Novel Magnetic Cross-Linked Cellulase Aggregates with a Potential Application in Lignocellulosic Biomass Bioconversion
Abstract
:1. Introduction
2. Results and Discussion
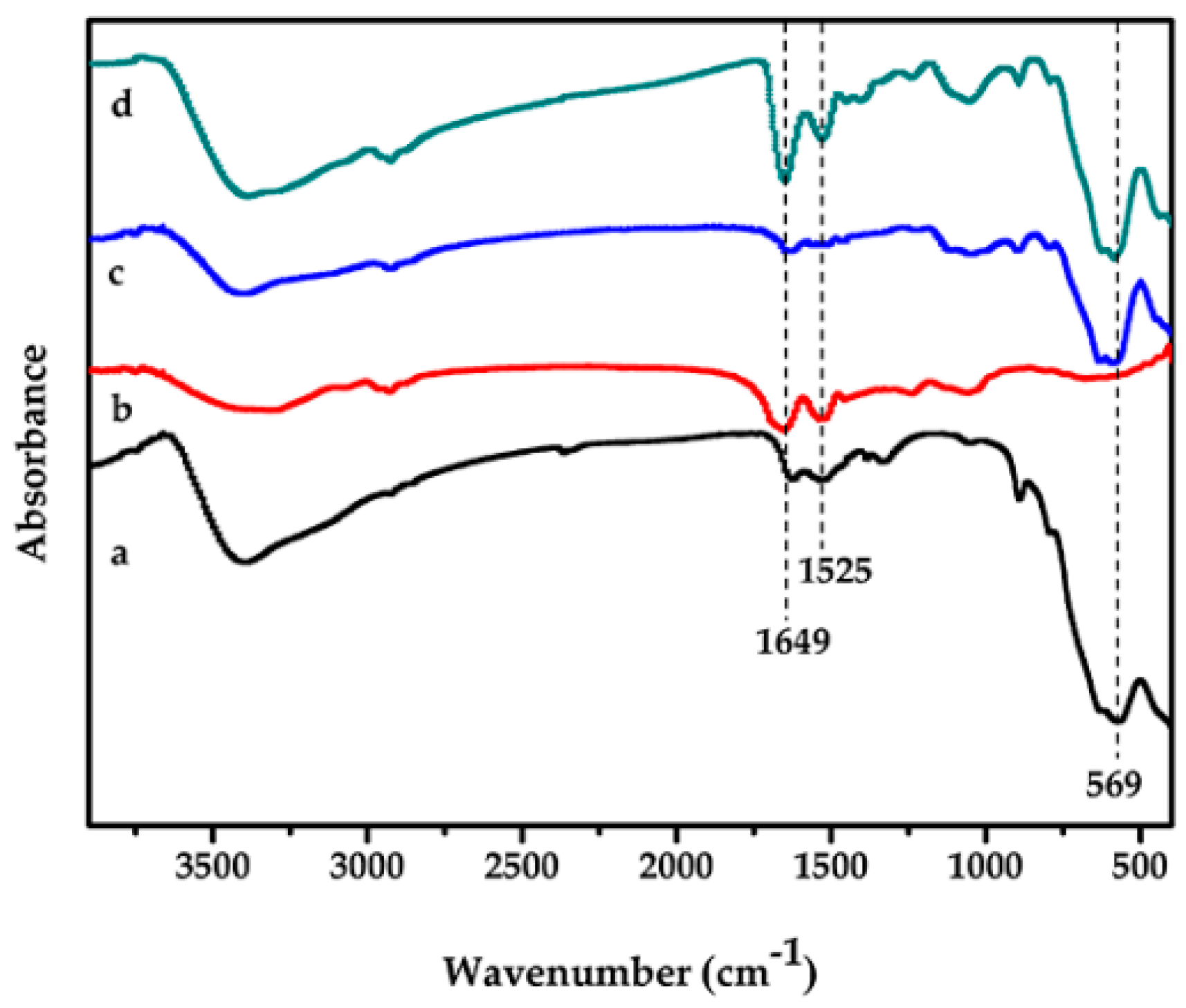
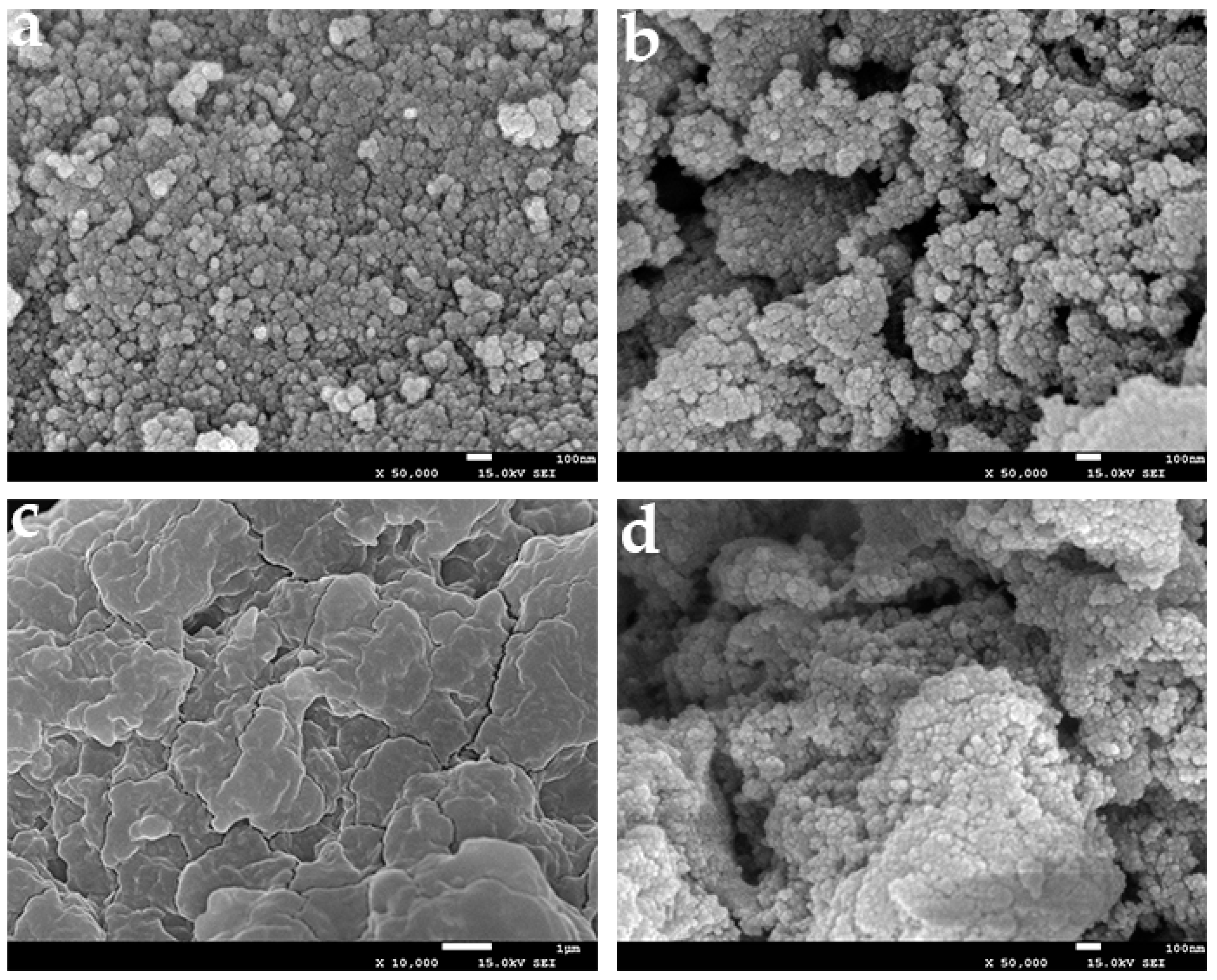
2.1. Chemical Characterisation and Analysis
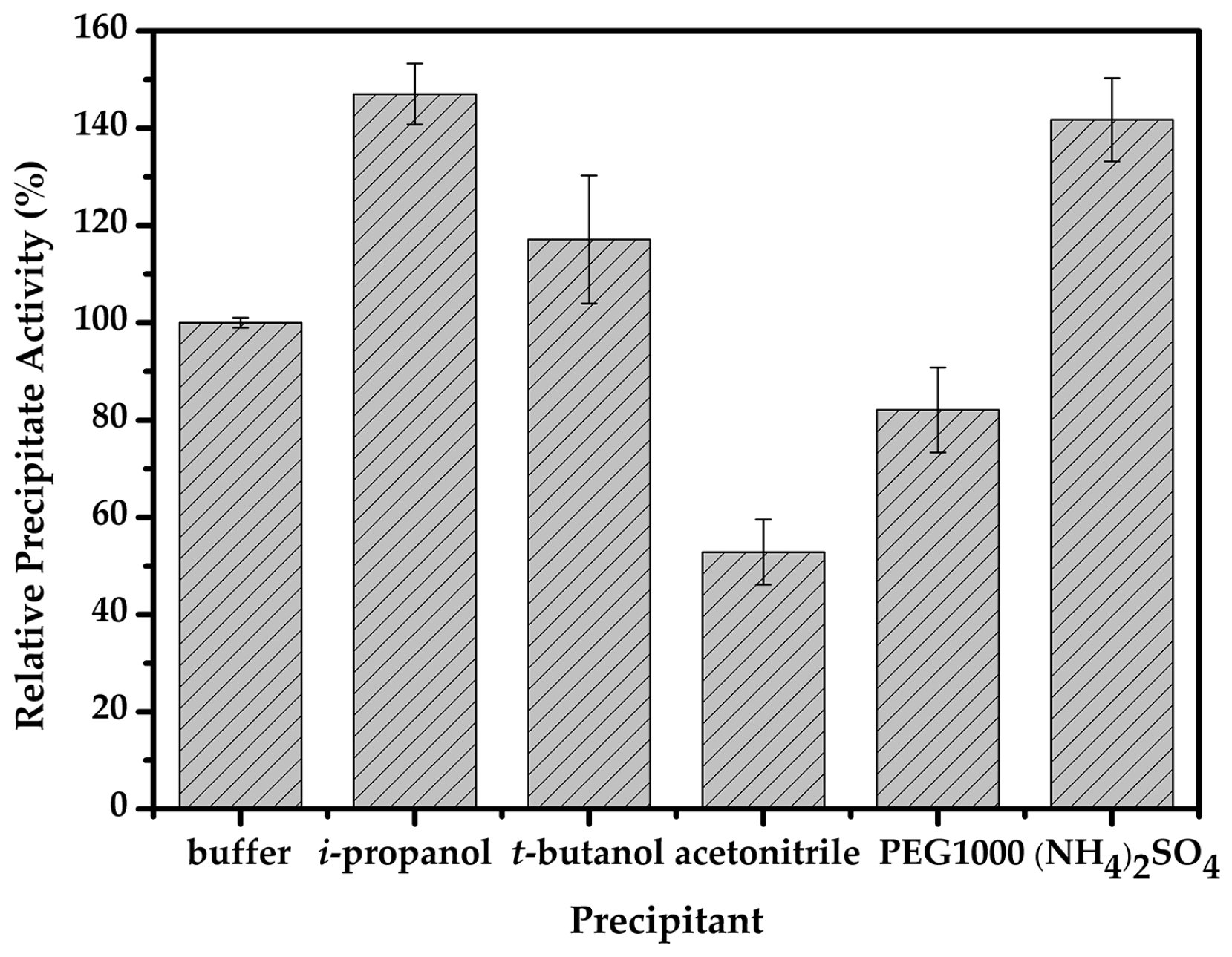
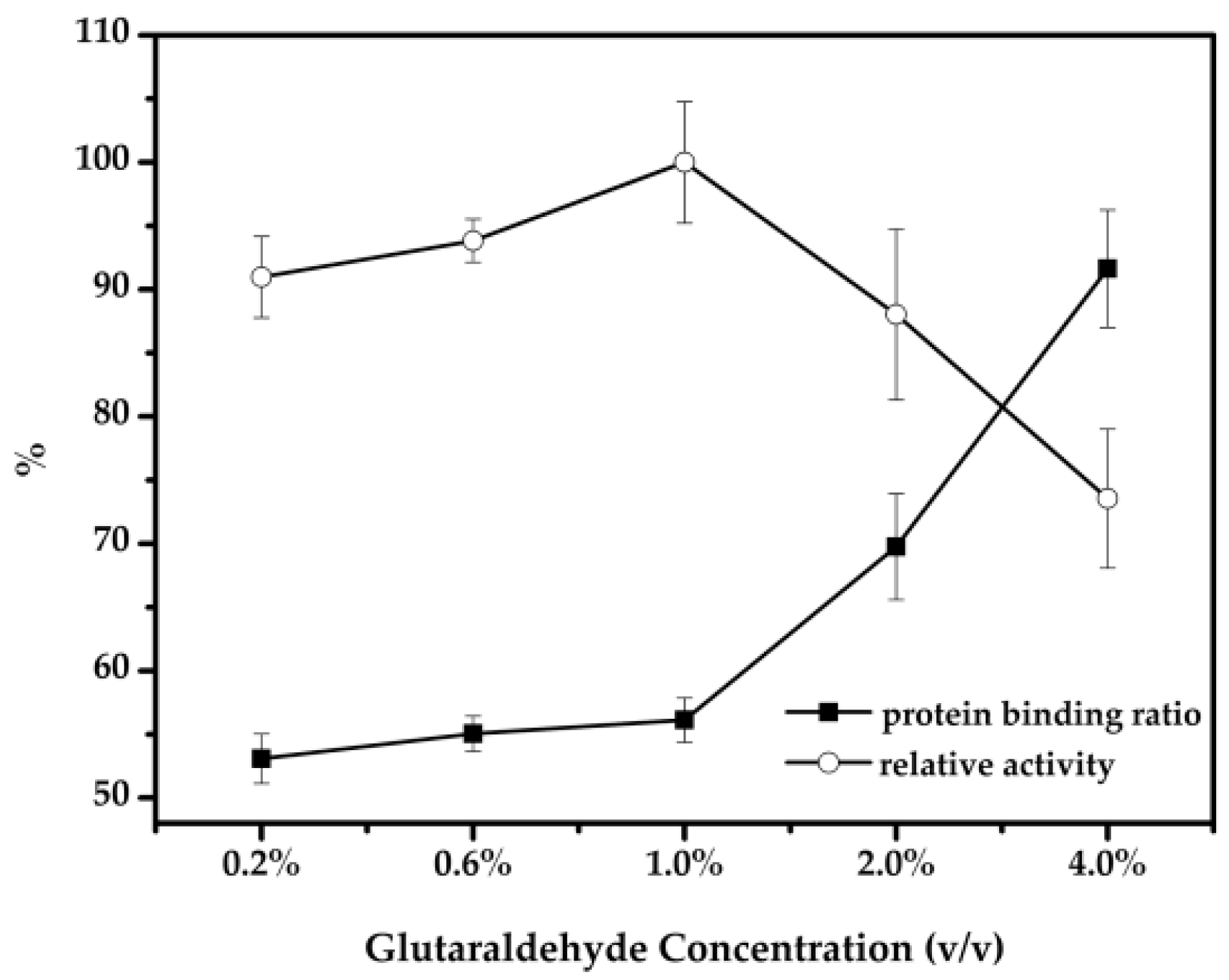
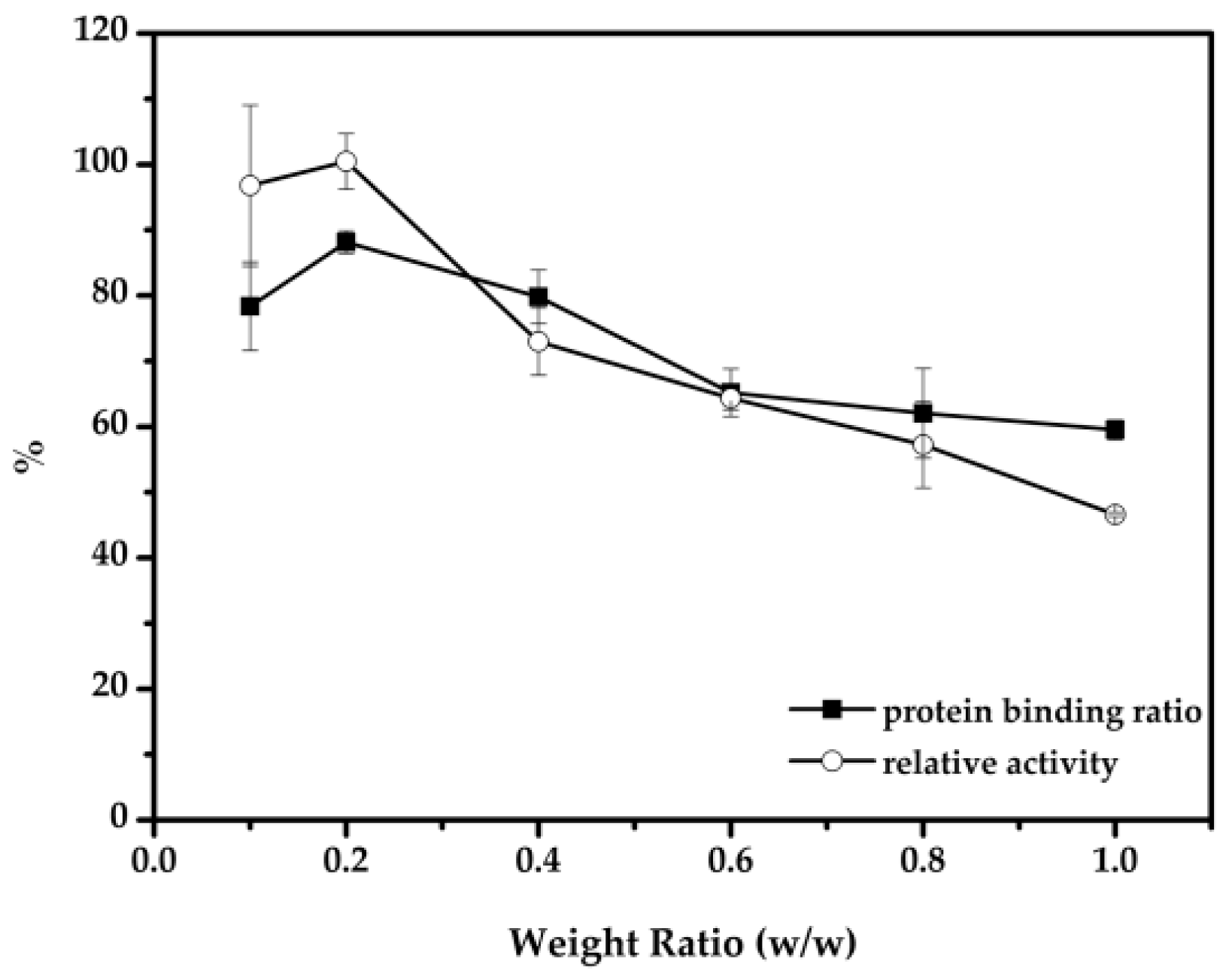
2.2. Optimal Conditions for the Immobilization of Cellulase
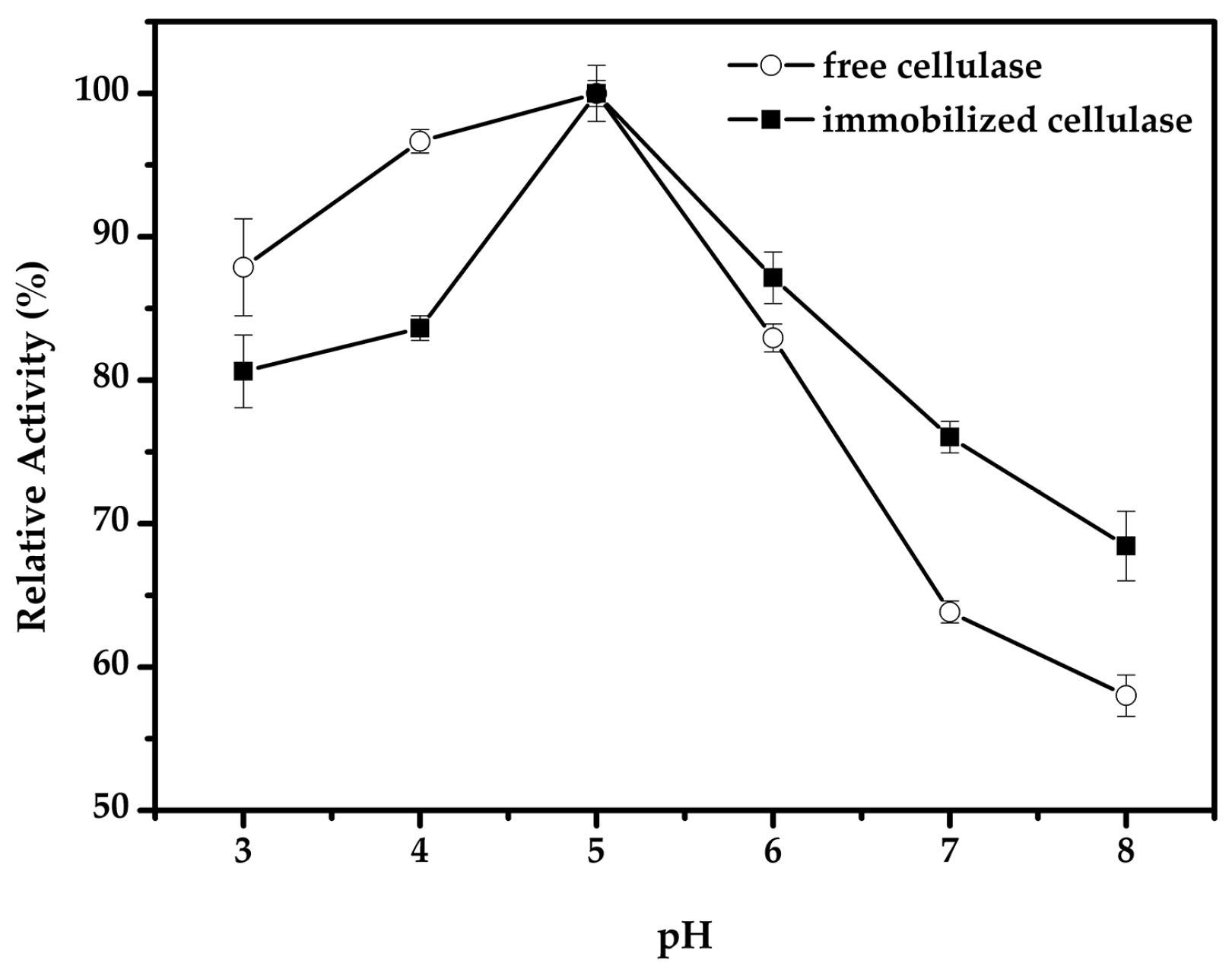
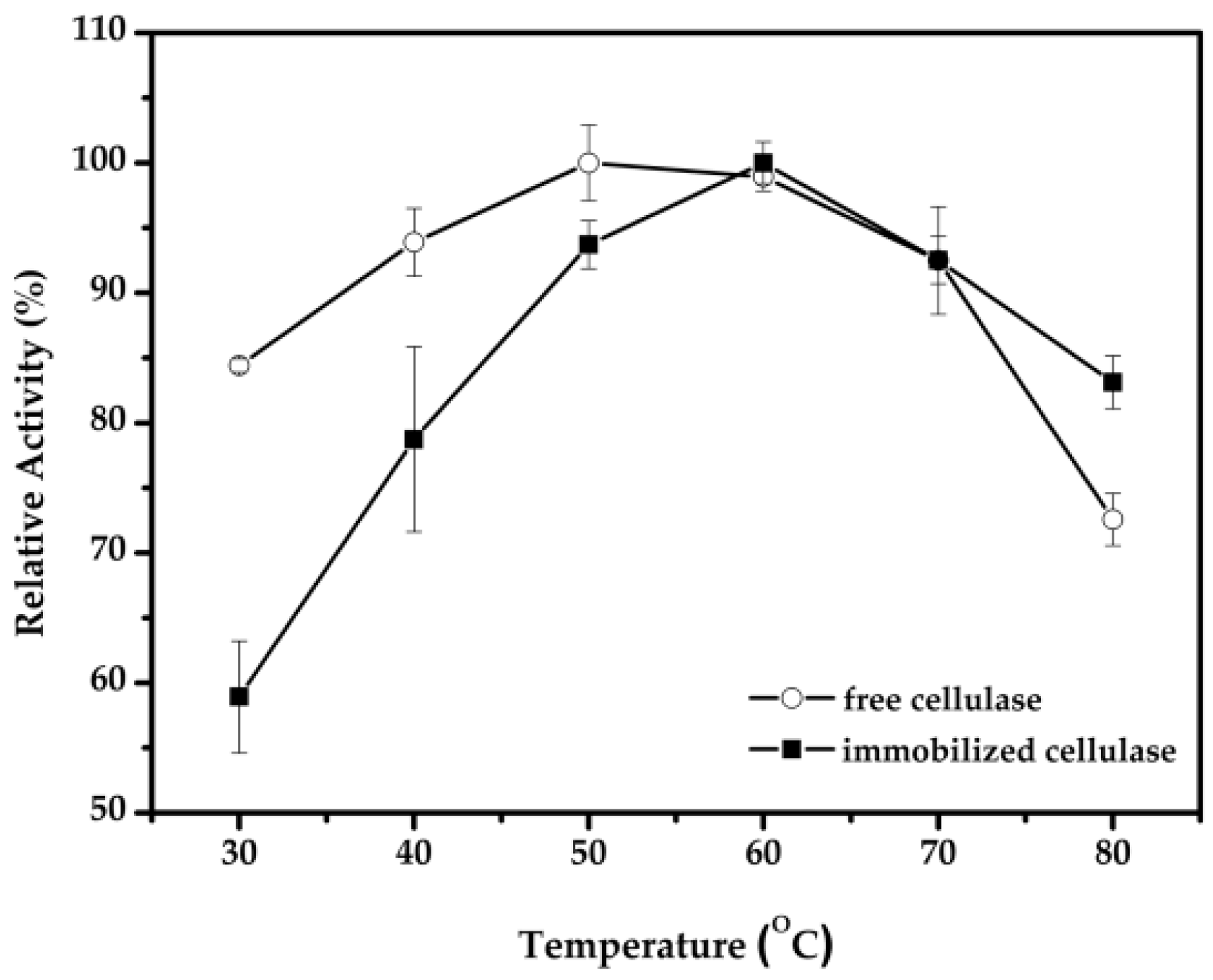
2.3. Effect of pH and Temperature for the Immobilized Cellulase
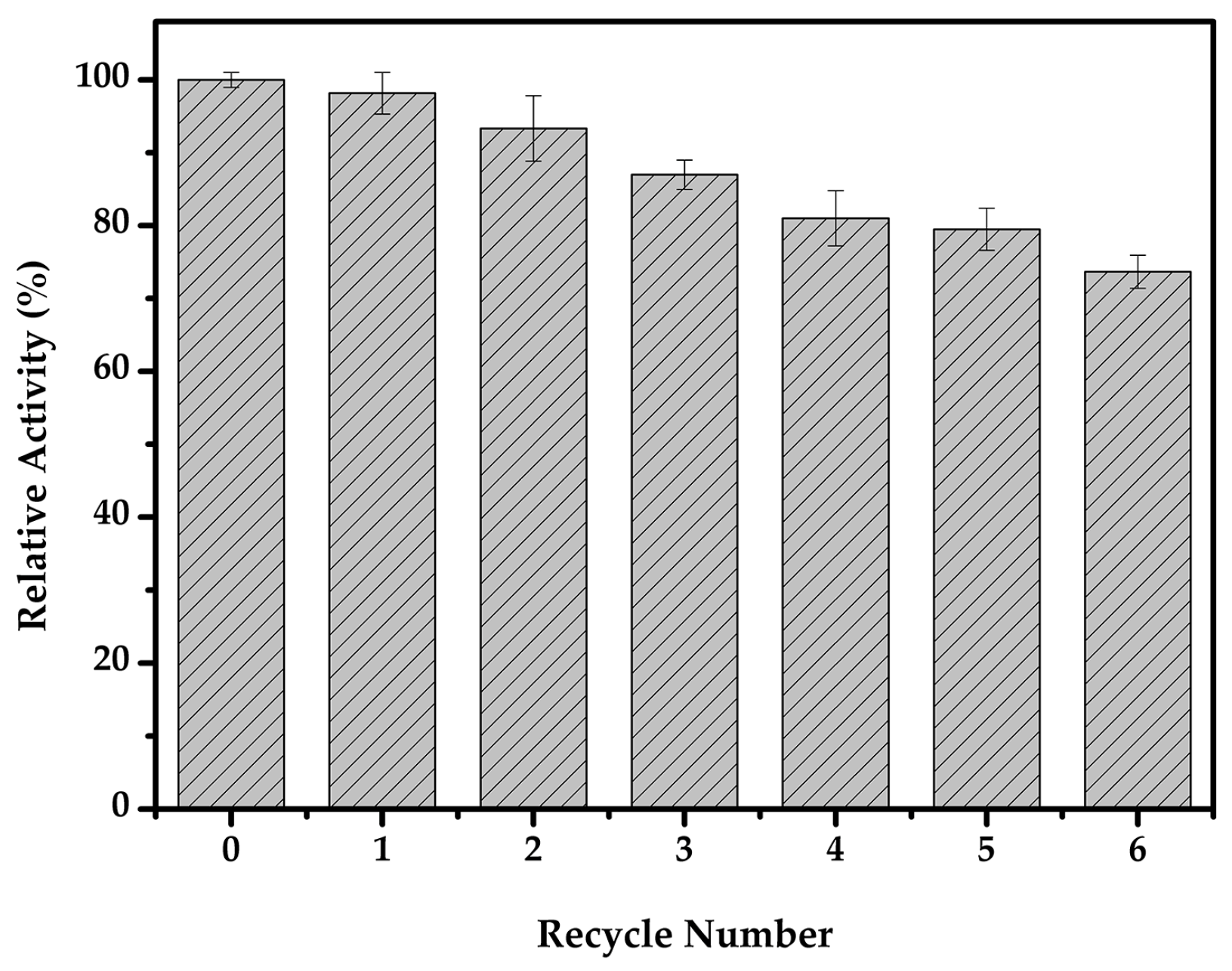
2.4. Carboxylmethyl Cellulose Reusability
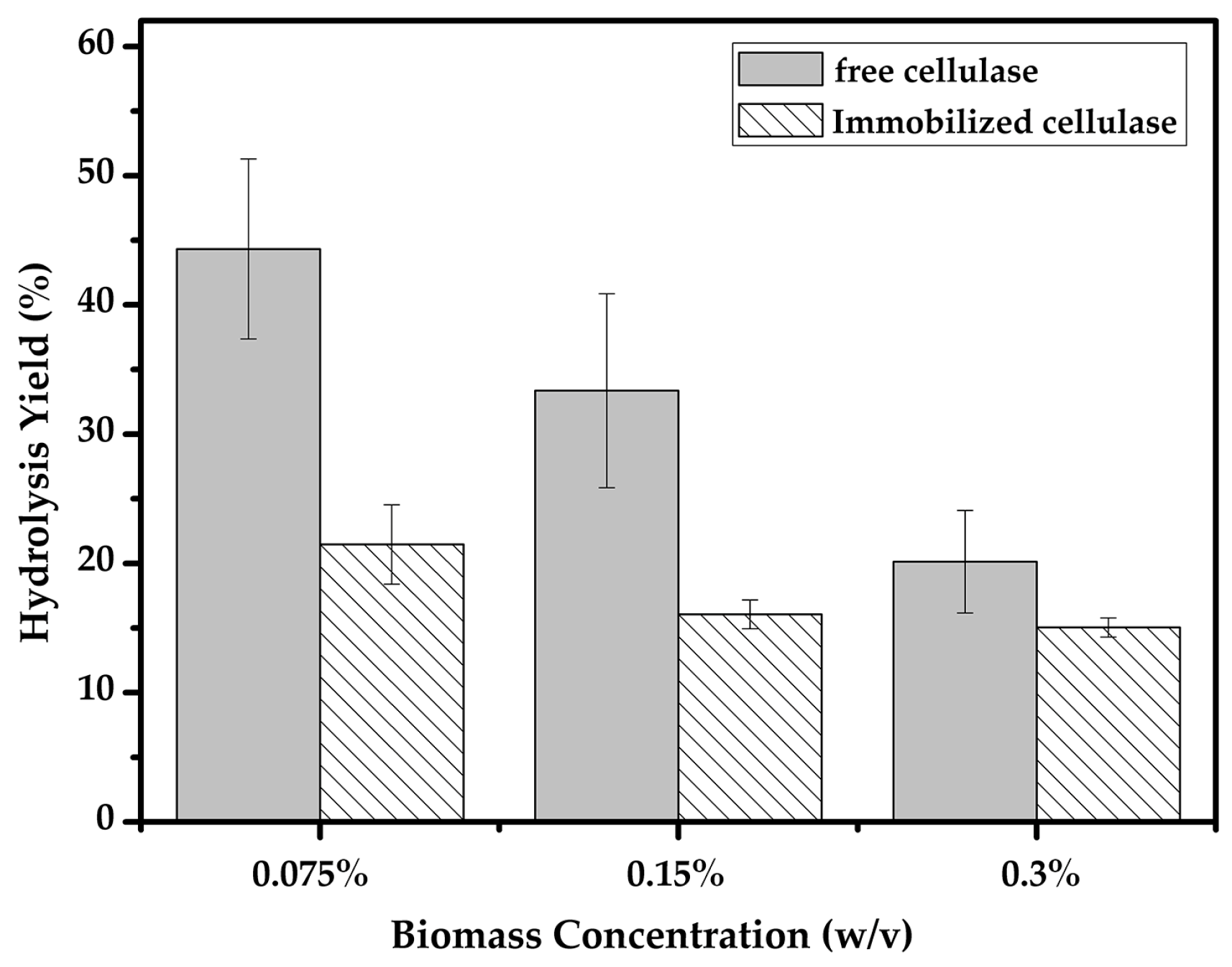
2.5. Hydrolysis of Biomass
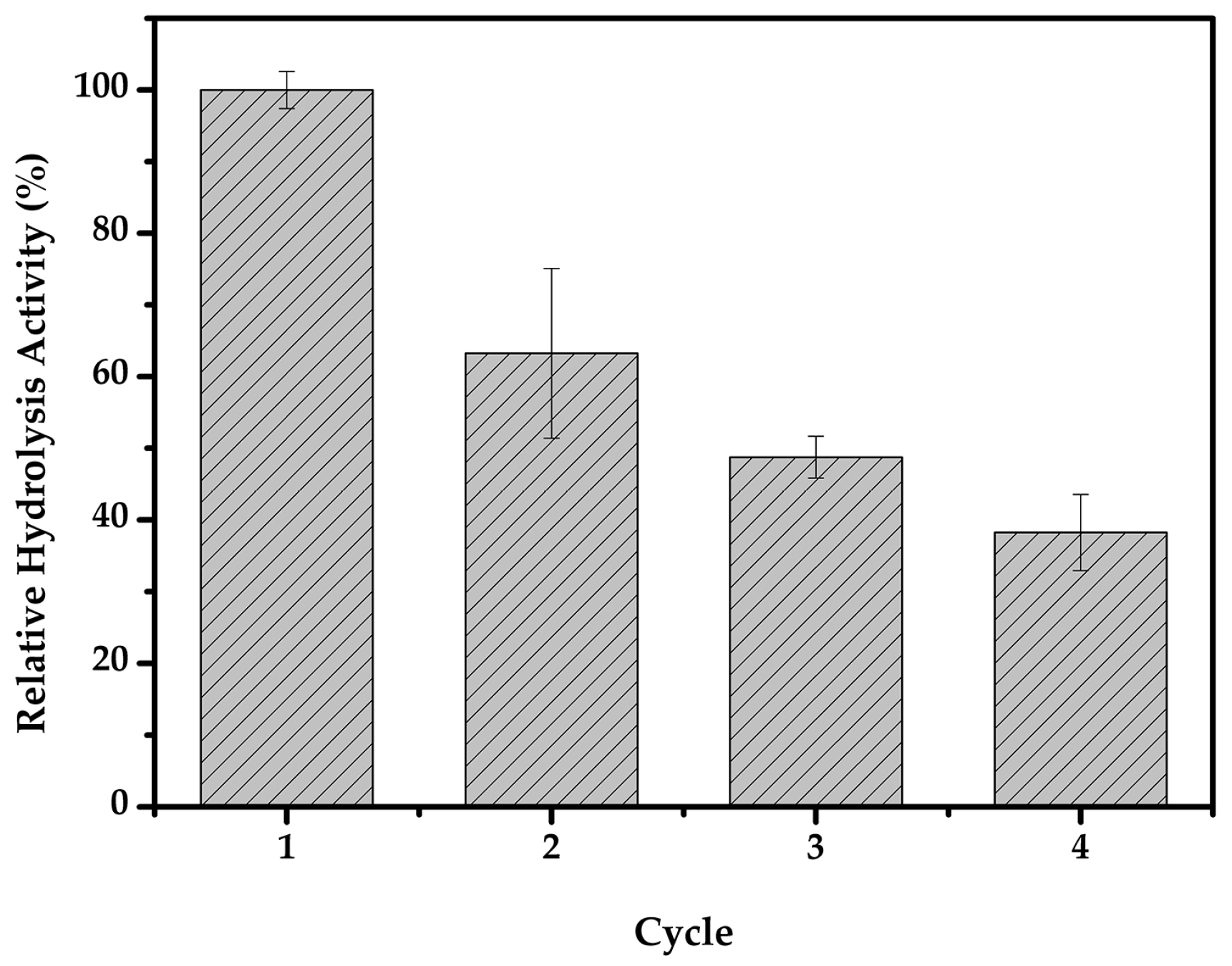
2.6. Biomass Hydrolysis Reusability
3. Materials and Methods
3.1. Materials
3.1.1. Enzymes and Chemicals
3.1.2. Cellulosic Biomass
3.2. Preparation of Modified Magnetic Fe3O4 Nanoparticles
3.3. Preparation of CLEAs and Magnetic CLEAs of Cellulase
3.4. Protein Binding Ratio and Enzyme Activity Measurements
3.5. Characterization
3.5.1. Fourier Transform Infrared Spectroscopy
3.5.2. Scanning Electron Microscope
3.5.3. UV-Vis
3.5.4. High Performance Liquid Chromatography
3.6. Optimization for Magnetic Cellulase CLEAs Preparation
3.7. Optimal Conditions for Cellulase Activity
3.8. CMC Reusability Study
3.9. Hydrolysis of Biomass
3.10. Biomass Hydrolysis Reusability
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, J.; Xi, J.; Wang, Y. Recent advances in the catalytic production of glucose from lignocellulosic biomass. Green Chem. 2015, 17, 737–751. [Google Scholar] [CrossRef]
- Lin, Y.; Tanaka, S. Ethanol fermentation from biomass resources: Current state and prospects. Appl. Microbiol. Biotechnol. 2006, 69, 627–642. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Fukuoka, A. Synthesis and utilisation of sugar compounds derived from lignocellulosic biomass. Green Chem. 2013, 15, 1740–1763. [Google Scholar] [CrossRef]
- Sun, S.N.; Cao, X.F.; Zhang, X.M.; Xu, F.; Sun, R.C.; Jones, G.L. Characteristics and enzymatic hydrolysis of cellulose-rich fractions from steam exploded and sequentially alkali delignified bamboo (Phyllostachys pubescens). Bioresour. Technol. 2014, 163, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Jeoh, T.; Ishizawa, C.I.; Davis, M.F.; Himmel, M.E.; Adney, W.S.; Johnson, D.K. Cellulase digestibility of pretreated biomass is limited by cellulose accessibility. Biotechnol. Bioeng. 2007, 98, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Harmer, M.A.; Fan, A.; Liauw, A.; Kumar, R.K. A new route to high yield sugars from biomass: Phosphoric-sulfuric acid. Chem. Commun. 2009, 43, 6610–6612. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wang, H.; Chen, Y.; She, Z.; Na, H.; Zhu, J. A novel facile two-step method for producing glucose from cellulose. Bioresour. Technol. 2013, 137, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, S.; Nakajima, K.; Kitano, M.; Yamaguchi, D.; Kato, H.; Hayashi, S.; Hara, M. Hydrolysis of cellulose by amorphous carbon bearing SO3H, COOH, and OH groups. J. Am. Chem. Soc. 2008, 130, 12787–12793. [Google Scholar] [CrossRef] [PubMed]
- Schüth, F.; Rinaldi, R.; Meine, N.; Käldström, M.; Hilgert, J.; Rechulski, M.D.K. Mechanocatalytic depolymerization of cellulose and raw biomass and downstream processing of the products. Catal. Today 2014, 234, 24–30. [Google Scholar] [CrossRef]
- Kobayashi, H.; Yabushita, M.; Komanoya, T.; Hara, K.; Fujita, I.; Fukuoka, A. High-yielding one-pot synthesis of glucose from cellulose using simple activated carbons and trace hydrochloric acid. ACS Catal. 2013, 3, 581–587. [Google Scholar] [CrossRef]
- Shimizu, K.; Satsuma, A. Toward a rational control of solid acid catalysis for green synthesis and biomass conversion. Energy Environ. Sci. 2011, 4, 3140–3153. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, Q.; Jiang, Z.; Yang, X.; Ji, Y. Enzymatic hydrolysis of pretreated soybean straw. Biomass Bioenergy 2007, 31, 162–167. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, W. Enzymatic hydrolysis of alkali-pretreated rice straw by Trichoderma reesei ZM4-F3. Biomass Bioenergy 2008, 32, 1130–1135. [Google Scholar] [CrossRef]
- Juturu, V.; Wu, J.C. Microbial cellulases: Engineering, production and applications. Renew. Sust. Energy Rev. 2014, 33, 188–203. [Google Scholar] [CrossRef]
- Verma, M.L.; Puri, M.; Barrow, C.J. Recent trends in nanomaterials immobilised enzymes for biofuel production. Crit. Rev. Biotechnol. 2016, 36, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.-Y.; Jiang, X.-P.; Ye, J.-J.; Zhang, Y.-W.; Zhang, X.-Y. Fabrication of graphene oxide decorated with Fe3O4@SiO2 for immobilization of cellulase. J. Nanopart. Res. 2015, 17, 1–12. [Google Scholar] [CrossRef]
- Cho, E.J.; Jung, S.; Kim, H.J.; Lee, Y.G.; Nam, K.C.; Lee, H.J.; Bae, H.J. Co-immobilization of three cellulases on Au-doped magnetic silica nanoparticles for the degradation of cellulose. Chem. Commun. 2012, 48, 886–888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qiu, J.; Feng, H.; Zang, L.; Sakai, E. Increase in stability of cellulase immobilized on functionalized magnetic nanospheres. J. Magn. Magn. Mater. 2015, 375, 117–123. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Bordbar, A.-K.; Zare, D.; Davoodi, D.; Noruzi, M.; Barkhi, M.; Tabatabaei, M. Immobilization of cellulase enzyme on superparamagnetic nanoparticles and determination of its activity and stability. Chem. Eng. J. 2011, 171, 669–673. [Google Scholar] [CrossRef]
- Alftrén, J.; Hobley, T.J. Immobilization of cellulase mixtures on magnetic particles for hydrolysis of lignocellulose and ease of recycling. Biomass Bioenergy 2014, 65, 72–78. [Google Scholar] [CrossRef]
- Zang, L.; Qiu, J.; Wu, X.; Zhang, W.; Sakai, E.; Wei, Y. Preparation of magnetic chitosan nanoparticles as support for cellulase immobilization. Ind. Eng. Chem. Res. 2014, 53, 3448–3454. [Google Scholar] [CrossRef]
- Jordan, J.; Kumar, C.S.S.R.; Theegala, C. Preparation and characterization of cellulase-bound magnetite nanoparticles. J. Mol. Catal. B Enzym. 2011, 68, 139–146. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.L.; Yuan, Z.H.; Qi, W.; Liu, Y.Y.; He, M.C. Artificial intelligence techniques to optimize the EDC/NHS-mediated immobilization of cellulase on Eudragit L-100. Int. J. Mol. Sci. 2012, 13, 7952–7962. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.T.; Meyer, A.S. Enzymatic cellulose hydrolysis: Enzyme reusability and visualization of beta-glucosidase immobilized in calcium alginate. Molecules 2014, 19, 19390–19406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talekar, S.; Joshi, A.; Joshi, G.; Kamat, P.; Haripurkar, R.; Kambale, S. Parameters in preparation and characterization of cross linked enzyme aggregates (CLEAs). RSC Adv. 2013, 3, 12485–12511. [Google Scholar] [CrossRef]
- Sheldon, R.A. Cross-linked enzyme aggregates (CLEA® s): Stable and recyclable biocatalysts. Biochem. Soc. Trans. 2007, 35, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Wilson, L.; Illanes, A.; Pessela, B.C.; Abian, O.; Fernandez-Lafuente, R.; Guisan, J.M. Encapsulation of crosslinked penicillin G acylase aggregates in lentikats: Evaluation of a novel biocatalyst in organic media. Biotechnol. Bioeng. 2004, 86, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Hilal, N.; Nigmatullin, R.; Alpatova, A. Immobilization of cross-linked lipase aggregates within microporous polymeric membranes. J. Membr. Sci. 2004, 238, 131–141. [Google Scholar] [CrossRef]
- Verma, M.L.; Barrow, C.J. Microbial Factories, 1st ed.; Springer: New Delhi, India, 2015; pp. 87–103. [Google Scholar]
- Hola, K.; Markova, Z.; Zoppellaro, G.; Tucek, J.; Zboril, R. Tailored functionalization of iron oxide nanoparticles for MRI, drug delivery, magnetic separation and immobilization of biosubstances. Biotechnol. Adv. 2015, 33, 1162–1176. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Sun, J.; Wang, Y.; Sheng, J.; Wang, F.; Sun, M. Application of iron magnetic nanoparticles in protein immobilization. Molecules 2014, 19, 11465–11486. [Google Scholar] [CrossRef] [PubMed]
- Talekar, S.; Ghodake, V.; Ghotage, T.; Rathod, P.; Deshmukh, P.; Nadar, S.; Mulla, M.; Ladole, M. Novel magnetic cross-linked enzyme aggregates (magnetic CLEAs) of alpha amylase. Bioresour. Technol. 2012, 123, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-W.; Yang, X.-L.; Jia, J.-Q.; Wang, N.; Hu, C.-L.; Yu, X.-Q. Surfactant-activated magnetic cross-linked enzyme aggregates (magnetic CLEAs) of Thermomyces lanuginosus lipase for biodiesel production. J. Mol. Catal. B Enzym. 2015, 115, 83–89. [Google Scholar] [CrossRef]
- Luo, Y.; Hu, L.; Tong, D.; Hu, C. Selective dissociation and conversion of hemicellulose in Phyllostachys heterocycla cv. var. pubescens to value-added monomers via solvent-thermal methods promoted by AlCl3. RSC Adv. 2014, 4, 24194–24206. [Google Scholar]
- Luo, Y.; Yi, J.; Tong, D.; Hu, C. Production of γ-valerolactone via selective catalytic conversion of hemicellulose in pubescens without addition of external hydrogen. Green Chem. 2016, 18, 848–857. [Google Scholar] [CrossRef]
- Bruce, I.J.; Sen, T. Surface modification of magnetic nanoparticles with alkoxysilanes and their application in magnetic bioseparations. Langmuir 2005, 21, 7029–7035. [Google Scholar] [CrossRef] [PubMed]
- Bohara, R.A.; Thorat, N.D.; Pawar, S.H. Immobilization of cellulase on functionalized cobalt ferrite nanoparticles. Korean J. Chem. Eng. 2015, 33, 216–222. [Google Scholar] [CrossRef]
- Wang, H.; Huang, J.; Wang, C.; Li, D.; Ding, L.; Han, Y. Immobilization of glucose oxidase using CoFe2O4/SiO2 nanoparticles as carrier. Appl. Surf. Sci. 2011, 257, 5739–5745. [Google Scholar] [CrossRef]
- Abraham, R.E.; Verma, M.L.; Barrow, C.J.; Puri, M. Suitability of magnetic nanoparticle immobilised cellulases in enhancing enzymatic saccharification of pretreated hemp biomass. Biotechnol. Biofuels 2014, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Palomo, J.M.; Fuentes, M.; Fernández-Lorente, G.; Mateo, C.; Guisan, J.M.; Fernández-Lafuente, R. General trend of lipase to self-assemble giving bimolecular aggregates greatly modifies the enzyme functionality. Biomacromolecules 2003, 4, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.L.; Rajkhowa, R.; Wang, X.; Barrow, C.J.; Puri, M. Exploring novel ultrafine Eri silk bioscaffold for enzyme stabilisation in cellobiose hydrolysis. Bioresour. Technol. 2013, 145, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Vaghari, H.; Jafarizadeh-Malmiri, H.; Mohammadlou, M.; Berenjian, A.; Anarjan, N.; Jafari, N.; Nasiri, S. Application of magnetic nanoparticles in smart enzyme immobilization. Biotechnol. Lett. 2016, 38, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, A.A.; Lu, J.; Lee, I. Immobilization of cellulase on magnetoresponsive nano-supports. J. Mol. Catal. B Enzym. 2013, 90, 76–86. [Google Scholar] [CrossRef]
- Kuo, C.-H.; Liu, Y.-C.; Chang, C.-M.J.; Chen, J.-H.; Chang, C.; Shieh, C.-J. Optimum conditions for lipase immobilization on chitosan-coated Fe3O4 nanoparticles. Carbohydr. Polym. 2012, 87, 2538–2545. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, C.; Wang, H. Pretreatment of bamboo residues with coriolus versicolor for enzymatic hydrolysis. J. Biosci. Bioeng. 2007, 104, 149–151. [Google Scholar] [CrossRef]
- Zhen, Q.; Wang, M.; Qi, W.; Su, R.; He, Z. Preparation of β-mannanase cleas using macromolecular cross-linkers. Catal. Sci. Technol. 2013, 3, 1937–1941. [Google Scholar] [CrossRef]
- He, T.; Jiang, Z.; Wu, P.; Yi, J.; Li, J.; Hu, C. Fractionation for further convension: From raw corn stover to lactic acid. Sci. Rep. 2016, 6, 38623. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Luo, Y.; Cai, B.; Li, J.; Tong, D.; Hu, C. The degradation of the lignin in Phyllostachys heterocycla cv. pubescens in an ethanol solvothermal system. Green Chem. 2014, 16, 3107–3116. [Google Scholar]
- Šulek, F.; Fernández, D.P.; Knez, Ž.; Habulin, M.; Sheldon, R.A. Immobilization of horseradish peroxidase as crosslinked enzyme aggregates (CLEAs). Process Biochem. 2011, 46, 765–769. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ghose, T.K. Measurement of cellulase activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Li, A.; Antizar-Ladislao, B.; Khraisheh, M. Bioconversion of municipal solid waste to glucose for bio-ethanol production. Bioprocess Biosyst. Eng. 2007, 30, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, J.; Zhang, W.; Yang, Z.; Yang, X.; Wang, N.; Yu, X. Novel Magnetic Cross-Linked Cellulase Aggregates with a Potential Application in Lignocellulosic Biomass Bioconversion. Molecules 2017, 22, 269. https://doi.org/10.3390/molecules22020269
Jia J, Zhang W, Yang Z, Yang X, Wang N, Yu X. Novel Magnetic Cross-Linked Cellulase Aggregates with a Potential Application in Lignocellulosic Biomass Bioconversion. Molecules. 2017; 22(2):269. https://doi.org/10.3390/molecules22020269
Chicago/Turabian StyleJia, Junqi, Weiwei Zhang, Zengjie Yang, Xianling Yang, Na Wang, and Xiaoqi Yu. 2017. "Novel Magnetic Cross-Linked Cellulase Aggregates with a Potential Application in Lignocellulosic Biomass Bioconversion" Molecules 22, no. 2: 269. https://doi.org/10.3390/molecules22020269



