Solvent and Copper Ion-Induced Synthesis of Pyridyl–Pyrazole-3-One Derivatives: Crystal Structure, Cytotoxicity
Abstract
:1. Introduction
2. Results and Discussion
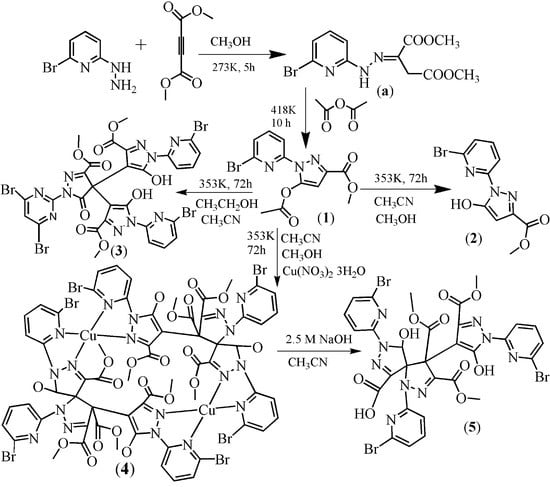
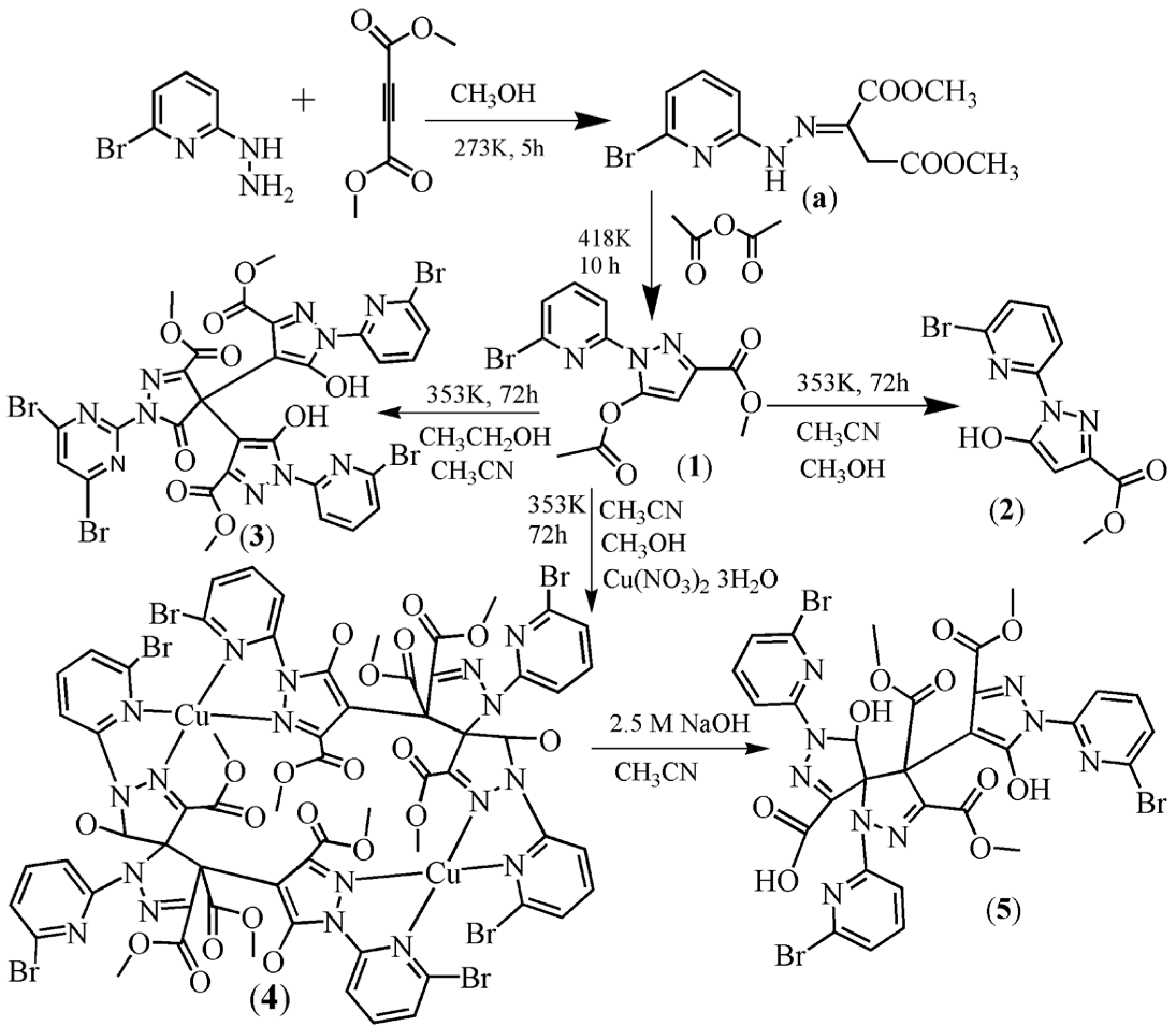
2.1. Synthesis
2.2. Description of the Crystal Structures
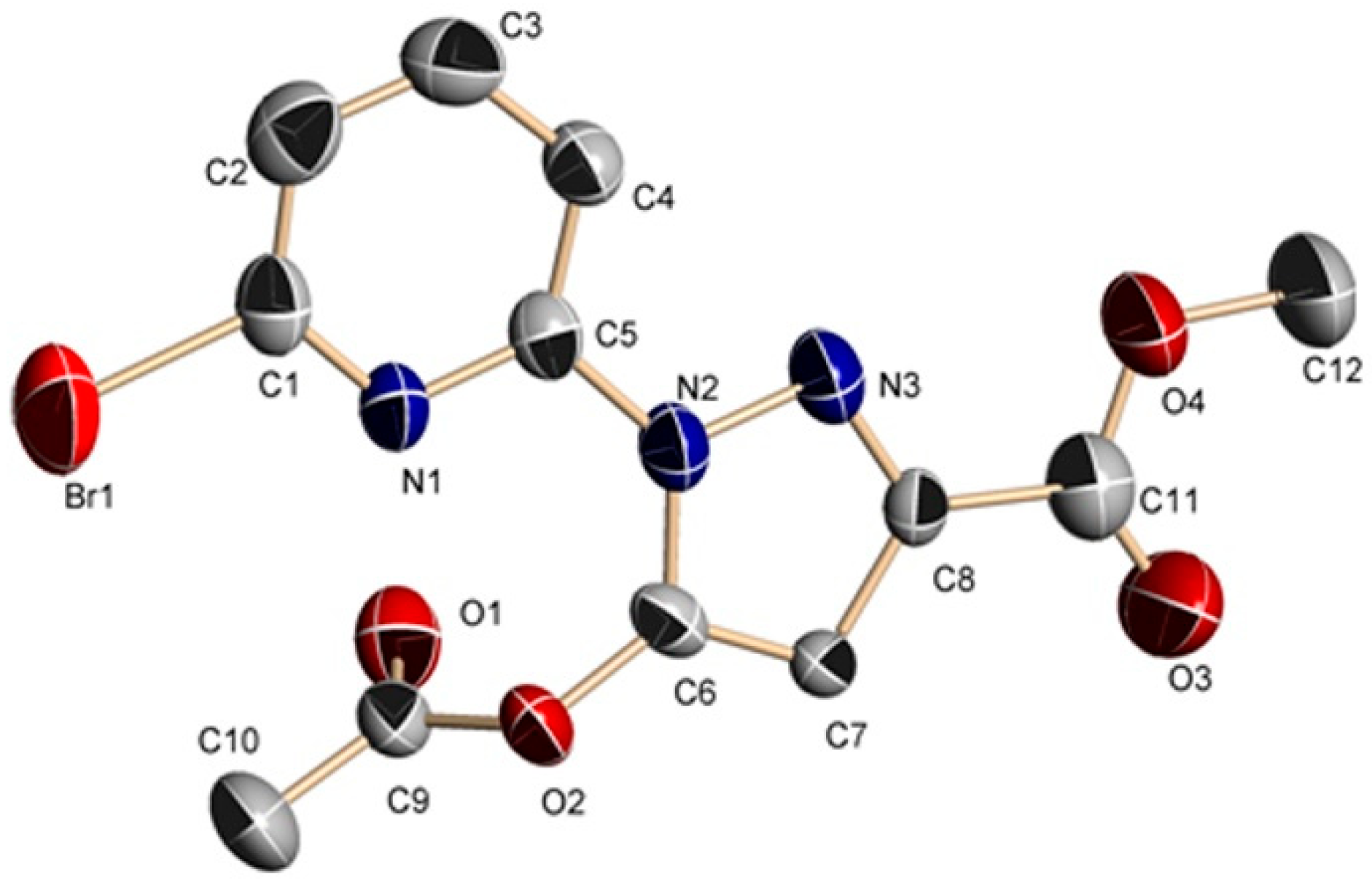
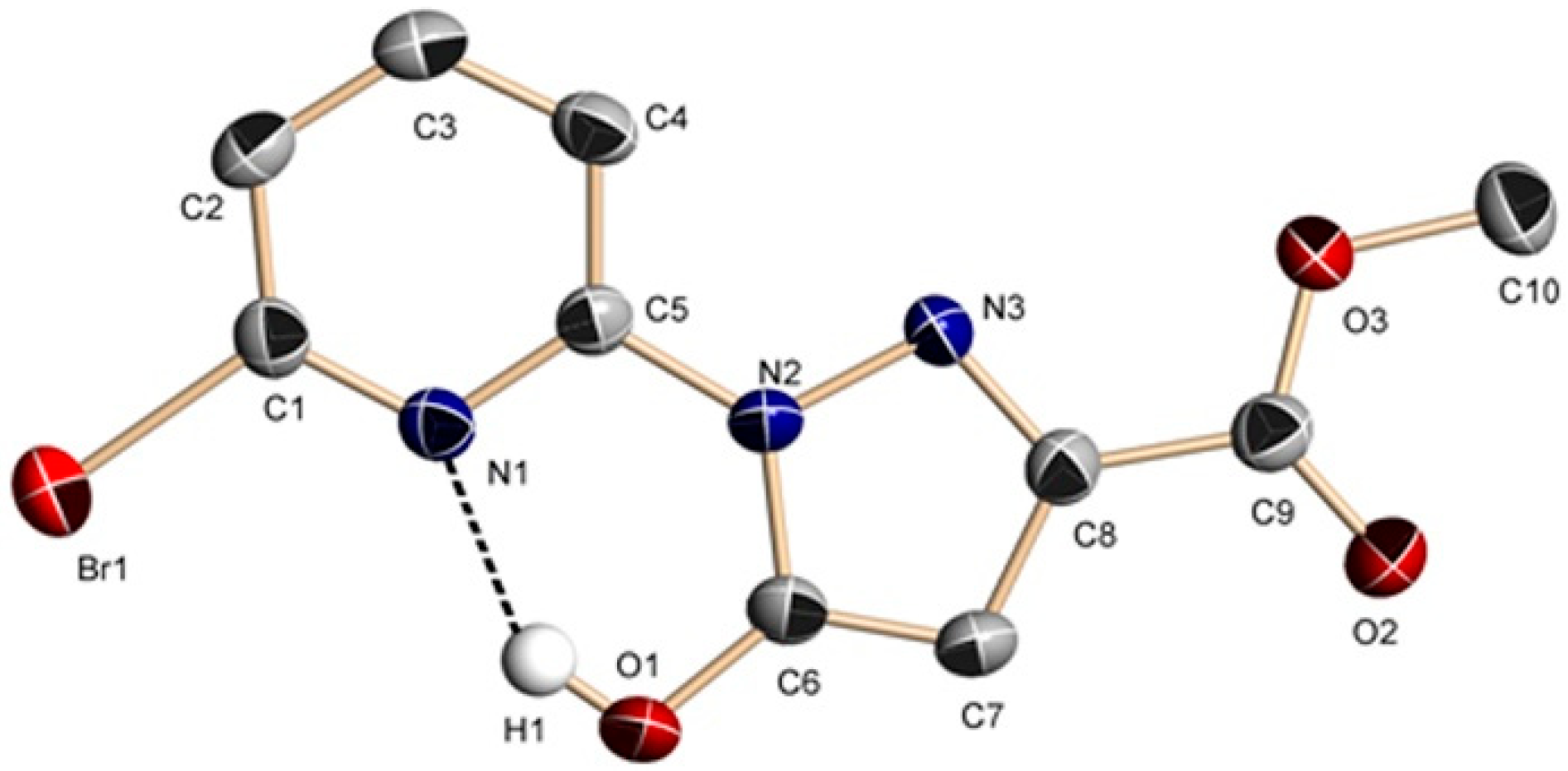
2.2.1. Crystal Structures of 1 and 2
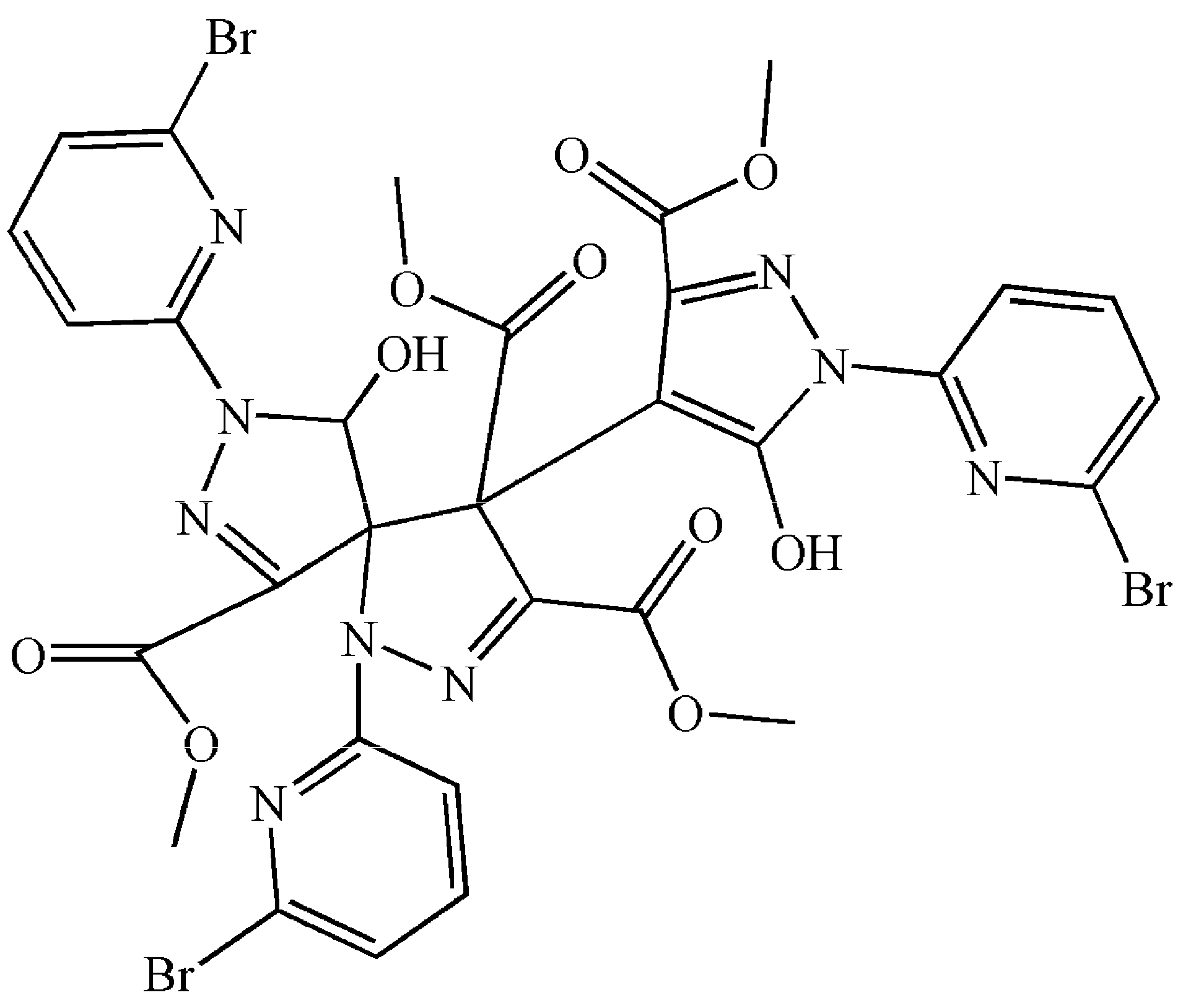
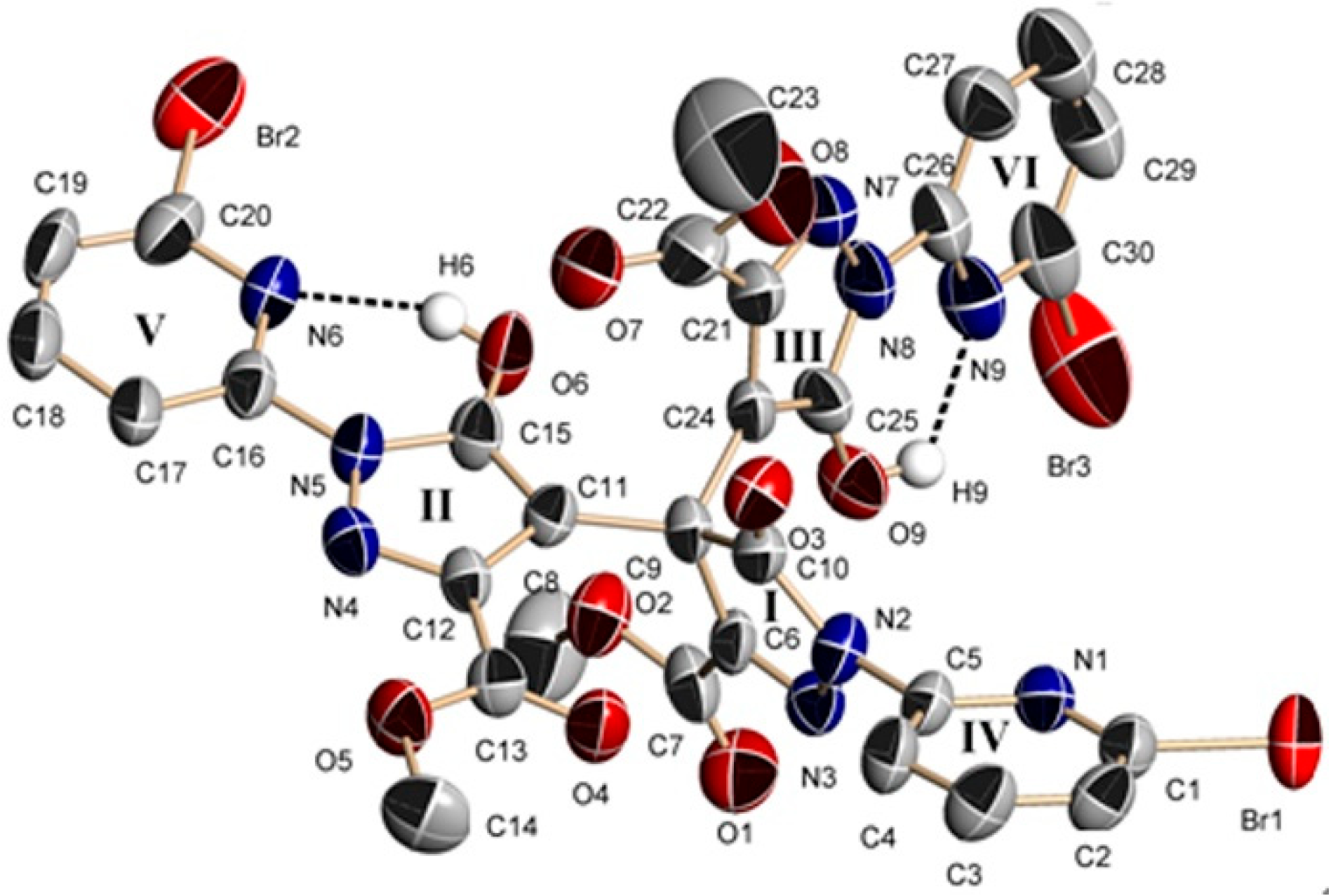
2.2.2. Crystal Structure of 3
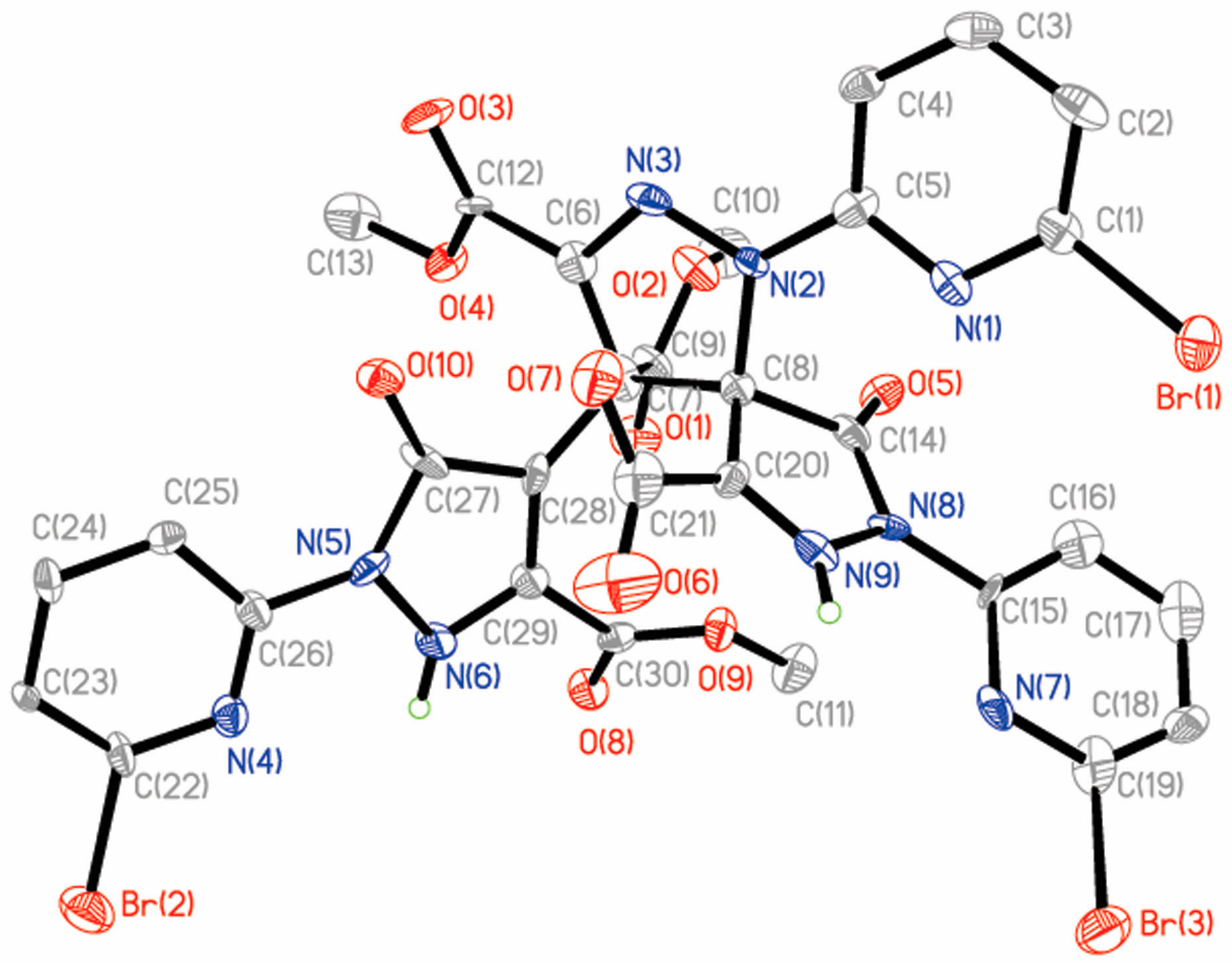
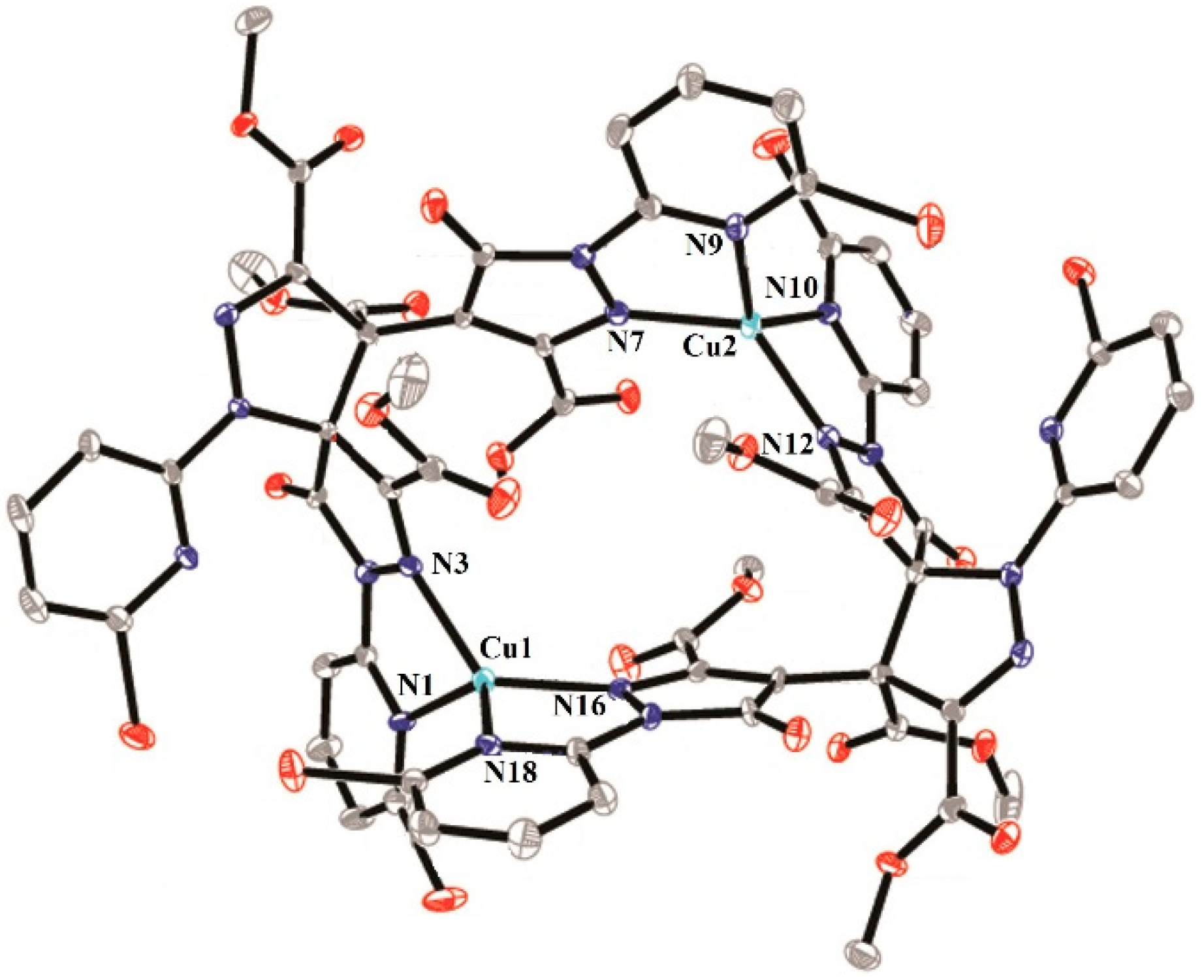
2.2.3. Crystal Structures of 4 and 5
2.3. In-Vitro Antitumor-Activity Screening by MTT Assay
3. Experimental Section
3.1. Materials and Instrumentation
3.2. Synthesis
3.3. X-ray Crystallography
3.4 Cell Culture and Treatment
3.5. In-Vitro Cytotoxicity MTT Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Milacic, V.; Chen, D.; Ronconi, L.; Landis-Piwowar, K.R.; Fregona, D.; Dou, Q.P. A Novel Anticancer Gold(III) Dithiocarbamate Compound Inhibits the Activity of a Purified 20S Proteasome and 26S Proteasome in Human Breast Cancer Cell Cultures and Xenografts. Cancer Res. 2006, 66, 10478–10486. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I. Gold coordination complexes as anticancer agents. Anticancer Agents Med. Chem. 2006, 6, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Lippard, S.J. Direct cellular responses to platinum-induced DNA damage. Chem. Rev. 2007, 107, 1387–1407. [Google Scholar] [CrossRef] [PubMed]
- Bruijnincx, P.C.A.; Sadler, P.J. New trends for metal complexes with anticancer activity. Chem. Biol. 2008, 12, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Hartinger, C.G.; Nazarov, A.A.; Ashraf, S.M.; Dyson, P.J.; Keppler, B.K. Carbohydrate-metal complexes and their potential as anticancer agents. Curr. Med. Chem. 2008, 15, 2574–2591. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.R.; Kalinowski, D.S.; Richardson, V.; Philip, C.S.; David, B.L.; Mohammad, I.; Bernhardt, P.V. 2-Acetylpyridine thiosemicarbazones are potent iron chelators and antiproliferative agents, redox activity, iron complexation and characterization of their antitumor activity. J. Med. Chem. 2009, 52, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Hemmert, C.; Fabié, A.; Fabre, A.; Françoise, B.; Heinz, G. Synthesis, structures, and antimalarial activities of some silver(I), gold(I) and gold(III) complexes involving N-heterocyclic carbene ligands. Eur. J. Med. Chem. 2013, 60, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Bielefeld, E.C.; Tanaka, C.; Coling, D.; Li, M.; Henderson, D.; Fetoni, A.R. An Src-protein tyrosine kinase inhibitor to reduce cisplatin ototoxicity while preserving its antitumor effect. Anti-Cancer Drugs 2013, 24, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, B.; Vancamp, L.; Trosko, J.E.; Mansour, V.H. Platinum compounds, a new class of potent antitumour agent. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Barry, N.P.E.; Sadler, P.J. Exploration of the medical periodic table, towards new targets. Chem. Commun. 2013, 49, 5106–5131. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Janaratne, T.; Krishnan, A.; Singhal, S.S.; Yadav, S.; Dayoub, A.S.; Hawkins, D.L.; Awasthi, S.; MacDonnell, F.M. Regression of lung cancer by hypoxia-sensitizing ruthenium polypyridyl complexes. Mol. Cancer Ther. 2013, 12, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Wang, W.; Chen, H.; Zhang, S.H.; Li, Y. Five novel dinuclear copper(II) complexes, Crystal structures, properties, Hirshfeld surface analysis and vitro antitumor activity study. Inorg. Chim. Acta 2016, 453, 507–515. [Google Scholar] [CrossRef]
- Christian, G.H.; Andrew, D.P.; Alexey, A.N. Polynuclear ruthenium, osmium and gold complexes. The quest for innovative anticancer chemotherapeutics. Curr. Top. Med. Chem. 2011, 11, 2688–2702. [Google Scholar]
- Chen, Z.F.; Gu, Y.Q.; Song, X.Y.; Peng, Y.; Liang, H. Synthesis, crystal structure, cytotoxicity and DNA interaction of 5,7-dichloro-8-quinolinolato-lanthanides. Eur. J. Med. Chem. 2013, 59, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Reed, J.C. Mitochondria and apoptosis. Science 1998, 281, 1309–1311. [Google Scholar] [CrossRef] [PubMed]
- Easmon, J.; Pürstinger, G.; Heinisch, G.; Roth, T.; Fiebig, H.H.; Holzer, W.; Jager, W.; Jenny, M.; Hofmann, J. Synthesis, cytotoxicity, and antitumor activity of copper(II) and iron(II) complexes of 4 N-azabicyclo [3.2.2] nonane thiosemicarbazones derived from acyl diazines. J. Med. Chem. 2001, 44, 2164–2171. [Google Scholar] [CrossRef] [PubMed]
- Griffith, D.; James, P.P.; Celine, J.M. Enzyme inhibition as a key target for the development of novel metal-based anti-cancer therapeutics. Anti-Cancer Agents Chem. 2010, 10, 354–370. [Google Scholar] [CrossRef]
- O’Connor, M.; Kellett, A.; McCann, M.; Rosair, G.; McNamara, M.; Howe, O.; Creaven, B.S.; McClean, S.; Kia, A.F.; O’Shea, D.; et al. Copper(II) complexes of salicylic acid combining superoxide dismutase mimetic properties with DNA binding and cleaving capabilities display promising chemotherapeutic potential with fast acting in vitro cytotoxicity against cisplatin sensitive and resistant cancer cell lines. J. Med. Chem. 2012, 55, 1957–1968. [Google Scholar] [PubMed]
- Tardito, S.; Barilli, A.; Bassanetti, I.; Tegoni, M.; Bussolati, O.; Franchi-Gazzola, R.; Mucchino, C.; Marchio, L. Copper-dependent cytotoxicity of 8-hydroxyquinoline derivatives correlates with their hydrophobicity and does not require caspase activation. J. Med. Chem. 2012, 55, 10448–10459. [Google Scholar] [CrossRef] [PubMed]
- Palanimuthu, D.; Shinde, S.V.; Somasundaram, K.; Samuelson, A.G. In vitro and in vivo anticancer activity of copper bis (thiosemicarbazone) complexes. J. Med. Chem. 2013, 56, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Raja, D.S.; Bhuvanesh, N.S.P.; Natarajan, K. Biological evaluation of a novel water soluble sulphur bridged binuclear copper (II) thiosemicarbazone complex. Eur. J. Med. Chem. 2011, 46, 4584–4594. [Google Scholar] [CrossRef] [PubMed]
- Manikandamathavan, V.M.; Parameswari, R.P.; Weyhermüller, T.; Vasanthi, H.R.; Nair, B.U. Cytotoxic copper(II) mixed ligand complexes, Crystal structure and DNA cleavage activity. Eur. J. Med. Chem. 2011, 46, 4537–4547. [Google Scholar] [CrossRef] [PubMed]
- Gürsoy, A.; Demirayak, Ş.; Çapan, G.; Erol, K.; Vural, K. Synthesis and preliminary evaluation of new 5-pyrazolinone derivatives as analgesic agents. Eur. J. Med. Chem. 2000, 35, 359–364. [Google Scholar] [CrossRef]
- Jiang, J.B.; Hesson, D.P.; Dusak, B.A.; Dexter, G.J.; Hamel, E. Synthesis and biological evaluation of 2-styrylquinazolin-4 (3H)-ones, a new class of antimitotic anticancer agents which inhibit tubulin polymerization. J. Med. Chem. 1990, 33, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Mohareb, R.M.; El-Sayed, N.N.E.; Abdelaziz, M.A. Uses of cyanoacetylhydrazine in heterocyclic synthesis, novel synthesis of pyrazole derivatives with anti-tumor activities. Molecules 2012, 17, 8449–8463. [Google Scholar] [CrossRef] [PubMed]
- Anzai, K.; Furuse, M.; Yoshida, A.; Matsuyama, A.; Moritake, T.; Tsuboi, K.; Ikota, N. In vivo radioprotection of mice by 3-methyl-1-phenyl-2-pyrazolin-5-one (Edaravone; Radicut®), a clinical drug. J. Radiat. Res. 2004, 45, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Soad, A.M.; Hawash, E.L.; Sayed, E.L.; Badawey, A.M.; Ibrahim, M.; Ashmawey, E.I. El–Ashmawey Nonsteroidal antiinflammatory agents-part 2 antiinflammatory, analgesic and antipyretic activity of some substituted 3-pyrazolin-5-ones and 1,2,4,5,6,7-3H-hexahydroindazol-3-ones. Eur. J. Med. Chem. 2006, 41, 155–165. [Google Scholar]
- Bailey, D.M.; Hansen, P.E.; Hlavac, A.G.; Baizman, E.R.; Pearl, J.; DeFelice, A.F.; Feigenson, M.E. 3,4-Diphenyl-1H-pyrazole-1-propanamine antidepressants. Eur. J. Med. Chem. 1985, 28, 256–260. [Google Scholar] [CrossRef]
- Badawey, E.S.A.M.; El-Ashmawey, I.M. Nonsteroidal antiinflammatory agents-Part 1, Antiinflammatory, analgesic and antipyretic activity of some new 1-(pyrimidin-2-yl)-3-pyrazolin-5-ones and 2-(pyrimidin-2-yl)-1,2,4,5,6,7-hexahydro-3H-indazol-3-ones. Eur. J. Med. Chem. 1998, 33, 349–361. [Google Scholar] [CrossRef]
- Ali, A.R.; El-Bendary, E.R.; Ghaly, M.A.; Shehata, I.A. Synthesis, in vitro anticancer evaluation and in silico studies of novel imidazo[2,1-b]thiazole derivatives bearing pyrazole moieties. Eur. J. Med. Chem. 2014, 75, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Verma, A.; Shrivastava, P.K.; Shravastava, S.K. Synthesis and biological evaluation of some new aryl pyrazol-3-one derivatives as potential hypoglycemic agents. Indian J. Chem. Technol. 2008, 47, 1555. [Google Scholar] [CrossRef]
- Shen, L.Q.; Huang, S.Y.; Diao, K.S.; Lei, F.H. Synthesis, crystal structure, computational study of 1-(6-chloro-pyridin-2-yl)-5-hydroxy-1H-pyrazole-3-carboxylic acid methyl ester and its 5-acetoxy analogs. J. Mol. Struct. 2012, 1021, 167–173. [Google Scholar] [CrossRef]
- Allen, F.H.; Davies, J.E.; Galloy, J.J.; Johnson, O.; Kennard, O.; Macrae, C.F.; Mitchelle, M.; Mitchell, G.F.; Smith, J.M.; Watson, D.G. The development of versions 3 and 4 of the Cambridge Structural Database System. J. Chem. Inf. Comp. Sci. 1991, 31, 187–204. [Google Scholar] [CrossRef]
- Dimova, V.; Jordanov, I.; Dimitrov, L. Uv Spectrophotometric Determination OF pK′s OF 1,2,4-triazoline-3-thiones IN sodium hydroxide solutions. J. Chil. Chem. Soc. 2016, 61, 3071–3075. [Google Scholar] [CrossRef]
- Ciolkowski, M.; Paneth, P.; Lorenz, I.P.; Rozalski, M.; Krajewska, U.; Budzisz, E. Tautomeric forms study of 1H-(2′-pyridyl)-3-methyl-5-hydroxypyrazole and 1H-(2′-pyridyl)-3-phenyl-5-hydroxypyrazole. Synthesis, structure, and cytotoxic activity of their complexes with palladium (II) ions. J. Enzym. Inhib. Med. Chem. 2009, 24, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Carey, F.A.; Sundberg, R.J. Advanced Organic Chemistry, Part A, Structure and Mechanisms, 5th ed; Springer Science + Business Media: Boston, MA, USA, 2007; Available online: https://link.springer.com/book/10.1007/978-0-387-44899-2 (accessed on 24 October 2017).
- Zou, H.H.; Wang, L.; Long, Z.X.; Qin, Q.P.; Song, Z.K.; Xie, T.; Zhang, S.H.; Liu, Y.C.; Lin, B.; Chen, Z.F. Preparation of 4-([2,2′,6′,2″-terpyridin]-4′-yl)-N,N-diethylaniline Ni II and Pt II complexes and exploration of their in vitro cytotoxic activities. Eur. J. Med. Chem. 2016, 108, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.; Jia, J.; Ma, X.; Zhou, M.; Fei, H. Membrane localized iridium(III) complex induces endoplasmic reticulum stress and mitochondria-mediated apoptosis in human cancer cells. J. Med. Chem. 2013, 56, 3636–3644. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
Sample Availability: Samples of the compounds 1–5 are available from the authors. |
| Compounds | BEL-7404 | HepG2 | NCI-H460 | T-24 | A549 | HL-7702 |
|---|---|---|---|---|---|---|
| 1 | 66.51 ± 1.13 | 37.15 ± 0.54 | 108.97 ± 1.65 | 70.51 ± 1.33 | 37.56 ± 1.03 | 110.65 ± 2.84 |
| 2 | 125.20 ± 1.87 | 29.75 ± 0.91 | 40.67 ± 0.54 | 46.21 ± 0.87 | 44.77 ± 1.12 | 118.36 ± 3.34 |
| 3 | 112.28 ± 2.78 | 54.74 ± 0.81 | 38.03 ± 0.89 | 85.38 ± 0.67 | 33.56 ± 1.07 | 150.83 ± 3.06 |
| 4 | 41.81 ± 0.37 | 10.66 ± 0.38 | 66.48 ± 0.57 | 42.89 ± 1.41 | 28.09 ± 1.01 | 96.14 ± 0.49 |
| 5 | 84.26 ± 1.01 | 175.23 ± 1.10 | 94.56 ± 0.41 | 76.03 ± 1.16 | 77.56 ± 0.72 | 102.26 ± 0.85 |
| Cisplatin b | 12.41 ± 0.38 | 9.48 ± 0.35 | 18.89 ± 1.02 | 28.07 ± 1.88 | 9.48 ± 0.35 | 5.63 ± 0.32 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Q.P.; Zhang, S.N.; Zhang, S.H.; Wang, K.; Xiao, Y. Solvent and Copper Ion-Induced Synthesis of Pyridyl–Pyrazole-3-One Derivatives: Crystal Structure, Cytotoxicity. Molecules 2017, 22, 1813. https://doi.org/10.3390/molecules22111813
Huang QP, Zhang SN, Zhang SH, Wang K, Xiao Y. Solvent and Copper Ion-Induced Synthesis of Pyridyl–Pyrazole-3-One Derivatives: Crystal Structure, Cytotoxicity. Molecules. 2017; 22(11):1813. https://doi.org/10.3390/molecules22111813
Chicago/Turabian StyleHuang, Qiu Ping, Shao Nan Zhang, Shu Hua Zhang, Kai Wang, and Yu Xiao. 2017. "Solvent and Copper Ion-Induced Synthesis of Pyridyl–Pyrazole-3-One Derivatives: Crystal Structure, Cytotoxicity" Molecules 22, no. 11: 1813. https://doi.org/10.3390/molecules22111813