentous fungi in Brazil. J. Basic. Microbiol. 2005, 45, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Zucca, P.; Fernandez-Lafuente, R.; Sanjust, E. Agarose and its derivatives as supports for enzyme immobilization. Molecules 2016, 21, 1577. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Abian, O.; Bernedo, M.; Cuenca, E.; Fuentes, M.; Fernández-Lorente, G.; Palomo, J.M.; Grazu, V.; Pessela, B.C.; Giacomini, C.; et al. Some special features of glyoxyl supports to immobilize proteins. Enzyme Microb. Techonol. 2005, 37, 456–462. [Google Scholar] [CrossRef]
- Lima, L.N.; Aragon, C.C.; Mateo, C.; Palomo, J.M.; Giordano, R.L.C.; Tardioloi, P.W.; Guisán, J.M.; Fernández-Lorente, G. Immobilization and stabilization of a bimolecular aggregate of the lipase from Pseudomonas fluorescens by multipoint covalent attachment. Process Biochem. 2013, 48, 118–123. [Google Scholar] [CrossRef]
- Miranda, J.S.; Silva, N.C.A.; Bassi, J.J.; Corradini, M.C.C.; Lage, F.A.P.; Hirata, D.B.; Mendes, A.A. Immobilization of Thermomyces lanuginosus lipase on mesoporous poly-hydroxybutyrate particles and application in alkyl esters synthesis: Isotherm, thermodynamic and mass transfer studies. Chem. Eng. J. 2014, 251, 392–403. [Google Scholar] [CrossRef]
- Prlainovic, N.Z.; Knezevic-Jugovic, Z.D.; Mijin, D.Z.; Bezbradica, D.I. Immobilization of lipase from Candida rugosa on Sepabeads: The effect of lipase oxidation by periodates. Bioprocess Biosyst. Eng. 2011, 34, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Sangster, J. Octanol-water partition coefficients of simple organic compounds. J. Phys. Chem. Ref. Data 1989, 18, 1111–1227. [Google Scholar] [CrossRef]
- Castro-Ochoa, L.D.; Rodríguez-Gómez, C.; Valerio-Alfaro, G.; Ros, R.O. Screening, purification and characterization of the thermoalkalophilic lipase produced by Bacillus thermoleovorans CCR11. Enzyme Microb. Technol. 2005, 37, 648–654. [Google Scholar] [CrossRef]
- Pereira, M.G.; Facchini, F.D.A.; Filó, L.E.C.; Polizeli, A.M.; Vici, A.C.; Jorge, J.A.; Fernández-Lorente, G.; Pessela, B.C.; Guisán, J.M.; Polizeli, M.L.T.M. Immobilized lipase from Hypocrea pseudokoningii on hydrophobic and ionic supports: Determination of thermal and organic solvent stabilities for applications in the oleochemical industry. Process Biochem. 2015, 50, 561–570. [Google Scholar] [CrossRef]
- Morais Júnior, W.G.; Fernández-Lorente, G.; Guisán, J.M.; Ribeiro, E.J.; Resende, M.M.; Pessela, B.C. Production of omega-3 polyunsatured fatty acids through hydrolysis of fish oil by Candida rugosa lipase immobilized and stabilized on different supports. Biocatal. Biotransform. 2017, 35, 63–73. [Google Scholar]
- Zhu, X.M.; Hu, J.N.; Shin, J.A.; Li, D.; Jin, J.; Adhikari, P.; Akoh, C.C.; Lee, J.H.; Choi, S.W.; Lee, K.T. Enrichment of pinolenic acid at the sn-2 position of triacylglycerol molecules through lipase-catalyzed reaction. Int. J. Food Sci. Nutr. 2010, 61, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Pérez, S.; Fernández-Lorente, G.; Guisán, J.M. Selective ethanolysis of fish oil catalyzed by immobilized lipases. J. Am. Oil Chem. Soc. 2014, 91, 63–69. [Google Scholar] [CrossRef]
- Cipolatti, E.P.; Moreno-Pérez, S.; Souza, L.T.A.; Valério, A.; Guisán, J.M.; de Araujo, P.H.H.; Sayer, C.; Ninow, J.L.; Oliveira, D.; Pessela, B.C. Synthesis and modification of polyurethane for immobilization of Thermomyces lanuginosus (TLL) lipase for ethanolysis of fish oil in solvent free system. J. Mol. Catal. B Enzyme 2015, 122, 163–169. [Google Scholar] [CrossRef]
- Fadiloglu, S.; Soylemez, Z. Olive oil hydrolysis by celite-immobilized Candida rugosa lipase. J. Agric. Food Chem. 1998, 46, 3411–3414. [Google Scholar] [CrossRef]
- Cabrera-Padilla, R.Y.; Lisboa, M.C.; Fricks, A.T.; Franceschi, E.; Lima, A.S.; Silva, D.P.; Soares, C.M. Immobilization of Candida rugosa lipase on poly(3-hydroxybutyrate-co-hydroxyvalerate) a new eco-friendly support. J. Ind. Microbiol. Biotechnol. 2012, 39, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.

| Derivative | Yield (%) | Expressed Activity (%) | Specific Activity (U/mg Support) |
|---|---|---|---|
| Butyl Sepharose (But) | 90.1 | 78.9 | 5.1 |
| Phenyl Sepharose (Phe) | 77.3 | 87.9 | 4.7 |
| Octyl Sepharose (Oct) | 71.4 | 144.9 | 20.9 |
| Toyopearl butyl 650 M (Toyo) | 90.3 | 73.6 | 3.7 |
| Lewatit VPOC 1600 (Lew) | 76.8 | 54.2 | 3.6 |
| Octadecyl Sepabeads (Sep) | 82.1 | 81.5 | 3.5 |
| Derivative | Parameter | Temperature (°C) | Stabilization c | |
|---|---|---|---|---|
| 50 | 60 | |||
| But | Kd a (h−1) | 0.04 | NM d | - |
| t1/2 b (h) | 18.05 | |||
| Phe | Kd a (h−1) | 0.10 | NM d | - |
| t1/2 b (h) | 6.05 | |||
| Oct | Kd a (h−1) | 0.07 | 0.40 | 30.78 |
| t1/2 b (h) | 9.74 | 1.72 | ||
| Toyo | Kd a (h−1) | 0.02 | 0.27 | 46.25 |
| t1/2 b (h) | 25.11 | 2.59 | ||
| Lew | Kd a (h−1) | 0.53 | NM d | - |
| t1/2 b (h) | 1.30 | |||
| Sep | Kd a (h−1) | 0.89 | NM d | - |
| t1/2 b (h) | 0.78 | |||
| CNBr | Kd a (h−1) | NM d | 12.36 | - |
| t1/2 b (h) | 0.06 | |||
| Solvent | Log P | Residual Activity (%) |
|---|---|---|
| Water | - | 100 |
| Glycerol | −1.67 | 93.11 |
| DMSO | −1.35 | 41.90 |
| Acetonitrile | −0.34 | 0.00 |
| Ethanol | −0.30 | 0.00 |
| Tert-amyl alcohol | 0.89 | 0.00 |
| Cyclohexane | 3.44 | 48.79 |
| Derivative | Initial Activity a | Selectivity b |
|---|---|---|
| Free enzyme | 0.078 | 4.78 |
| But | 0.032 | 3.47 |
| Phe | 0.093 | 5.68 |
| Oct | 0.073 | 2.27 |
| CNBr | 0.055 | 11.60 |
| Solvent a | Derivative | Initial Activity b | Selectivity c |
|---|---|---|---|
| - | Sep | 0.130 | 2.82 |
| Lew | 0.172 | 2.31 | |
| Toyo | 0.074 | 1.44 | |
| Cyclohexane | Sep | 0.012 | 1.97 |
| Lew | 0.189 | 1.93 | |
| Toyo | - | - | |
| Tert-amyl alcohol | Sep | 0.020 | 2.20 |
| Lew | 0.988 | 2.07 | |
| Toyo | 0.017 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turati, D.F.M.; Morais Júnior, W.G.; Terrasan, C.R.F.; Moreno-Perez, S.; Pessela, B.C.; Fernandez-Lorente, G.; Guisan, J.M.; Carmona, E.C. Immobilization of Lipase from Penicillium sp. Section Gracilenta (CBMAI 1583) on Different Hydrophobic Supports: Modulation of Functional Properties. Molecules 2017, 22, 339. https://doi.org/10.3390/molecules22020339
Turati DFM, Morais Júnior WG, Terrasan CRF, Moreno-Perez S, Pessela BC, Fernandez-Lorente G, Guisan JM, Carmona EC. Immobilization of Lipase from Penicillium sp. Section Gracilenta (CBMAI 1583) on Different Hydrophobic Supports: Modulation of Functional Properties. Molecules. 2017; 22(2):339. https://doi.org/10.3390/molecules22020339
Chicago/Turabian StyleTurati, Daniela F. M., Wilson G. Morais Júnior, César R. F. Terrasan, Sonia Moreno-Perez, Benevides C. Pessela, Gloria Fernandez-Lorente, Jose M. Guisan, and Eleonora C. Carmona. 2017. "Immobilization of Lipase from Penicillium sp. Section Gracilenta (CBMAI 1583) on Different Hydrophobic Supports: Modulation of Functional Properties" Molecules 22, no. 2: 339. https://doi.org/10.3390/molecules22020339
APA StyleTurati, D. F. M., Morais Júnior, W. G., Terrasan, C. R. F., Moreno-Perez, S., Pessela, B. C., Fernandez-Lorente, G., Guisan, J. M., & Carmona, E. C. (2017). Immobilization of Lipase from Penicillium sp. Section Gracilenta (CBMAI 1583) on Different Hydrophobic Supports: Modulation of Functional Properties. Molecules, 22(2), 339. https://doi.org/10.3390/molecules22020339