Immobilized Trienzymatic System with Enhanced Stabilization for the Biotransformation of Lactose
Abstract
:1. Introduction
2. Results and Discussion
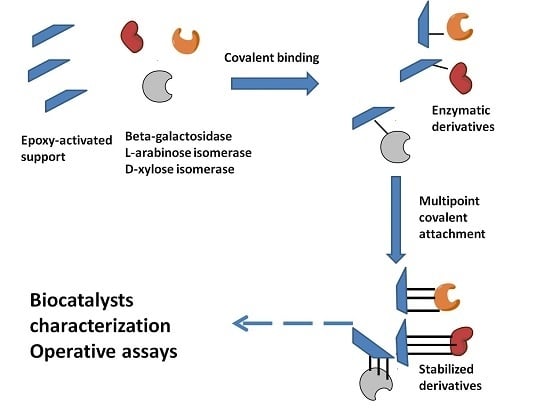
2.1. Immobilization and Stabilization
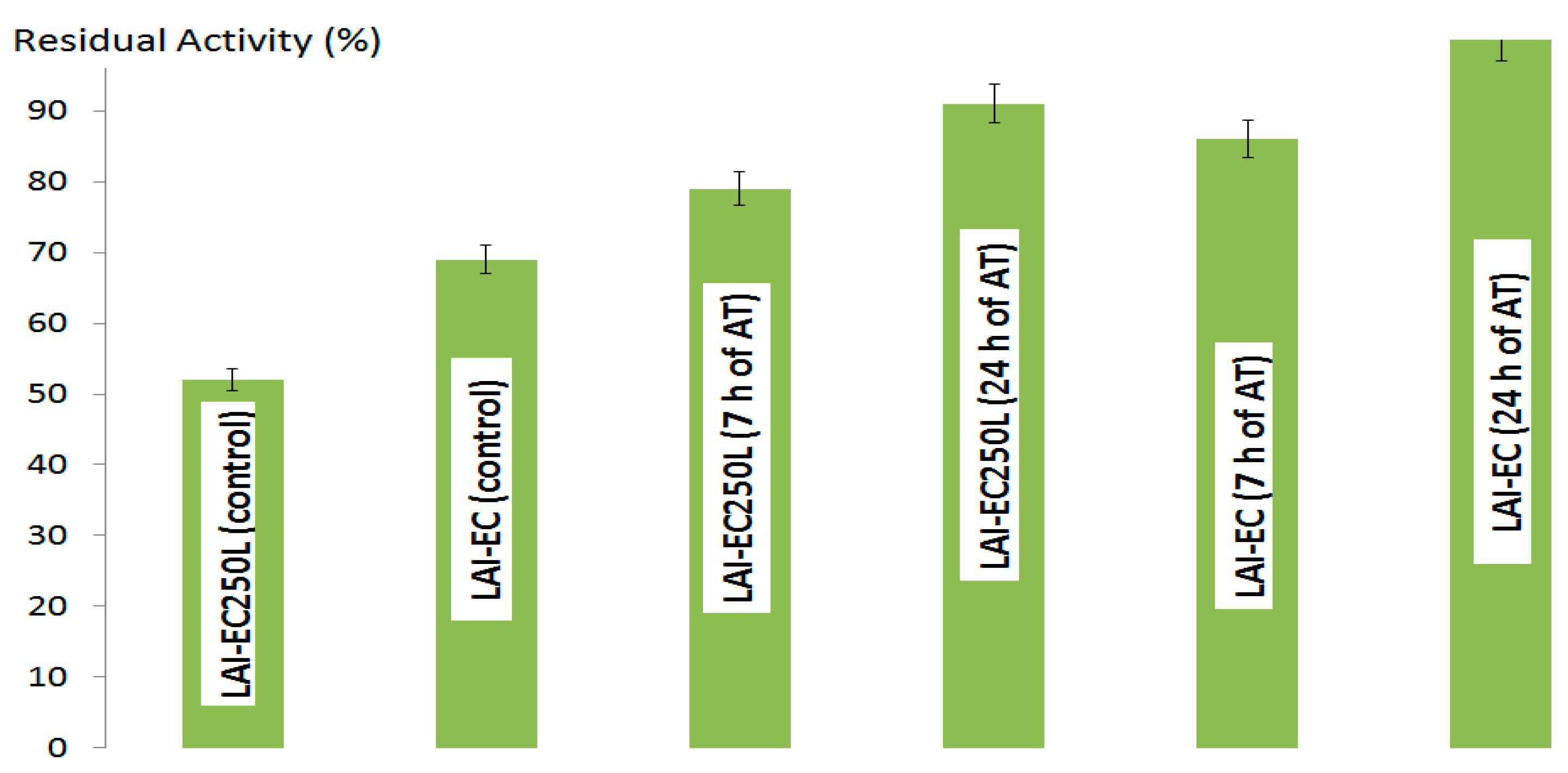
2.1.1. l-Arabinose Isomerase
2.1.2. d-Xylose Isomerase
2.2. Characterization of Derivatives
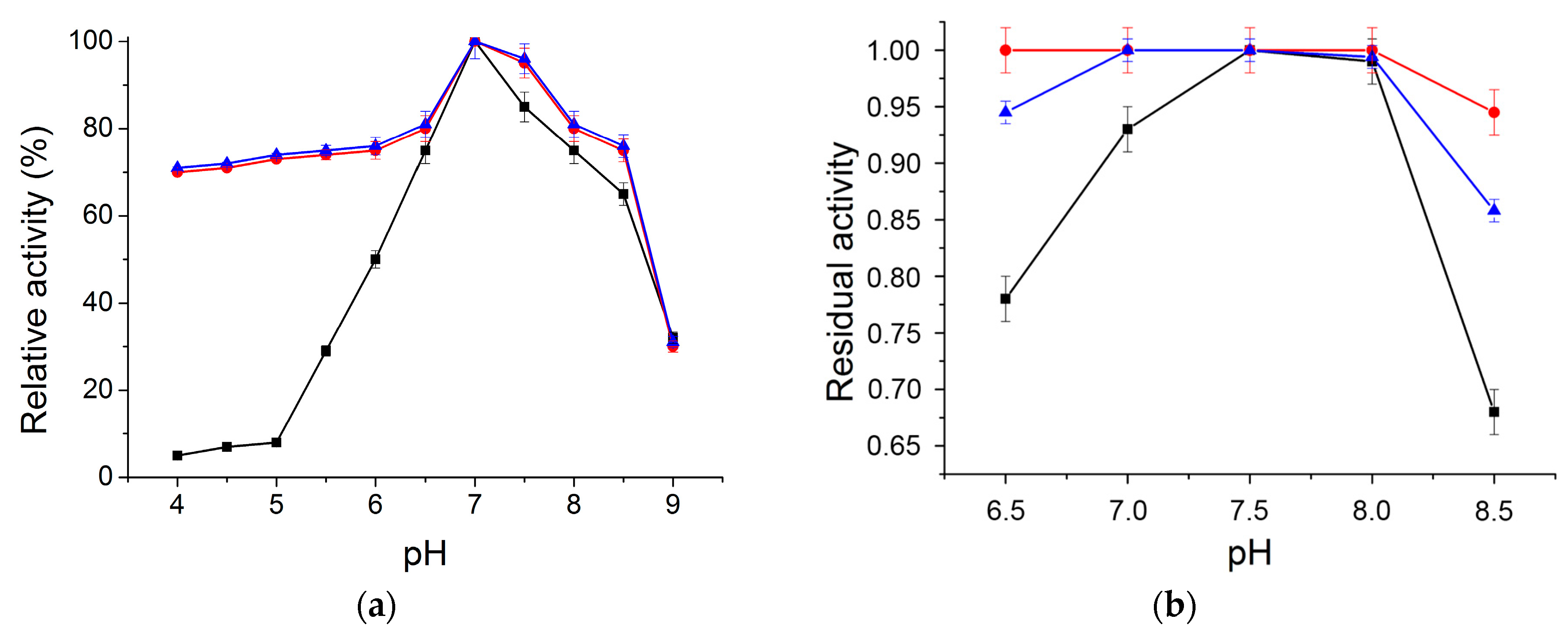
2.2.1. Optimum pH and pH Stability Profile
2.2.2. Optimum Temperature and Thermal Stability
2.2.3. Kinetic Parameters
2.3. Performance of Biocatalysts
2.3.1. d-Galactose and d-Glucose Isomerization by Immobilized Ketohexose Isomerases
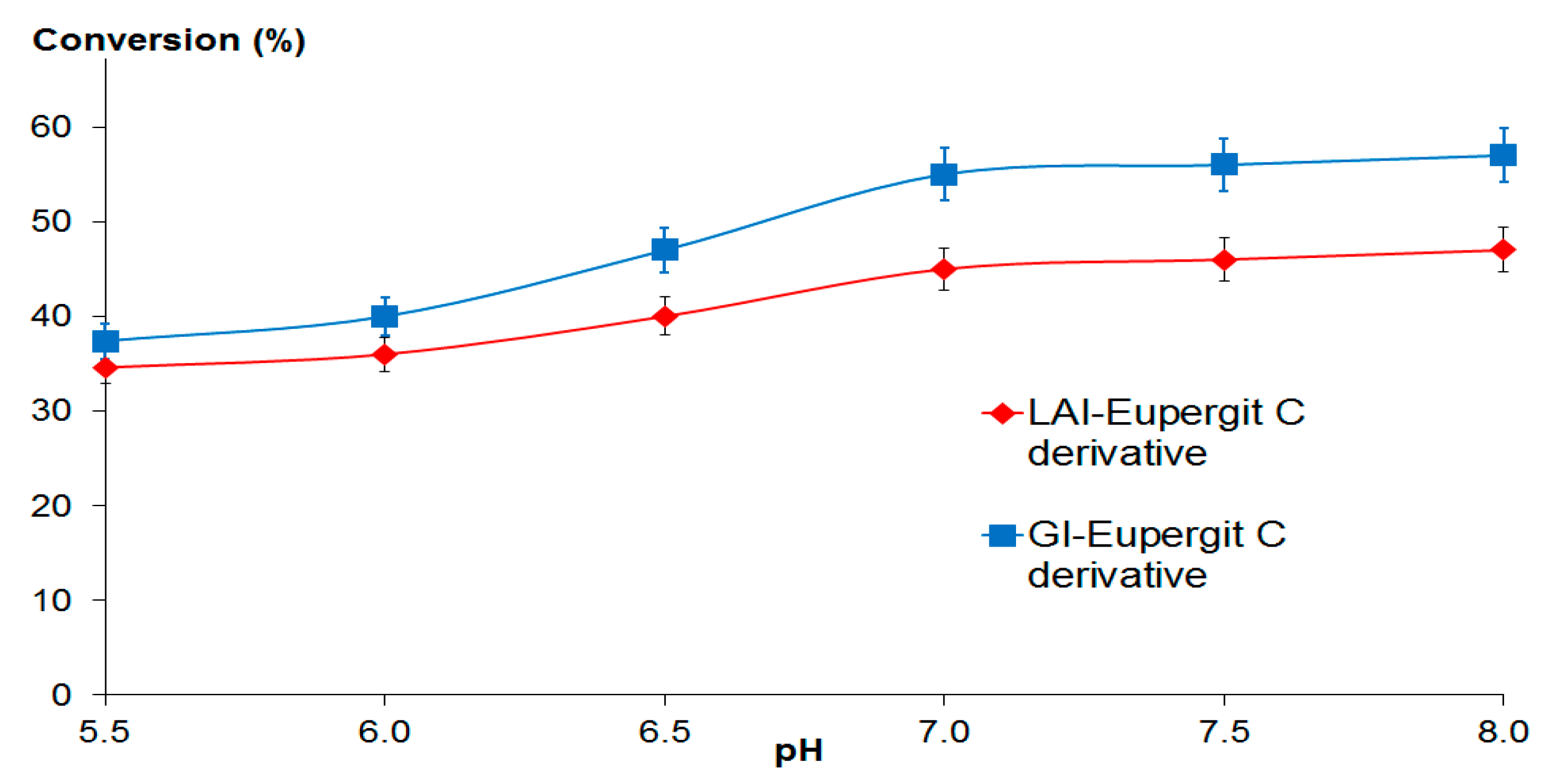
2.3.2. Lactose Bioconversion
3. Materials and Methods
3.1. Materials
3.2. Sugar Analysis
3.3. Protein Determination
3.4. Soluble Enzyme Treatment
3.5. Enzyme Immobilization
3.6. Enzyme Assays
3.7. Properties of Insoluble Derivatives
3.7.1. Effect of Temperature on Activity
3.7.2. Thermal Stability
3.7.3. Optimum pH and pH Stability
3.7.4. Determination of Kinetic Parameters
3.8. Applications with Substrates
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Choi, J.-M.; Han, S.-S.; Kim, H.-S. Industrial application of enzyme biocatalysis: Current status and future aspects. Biotechnol. Adv. 2015, 33, 1443–1454. [Google Scholar] [CrossRef] [PubMed]
- Brady, D.; Jordaan, J. Advances in enzyme immobilisation. Biotechnol. Lett. 2009, 31, 1639–1650. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernández-Lafuente, R. Strategies for the one-step immobilization-purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, P. Immobilisation and application of lipases in organic media. Chem. Soc. Rev. 2013, 42, 6406–6436. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Katchalski-Katzir, E.; Kraemer, D.M. Eupergit, a carrier for immobilization of enzymes of industrial potential. J. Mol. Catal. B Enzym. 2000, 10, 157–176. [Google Scholar] [CrossRef]
- Martín, M.T.; Plou, F.J.; Alcalde, M.; Ballesteros, A. Immobilization on Eupergit C of cyclodextrin glucosyltransferase (CGTase) and properties of immobilized biocatalyst. J. Mol. Catal. B Enzym. 2003, 21, 299–308. [Google Scholar] [CrossRef]
- Mateo, C.; Abian, O.; Fernandez-Lafuente, R.; Guisan, J.M. Increase in conformational stability of enzymes immobilized on epoxy-activated supports by favoring additional multipoint covalent attachment. Enzym. Microb. Technol. 2000, 26, 509–515. [Google Scholar] [CrossRef]
- Mateo, C.; Abian, O.; Fernández-Lorente, G.; Pedroche, J.; Fernández-Lafuente, R.; Guisan, J.M.; Tam, A.; Daminati, M. Epoxy Sepabeads: A novel epoxy support for stabilization of industrial enzymes via very intense multipoint covalent attachment. Biotechnol. Prog. 2002, 18, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Illanes, A. Whey upgrading by enzyme biocatalysis. Electron. J. Biotechnol. 2011, 14. [Google Scholar] [CrossRef]
- Torres, P.; Batista-Viera, F. Immobilization of β-galactosidase from Bacillus circulans onto epoxy-activated acrylic supports. J. Mol. Catal. B: Enzym. 2012, 74, 230–235. [Google Scholar] [CrossRef]
- Vetere, A.; Paoletti, S. Separation and characterization of three β-galactosidases from Bacillus circulans. Biochim. Biophys. Acta 1998, 1380, 225–231. [Google Scholar] [CrossRef]
- Mozaffar, Z.; Nakanishi, K.; Matsuno, R. Formation of oligosaccharides during hydrolysis of lactose in milk using β-galactosidase from Bacillus circulans. J. Food Sci. 1985, 50, 1602–1606. [Google Scholar] [CrossRef]
- Dekker, P.J.T.; Daamen, C.B.G. Enzymes exogenous to milk in dairy technology. β-galactosidase. In Encyclopedia of Dairy Science, 2nd ed.; Fuquay, J.W., Ed.; Academic Press: London, UK, 2011; pp. 276–283. [Google Scholar]
- Prazeres, A.R.; Carvalho, F.; Rivas, J. Cheese whey management: A review. J. Environ. Manag. 2012, 110, 48–68. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.; Batista-Viera, F. Improved biocatalysts based on Bacillus circulans β-galactosidase immobilized onto epoxy-activated acrylic supports: Applications in whey processing. J. Mol. Catal. B Enzym. 2012, 83, 57–64. [Google Scholar] [CrossRef]
- Yamanaka, K. l-arabinose isomerase from Lactobacillus gayonii. Methods Enzymol. 1975, 41, 458–461. [Google Scholar] [PubMed]
- Yeom, S.-J.; Ji, J.-H.; Yoon, R.-Y.; Oh, D.-K. l-ribulose production from l-arabinose by an l-arabinose isomerase mutant from Geobacillus thermodenitrificans. Biotechnol. Lett. 2008, 30, 1789–1793. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Kim, P.; Oh, D.-K. Properties of l-arabinose isomerase from Escherichia coli as biocatalyst for tagatose production. World J. Microbiol. Biotechnol. 2003, 19, 47–51. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, B.; Pan, B. Purification and characterization of l-arabinose isomerase from Lactobacillus plantarum producing d-tagatose. World J. Microbiol. Biotechnol. 2007, 23, 641–646. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Prabhu, P.; Lee, J.K. Immobilization of Bacillus licheniformis l-arabinose isomerase for semi-continuous l-ribulose production. Biosci. Biotechnol. Biochem. 2009, 73, 2234–2239. [Google Scholar] [CrossRef] [PubMed]
- Rhimi, M.; Messaoud, E.B.; Borgi, M.A.; Ben Khadra, K.; Bejar, S. Co-expression of l-arabinose isomerase and d-glucose isomerase in E. coli and development of an efficient process producing simultaneously d-tagatose and d-fructose. Enzym. Microb. Technol. 2007, 40, 1531–1537. [Google Scholar] [CrossRef]
- Muddada, S. Tagatose: The multifunctional food ingredient and potential drug. J. Pharm. Res. 2012, 5, 626–631. [Google Scholar]
- Muniruzzaman, S.; Tokunaga, H.; Izumori, K. Isolation of Enterobacter agglomerans strain 221e from soil, a potent d-tagatose producer from galactitol. J. Ferment. Bioeng. 1994, 78, 145–148. [Google Scholar] [CrossRef]
- Ibrahim, O.O.; Spradlin, J.E. Process for Manufacturing d-tagatose. US Patent 6057135A, 2 May 2000. [Google Scholar]
- Xu, Z.; Li, S.; Feng, X.H.; Liang, J.; Xu, H. l-Arabinose isomerase and its use for biotechnological production of rare sugars. Appl. Microbiol. Biotechnol. 2014, 98, 8869–8878. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Xu, Z.; Tang, B.; Li, S.; Gao, J.; Chi, B.; Xu, H. Construction and co-expression of polycistronic plasmids encoding thermophilic l-arabinose isomerase and hyperthermophilic β-galactosidase for single-step production of d-tagatose. Biochem. Eng. J. 2016, 109, 28–34. [Google Scholar] [CrossRef]
- Manzo, R.M.; Simonetta, A.C.; Rubiolo, A.C.; Mammarella, E.J. Screening and selection of wild strains for l-arabinose isomerase production. Braz. J. Chem. Eng. 2013, 30, 711–720. [Google Scholar] [CrossRef]
- Torres, P.; Manzo, R.; Rubiolo, A.; Batista-Viera, F.; Mammarella, E. Purification of an l-arabinose isomerase from Enterococcus faecium DBFIQ E36 employing a biospecific affinity strategy. J. Mol. Catal. B Enzym. 2014, 102, 99–105. [Google Scholar] [CrossRef]
- Kazenwadel, F.; Franzreb, M.; Rapp, B.E. Synthetic enzyme supercomplexes: Co-immobilization of enzyme cascades. Anal. Methods 2015, 7, 4030–4037. [Google Scholar] [CrossRef]
- García-Galan, C.; Berenguer-Murcia, A.; Fernández-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Peirce, S.; Virgen-Ortíz, J.J.; Tacias-Pascacio, V.G.; Rueda, N.; Bartolomé-Cabrero, R.; Fernández-Lopez, L.; Russo, M.E.; Marzocchella, A.; Fernández-Lafuente, R. Development of simple protocols to solve the problems of enzyme coimmobilization. Application to coimmobilize a lipase and a β-galactosidase. RSC Adv. 2016, 6, 61707–61715. [Google Scholar] [CrossRef]
- López-Gallego, F. Desarrollo de Nuevos Catalizadores Enzimáticos Para la Producción Directa de Cefalosporinas Semisintéticas a Partir de Cefalosporina C. Ph.D. Thesis, Faculty of Sciences, Universidad Autónoma de Madrid, Madrid, Spain, 2006. [Google Scholar]
- Iyer, P.V.; Ananthanarayan, L. Enzyme stability and stabilization—Aqueous and non aqueous environment. Process Biochem. 2008, 43, 1019–1032. [Google Scholar] [CrossRef]
- Kovalevsky, A.Y.; Hanson, L.; Fisher, S.Z.; Mustyakimov, M.; Mason, S.A.; Forsyth, V.T.; Blakeley, M.P.; Keen, D.A.; Wagner, T.; Carrell, H.L.; et al. Metal ions roles and the movement of hydrogen during reaction catalyzed by d-xylose isomerase: A joint X-ray and neutron diffraction study. Structure 2010, 18, 688–699. [Google Scholar] [CrossRef]
- Tükel, S.S.; Alagöz, D. Catalytic efficiency of immobilized glucose isomerase in isomerisation of glucose to fructose. Food Chem. 2008, 111, 658–662. [Google Scholar] [CrossRef]
- Hernaiz, M.J.; Crout, D.H.G. Immobilization/stabilization on Eupergit C of the β-galactosidase from Bacillus circulans and an α-galactosidase from Aspergillus oryzae. Enzym. Microb. Technol. 2000, 27, 26–32. [Google Scholar] [CrossRef]
- Cho, Y.-J.; Park, O.-J.; Shin, H.-J. Immobilization of thermostable trehalose synthase for the production of trehalose. Enzym. Microb. Technol. 2006, 39, 108–113. [Google Scholar] [CrossRef]
- Mateo, C.; Palomo, J.M.; Fernández-Lorente, G.; Guisán, J.M.; Fernández-Lafuente, R. Improvement of enzyme activity, stability and selectivity by immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Cowan, D.A.; Fernández-Lafuente, R. Enhancing the functional properties of thermophilic enzymes by chemical modification and immobilization. Enzym. Microb. Technol. 2011, 49, 326–346. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Lafuente, R. Stabilization of multimeric enzymes: Strategies to prevent subunit dissociation. Enzym. Microb. Technol. 2009, 45, 405–418. [Google Scholar] [CrossRef]
- Guzik, U.; Hupert-Kocurek, K.; Wojcieszynska, D. Immobilization as a strategy for improving enzyme properties-Application to oxidoreductases. Molecules 2014, 19, 8995–9018. [Google Scholar] [CrossRef] [PubMed]
- Pessela, B.C.C.; Mateo, C.; Fuentes, M.; Vian, A.; García, J.L.; Carrascosa, A.V.; Guisán, J.M.; Fernández-Lafuente, R. The immobilization of a thermophilic β-galactosidase on Sepabeads supports decreases product inhibition. Enzym. Microb. Technol. 2003, 33, 199–205. [Google Scholar] [CrossRef]
- Balcão, V.M.; Vila, M.M. Structural and functional stabilization of protein entities: State-of-the-art. Adv. Drug Deliv. Rev. 2015, 93, 25–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trinder, P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem. 1969, 6, 24–27. [Google Scholar] [CrossRef]
- Dische, Z.; Borenfreund, E. A new spectrophotometric method for the detection and determination of keto sugars and trioses. J. Biol. Chem. 1951, 192, 583–587. [Google Scholar] [PubMed]
- Smith, P.K.; Khron, R.I.; Hermanson, G.F.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klent, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Vashit, S.K.; Dixit, C.K. Interference with bicinchoninic acid protein assay. Biochem. Biophys. Res. Commun. 2011, 411, 455–457. [Google Scholar] [CrossRef] [PubMed]
- Giacomini, C.; Villarino, A.; Franco Fraguas, L.; Batista-Viera, F. Immobilization of β-galactosidase from Kluyveromyces lactis on silica and agarose: Comparison of different methods. J. Mol. Catal. B Enzym. 1998, 4, 313–327. [Google Scholar] [CrossRef]
- Sadana, A.; Henley, J.P. Single-step unimolecular non-first-order enzyme deactivation kinetics. Biotechnol. Bioeng. 1987, 30, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not Available.


| Support | Alkaline Incubation at pH 8.5 (h) | Applied Protein (mg/g of gel) | Protein Imm. Yield (%) | Imm. Activity Yield (%) | Half Life (h) |
|---|---|---|---|---|---|
| Eupergit C 250 L | 0 | 4.1 ± 0.1 | 100 ± 1 | 89 ± 3 | 65 ± 6 |
| 7 | ---------- | ----------- | 81 ± 3 | 214 ± 6 | |
| 24 | ----------- | ----------- | 73 ± 6 | 308 ± 2 | |
| Eupergit C | 0 | 4.1 ± 0.1 | 100 ± 1 | 71 ± 6 | 95 ± 3 |
| 7 | ---------- | ----------- | 59 ± 3 | 330 ± 8 | |
| 24 | ---------- | ---------- | 51 ± 3 | 379 ± 2 |
| Support | Alkaline Incubation at pH 8.5 (h) | Applied Protein (mg/g of gel) | Protein Imm. Yield (%) | Imm. Activity Yield (%) | Half Life (h) |
|---|---|---|---|---|---|
| Eupergit C 250 L | 0 | 1.9 ± 0.2 | 100 ± 1 | 98 ± 1 | 210 ± 6 |
| 7 | ---------- | ----------- | 91 ± 1 | 360 ± 6 | |
| 24 | ----------- | ----------- | 84 ± 1 | 491 ± 2 | |
| Eupergit C | 0 | 1.9 ± 0.2 | 100 ± 1 | 91 ± 1 | 230 ± 2 |
| 7 | ---------- | ----------- | 79 ± 1 | 389 ± 6 | |
| 24 | ---------- | ---------- | 66 ± 1 | 554 ± 6 |
| Biocatalyst | Temperature (°C) | Km (mM) | Vmax (nM/min) |
|---|---|---|---|
| Native enzyme | 30 | 100 ± 7 | 10 ± 3 |
| 40 | 70 ± 2 | 40 ± 3 | |
| 50 | 34 ± 2 | 80 ± 6 | |
| Eupergit C derivative | 30 | 170 ± 2 | 1 ± 0.7 |
| 40 | 120 ± 2 | 35 ± 1 | |
| 50 | 50 ± 2 | 60 ± 6 | |
| Eupergit C 250 L derivative | 30 | 127 ± 6 | 3 ± 0.1 |
| 40 | 100 ± 2 | 45 ± 1 | |
| 50 | 40 ± 2 | 70 ± 2 |
| Biocatalyst | Temperature (°C) | Km (mM) | Vmax (nM/min) |
|---|---|---|---|
| Native enzyme | 30 | 365 ± 3 | 49 ± 8 |
| 40 | 315 ± 2 | 68 ± 4 | |
| 50 | 265 ± 2 | 176 ± 2 | |
| Eupergit C derivative | 30 | 925 ± 3 | 17 ± 2 |
| 40 | 802 ± 2 | 59 ± 5 | |
| 50 | 750 ± 2 | 159 ± 2 | |
| Eupergit C 250 L derivative | 30 | 905 ± 3 | 31 ± 2 |
| 40 | 775 ± 6 | 65 ± 5 | |
| 50 | 672 ± 7 | 161 ± 6 |
| System | Lactolysis (%) | Tagatose (%) 2 | Fructose (%) 2 |
|---|---|---|---|
| Soluble enzymes | 76 ± 1 | 22 ± 3 | 21 ± 1 |
| Immobilized derivatives (sequential use) | 86 ± 1 | 31 ± 2 | 24 ± 2 |
| Immobilized derivatives (simultaneous use) | 93 ± 3 | 40 ± 1 | 29 ± 3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, P.; Batista-Viera, F. Immobilized Trienzymatic System with Enhanced Stabilization for the Biotransformation of Lactose. Molecules 2017, 22, 284. https://doi.org/10.3390/molecules22020284
Torres P, Batista-Viera F. Immobilized Trienzymatic System with Enhanced Stabilization for the Biotransformation of Lactose. Molecules. 2017; 22(2):284. https://doi.org/10.3390/molecules22020284
Chicago/Turabian StyleTorres, Pedro, and Francisco Batista-Viera. 2017. "Immobilized Trienzymatic System with Enhanced Stabilization for the Biotransformation of Lactose" Molecules 22, no. 2: 284. https://doi.org/10.3390/molecules22020284