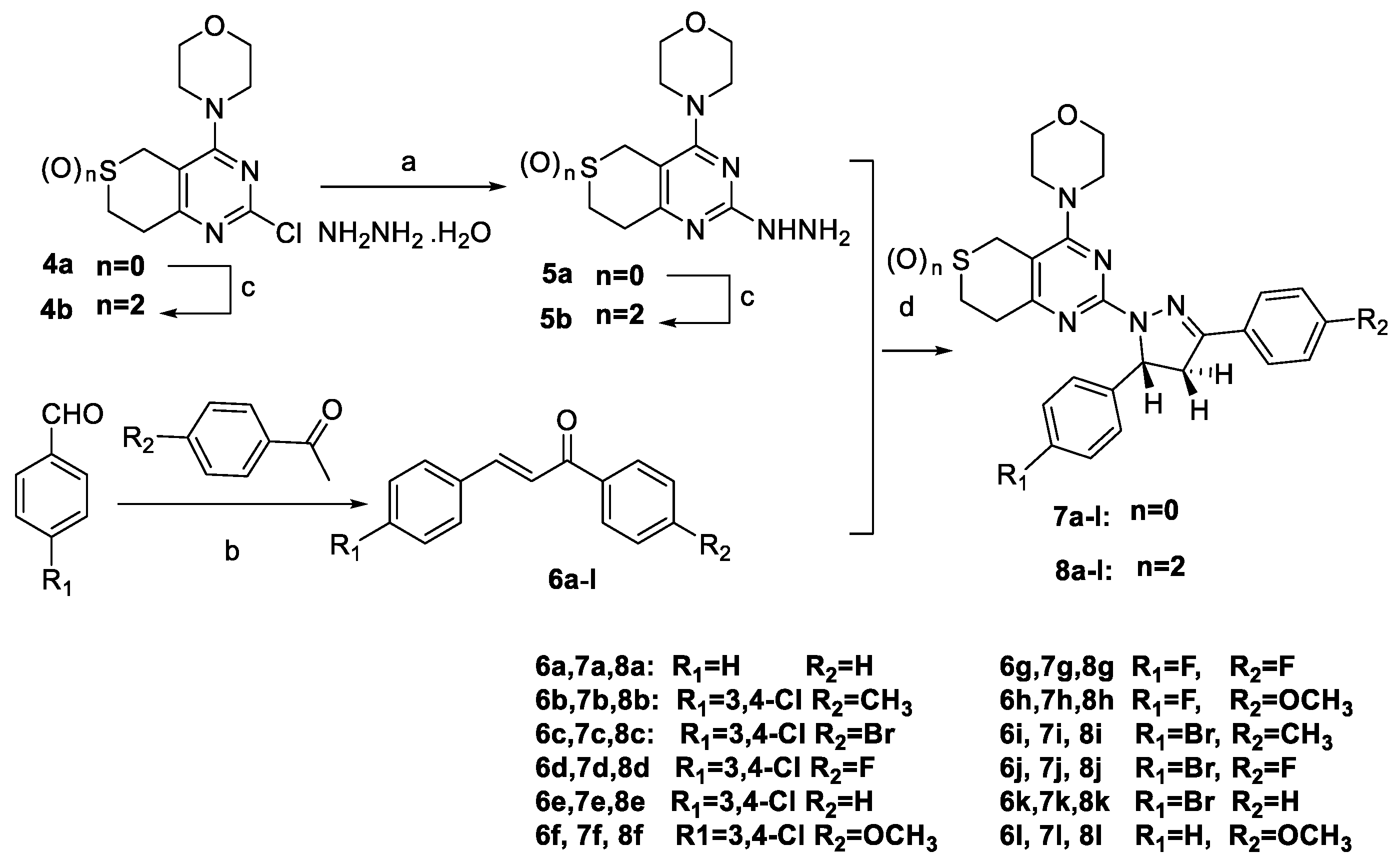
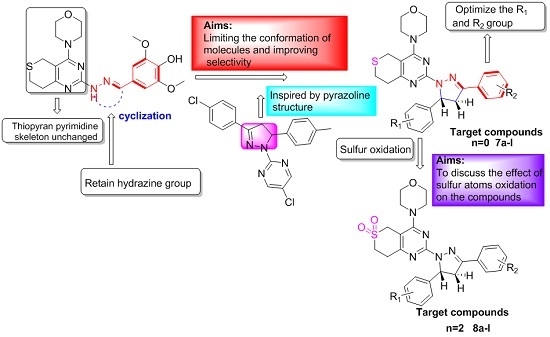
3.2.3. General Procedure for the Preparation of Target Compounds 7a–l and 8a–l
Substituted chalcone (0.001 mol) and substituted 4-(2-hydrazinyl-7,8-dihydro-5
H-thiopyrano[4,3-
d]pyrimidin-4-yl)morpholine (0.001 mol) or 2-hydrazinyl-4-7,8-dihydro-5
H-thiopyrano[4,3-
d]pyrimidine 6,6-dioxide (0.001 mol) were dissolved in 20 mL of glacial acetic acid. Catalytic amount of conc. H
2SO
4 was added. We then obtained the target compounds,
7a–
l and
8a–
l, according to the method reported in the literature [
6].
(R)-4-(2-(3,5-Diphenyl-4,5-dihydro-1H-pyrazol-1-yl)-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-4-yl)morpholine (7a). A brown yellow solid. Yield: 70%. m.p. 139–142 °C. ESI-MS m/z: [M + H]+ 457.6; 1H-NMR (400 MHz, DMSO) δ 7.76 (d, J = 7.9 Hz, 2H, Ar-H), 7.43 (d, J = 7.8 Hz, 3H, Ar-H), 7.27 (d, J = 7.7 Hz, 2H, Ar-H), 7.20 (d, J = 7.6 Hz, 3H, Ar-H), 5.63 (dd, J = 11.9, 5.5 Hz, 1H, pyrazoline), 3.87 (dd, J = 17.7, 12.3 Hz, 1H, pyrazoline), 3.55 (d, J = 14.9 Hz, 3H, thiopyrano and morpholine hydrogen), 3.52–3.43 (m, 3H, thiopyrano and morpholine hydrogen), 3.40 (s, 1H, pyrazoline), 3.10 (dd, J = 17.3, 5.4 Hz, 3H, thiopyrano and morpholine hydrogen), 2.99–2.88 (m, 5H, thiopyrano and morpholine hydrogen).13C-NMR (100 MHz, DMSO) δ 165.44, 164.41, 155.51, 151.31, 144.43, 132.53, 129.78 129.13(2C), 128.97(2C), 127.34, 126.69(2C), 126.02(2C), 108.86, 66.28(2C), 62.72, 49.14(2C), 42.36, 33.81, 26.19, 26.06. Purity: 98.27% by high performance liquid chromatography (HPLC) (80:20 MeOH/H2O).
(R)-4-(2-(5-(3,4-Dichlorophenyl)-3-(p-tolyl)-4,5-dihydro-1H-pyrazol-1-yl)-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-4-yl)morpholine (7b). A pale yellow solid. Yield: 72%. m.p. 194–196 °C. ESI-MS m/z: [M + H]+ 540.5; 1H-NMR (400 MHz, DMSO) δ 7.64 (d, J = 7.9 Hz, 2H, Ar-H), 7.56–7.49 (m, 2H, Ar-H ), 7.24 (d, J = 7.9 Hz, 2H, Ar-H), 7.17 (d, J = 8.3 Hz, 1H, Ar-H), 5.60 (dd, J = 12.1, 6.1 Hz, 1H, pyrazoline), 3.82 (dd, J = 17.8, 12.2 Hz, 1H, pyrazoline ), 3.51 (dt, J = 23.0, 13.2 Hz, 7H, thiopyrano, morpholine and pyrazoline hydrogen), 3.17–3.07 (m, 3H, thiopyrano and morpholine hydrogen), 2.95–2.86 (m, 5H, thiopyrano and morpholine hydrogen), 2.33 (s, 3H, –CH3). Purity: 97.50% by HPLC (80:20 MeOH/H2O).
(R)-4-(2-(3-(4-Bromophenyl)-5-(3,4-dichlorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-4-yl)morpholine (7c). A pale yellow solid. Yield: 78%. m.p. 185–186 °C. ESI-MS m/z: [M + H]+ 605.38;1H-NMR (400 MHz, DMSO) δ 7.68 (d, J = 8.5 Hz, 2H, Ar-H), 7.63 (d, J = 8.6 Hz, 2H, Ar-H), 7.54 (d, J = 8.3 Hz, 2H, Ar-H), 7.18 (d, J = 8.1 Hz, 1H, Ar-H), 5.63 (dd, J = 12.2, 6.2 Hz, 1H, pyrazoline ), 3.84 (dd, J = 17.8, 12.4 Hz, 1H, pyrazoline), 3.67–3.32 (m, 7H ,thiopyrano, morpholine and pyrazoline hydrogen), 3.19–3.05 (m, 3H, thiopyrano and morpholine hydrogen), 2.98–2.87 (m, 5H, thiopyrano and morpholine hydrogen ).13C-NMR (100 MHz, DMSO) δ 166.02, 164.52, 162.57, 155.66, 150.31, 145.42, 132.08, 131.63, 131.34, 129.84(2C), 128.829(2C), 126.46, 123.04, 109.43, 66.30(2C), 62.02, 49.22(2C), 41.78, 33.97, 26.22, 26.09. Purity: 98.60% by HPLC (80:20 MeOH/H2O).
(R)-4-(2-(5-(3,4-Dichlorophenyl)-3-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-4-yl)morpholine (7d). A pale yellow solid. Yield: 71%. m.p. 207–211 °C. ESI-MS m/z: [M + H]+ 544.47; 1H-NMR (400 MHz, DMSO) δ 7.68 (d, J = 8.5 Hz, 2H, Ar-H), 7.63 (d, J = 8.6 Hz, 2H, Ar-H), 7.54 (d, J = 8.3 Hz, 2H, Ar-H), 7.18 (d, J = 8.1 Hz, 1H, Ar-H), 5.63 (dd, J = 12.2, 6.2 Hz, 1H, pyrazoline), 3.84 (dd, J = 17.8, 12.4 Hz, 1H, pyrazoline), 3.67–3.32 (m, 7H, thiopyrano, morpholine and pyrazoline hydrogen), 3.19–3.05 (m, 3H, thiopyrano and morpholine hydrogen), 2.98–2.87 (m, 5H, thiopyrano and morpholine hydrogen). Purity: 95.31% by HPLC (80:20 MeOH/H 2O).
(R)-4-(2-(5-(3,4-Dichlorophenyl)-3-phenyl-4,5-dihydro-1H-pyrazol-1-yl)-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-4-yl)morpholine (7e). A yellow solid. Yield: 73%. m.p. 288–289 °C. ESI-MS m/z: [M + H]+ 556.5; 1H-NMR (400 MHz, DMSO) δ 7.75 (d, J = 6.7 Hz, 2H, Ar-H), 7.55 (d, J = 8.2 Hz, 2H, Ar-H), 7.44 (d, J = 7.7 Hz, 3H, Ar-H), 7.18 (d, J = 8.3 Hz, 1H, Ar-H ), 5.63 (dd, J = 12.1, 6.1 Hz, 1H, pyrazoline), 3.86 (dd, J = 17.7, 12.0 Hz, 1H, pyrazoline), 3.63–3.42 (m, 7H, thiopyrano, morpholine and pyrazoline hydrogen), 3.13 (d, J = 14.0 Hz, 3H, thiopyrano and morpholine hydrogen), 3.00–2.88 (m, 5H, thiopyrano and morpholine hydrogen). Purity: 97.40% by HPLC (80:20 MeOH/H2O).
(R)-4-(2-(5-(3,4-Dichlorophenyl)-3-(4-methoxyphenyl)-4,5-dihydro-1H-pyrazol-1-yl)-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-4-yl)morpholine (7f). A yellow solid. Yield: 75%. m.p. 197–209 °C. ESI-MS m/z: [M + H]+ 464.1; 1H-NMR (400 MHz, DMSO) δ 7.81 (d, J = 8.7 Hz, 2H, Ar-H), 7.60–7.55 (m, 2H, Ar-H), 7.22 (dd, J = 8.4, 1.8 Hz, 1H, Ar-H), 7.04 (d, J = 8.8 Hz, 2H, Ar-H), 5.65 (dd, J = 11.8, 6.1 Hz, 1H, pyrazoline), 3.93 (dd, J = 18.1, 11.9 Hz, 1H, pyrazoline), 3.81 (s, 3H,–OCH3), 3.56 (dd, J = 3.6 Hz, 4H, morpholine), 3.42 (d, J = 14.1, 6.9 Hz, 3H, thiopyrano and pyrazoline hydrogen), 3.26–2.83 (m, 8H, thiopyrano and morpholine hydrogen). Purity: 97.04% by HPLC (80:20 MeOH/H2O).
(R)-4-(2-(3,5-Bis(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-4-yl)morpholine (7g). A pale yellow solid. Yield: 70%. m.p. 144–146°C.. ESI-MS m/z: [M + H]+ 493.58; 1H-NMR (400 MHz, DMSO) δ 8.08 (d, J = 2.8 Hz, 2H, Ar-H), 7.60–7.50 (m, 4H, Ar-H), 7.39 (s, 2H, Ar-H), 5.63 (dd, J = 12.1, 6.1 Hz, 1H, pyrazoline), 3.86 (dd, J = 17.7, 12.0 Hz, 1H, pyrazoline), 3.63–3.42 (m, 7H, thiopyrano, morpholine and pyrazoline hydrogen), 3.13 (d, J = 14.0 Hz, 3H, thiopyrano and morpholine hydrogen), 3.00–2.88 (m, 5H, thiopyrano and morpholine hydrogen). 13C-NMR (100 MHz, DMSO) δ 165.87, 164.55, 162.75, 161.92, 160.33, 155.77, 150.21, 140.53, 129.16, 128.90, 128.83, 128.11, 128.04, 116.25, 116.03, 115.78, 115.57, 109.03, 66.28(2C), 62.10(2C), 49.17, 42.32, 26.25, 26.07. Purity: 95.20% by HPLC (80:20 MeOH/H2O).
(R)-4-(2-(5-(4-Fluorophenyl)-3-(4-methoxyphenyl)-4,5-dihydro-1H-pyrazol-1-yl)-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-4-yl)morpholine (7h). A yellow solid. Yield: 68%. m.p. 116–118 °C. ESI-MS m/z: [M + H]+ 505.6; 1H-NMR (400 MHz, DMSO) δ 8.06 (d, J = 8.3 Hz, 2H, Ar-H), 7.58–7.52 (m, 2H, Ar-H), 7.41 (t, J = 8.6 Hz, 2H,Ar-H), 7.30 (d, J = 8.4 Hz, 2H, Ar-H), 5.92 (dd, J = 11.7, 5.6 Hz, 1H, pyrazoline), 4.18 (dd, J = 17.9, 12.1 Hz, 1H, pyrazoline), 4.08 (s, 3H, –OCH3), 3.84–3.72 (m, 7H, thiopyrano, morpholine and pyrazoline hydrogen), 3.50 (dd, J = 26.7, 8.6 Hz, 4H, thiopyrano and morpholine hydrogen), 3.22 (dd, J = 29.1, 5.3 Hz, 4H, thiopyrano and morpholine hydrogen). Purity: 95.80% by HPLC (80:20 MeOH/H2O).
(R)-4-(2-(5-(4-Bromophenyl)-3-(p-tolyl)-4,5-dihydro-1H-pyrazol-1-yl)-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-4-yl)morpholine (7i). A yellow solid. Yield: 77%. m.p. 142–145 °C. ESI-MS m/z: [M + H]+ 475.59; 1H-NMR (400 MHz, DMSO) δ 7.64 (d, J = 7.2 Hz, 2H, Ar-H), 7.47 (d, J = 7.2 Hz, 2H, Ar-H), 7.24 (d, J = 7.4 Hz, 2H, Ar-H), 7.15 (d, J = 7.2 Hz, 2H, Ar-H), 5.63 (dd, J = 12.1, 6.1 Hz, 1H, pyrazoline), 3.86 (dd, J = 17.7, 12.0 Hz, 1H, pyrazoline), 3.63–3.42 (m, 7H, thiopyrano, morpholine and pyrazoline hydrogen), 3.13 (d, J = 14.0 Hz, 3H, thiopyrano and morpholine hydrogen), 3.00–2.88 (m, 5H, thiopyrano and morpholine hydrogen), 2.32 (s, 3H, CH3).13C-NMR (100 MHz, DMSO) δ 165.91, 164.53, 155.78, 151.17, 144.00, 139.50, 131.84(2C), 129.72(2C), 128.41(2C), 126.67(2C), 120.18, 108.94, 66.29(2C), 62.24, 116.03, 49.19(2C),42.16, 33.97, 26.25, 26.05, 21.45. Purity: 95.54% by HPLC (80:20 MeOH/H2O).
(R)-4-(2-(5-(4-Bromophenyl)-3-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-4-yl)morpholine (7j). A pale yellow solid. Yield: 71%. m.p. 220–221 °C. ESI-MS m/z: [M + H]+ 550.5; 1H-NMR (400 MHz, DMSO) δ 7.79 (s, 2H, Ar-H), 7.30–7.20 (m, 4H, Ar-H), 7.11 (d, J = 7.9 Hz, 2H, Ar-H), 5.68–5.57 (m, 1H, pyrazoline), 3.83 (dd, J = 16.9, 12.6 Hz, 1H, pyrazoline), 3.64–3.41 (m, 7H, thiopyrano, morpholine and pyrazoline hydrogen), 3.08 (d, J = 12.2 Hz, 3H, thiopyrano and morpholine hydrogen), 2.95–2.85 (m, 5H, thiopyrano and morpholine hydrogen). Purity: 97.34% by HPLC (80:20 MeOH/H2O).
(R)-4-(2-(5-(4-Bromophenyl)-3-phenyl-4,5-dihydro-1H-pyrazol-1-yl)-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-4-yl)morpholine (7k). A yellow solid. Yield: 69%. m.p. 167–168 °C. ESI-MS m/z: [M + H]+ 536.49; 1H-NMR (400 MHz, DMSO) δ 7.79–7.71 (m, 2H, Ar-H), 7.48–7.37 (m, 3H, Ar-H), 7.24 (dd, J = 8.5, 5.6 Hz, 2H, Ar-H), 7.10 (t, J = 8.8 Hz, 2H, Ar-H), 5.65 (dd, J = 12.1, 5.6 Hz, 1H, pyrazoline), 3.85 (dd, J = 17.7, 12.2 Hz, 1H, pyrazoline), 3.67–3.37 (m, 7H, thiopyrano, morpholine and pyrazoline hydrogen), 3.16–3.05 (m, 3H, thiopyrano and morpholine hydrogen), 2.97–2.87 (m, 5H, thiopyrano and morpholine hydrogen). Purity: 96.03% by HPLC (80:20 MeOH/H2O).
(R)-4-(2-(3-(4-Methoxyphenyl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl)-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidin-4-yl)morpholine (7l). A pale yellow solid. Yield: 67%. m.p. >300 °C. ESI-MS m/z: [M + H]+ 487.6; 1H -NMR (400 MHz, DMSO) δ 7.72 (d, J = 8.4 Hz, 2H, Ar-H), 7.28 (d, J = 7.0 Hz, 2H, Ar-H), 7.21 (d, J = 7.6 Hz, 3H, Ar-H), 7.01 (d, J = 8.5 Hz, 2H, Ar-H), 5.63 (s, 1H, pyrazoline), 3.86 (d, J = 17.3 Hz, 1H, pyrazoline), 3.81 (s, 3H, –OCH3), 3.56 (dd, J = 3.6 Hz, 4H, morpholine), 3.42 (d, J = 14.1, 6.9 Hz, 3H, thiopyrano and pyrazoline hydrogen), 3.26–2.83 (m, 8H, thiopyrano and morpholine hydrogen). Purity: 98.86% by HPLC (80:20 MeOH/H2O).
(R)-2-(3,5-Diphenyl-4,5-dihydro-1H-pyrazol-1-yl)-4-morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidine 6,6-dioxide (8a). A pale yellow solid. Yield: 70%. m.p. 219–220 °C. ESI-MS m/z: [M + H]+ 489.5; 1H-NMR (400 MHz, DMSO) δ 7.90 (d, J = 6.3 Hz, 2H, Ar-H), 7.40 (d, J = 7.3 Hz, 4H, Ar-H), 7.28 (d, J = 3.2 Hz, 2H, Ar-H), 7.23 (d, J = 7.6 Hz, 2H, Ar-H ), 5.60 (dd, J = 11.9, 6.2 Hz, 1H, pyrazoline),4.12 (dd, J = 39.3, 15.5 Hz, 2H, thiopyrano), 3.87 (dd, J = 17.7, 12.4 Hz, 1H, pyrazoline), 3.56 (s, 2H, morpholine), 3.51 (s, 2H, morpholine), 3.43 (s, 2H, thiopyrano), 3.25–3.15 (m, 3H, morpholine and pyrazoline hydrogen), 3.08 (s, 2H, morpholine), 2.97 (s, 2H, thiopyrano). Purity: 97.60% by HPLC (80:20 MeOH/H2O).
(R)-2-(5-(3,4-Dichlorophenyl)-3-(p-tolyl)-4,5-dihydro-1H-pyrazol-1-yl)-4-morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidine 6,6-dioxide (8b). A yellow solid. Yield: 63%. m.p. 225–226 °C. ESI-MS m/z: [M + H]+ 572.50; 1H-NMR (400 MHz, DMSO) δ 7.65 (d, J = 8.0 Hz, 2H,Ar-H), 7.54 (d, J = 8.8 Hz, 2H, Ar-H), 7.25 (d, J = 8.0 Hz, 2H, Ar-H), 7.16 (d, J = 8.2 Hz, 1H, Ar-H), 5.62 (dd, J = 12.1, 5.9 Hz, 1H, pyrazoline), 4.16 (d, J = 15.6 Hz, 1H, thiopyrano), 4.06 (d, J = 15.5 Hz, 1H, thiopyrano), 3.85 (dd, J = 17.9, 12.3 Hz, 1H, pyrazoline), 3.56 (s, 2H, morpholine), 3.49 (d, J = 9.0 Hz, 2H, morpholine), 3.43 (s, 2H, thiopyrano), 3.16 (dd, J = 17.9, 5.9 Hz, 3H, morpholine and pyrazoline hydrogen), 3.12–3.05 (m, 2H, morpholine), 2.97 (s, 2H, thiopyrano), 2.33 (s, 3H, –CH3). Purity: 99.45% by HPLC (80:20 MeOH/H2O).
(R)-2-(3-(4-Bromophenyl)-5-(3,4-dichlorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-4-morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidine 6,6-dioxide (8c). A yellow solid. Yield: 59%. m.p. 162–163 °C. ESI-MS m/z: [M + H]+ 637.37; 1H-NMR (400 MHz, DMSO) δ 7.68 (d, J = 8.4 Hz, 2H, Ar-H), 7.62 (d, J = 8.3 Hz, 2H, Ar-H), 7.54 (d, J = 6.9 Hz, 2H,Ar-H), 7.17 (d, J = 8.4 Hz, 1H, Ar-H), 5.62 (dd, J = 12.1, 6.0 Hz, 1H, pyrazoline), 4.16 (d, J = 15.6 Hz, 1H, thiopyrano), 4.08 (d, J = 15.6 Hz, 1H,thiopyrano), 3.86 (dd, J = 17.8, 12.3 Hz, 1H, pyrazoline), 3.56 (d, J = 5.3 Hz, 2H,morpholine), 3.53–3.46 (m, 2H, morpholine), 3.43 (s, 2H, thiopyrano), 3.24–3.14 (m, 3H, morpholine and pyrazoline hydrogen), 3.09 (d, J = 11.8 Hz, 2H,morpholine), 2.97 (s, 2H, thiopyrano). Purity: 96.89% by HPLC (80:20 MeOH/H2O).
(R)-2-(5-(3,4-Dichlorophenyl)-3-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-4-morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidine 6,6-dioxide (8d). A yellow solid. Yield: 61%. m.p. 150–153 °C. ESI-MS m/z: [M + H]+ 576.47; 1H-NMR (400 MHz, DMSO) δ 7.85–7.77 (m, 2H, Ar-H), 7.55 (d, J = 6.6 Hz, 2H, Ar-H), 7.28 (t, J = 8.4 Hz, 2H, Ar-H), 7.18 (d, J = 8.1 Hz, 1H, Ar-H), 5.63 (dd, J = 11.9, 5.7 Hz, 1H, pyrazoline), 4.12 (dd, J = 39.3, 15.5 Hz, 2H, thiopyrano), 3.87 (dd, J = 17.7, 12.4 Hz, 1H, pyrazoline), 3.56 (s, 2H, morpholine), 3.51 (s, 2H, morpholine), 3.43 (s, 2H, thiopyrano), 3.25–3.15 (m, 3H, morpholine and pyrazoline hydrogen), 3.08 (s, 2H, morpholine), 2.97 (s, 2H, thiopyrano). 13C-NMR (100 MHz, DMSO) δ 165.77, 164.58, 162.55, 162.12, 156.38, 151.39, 145.22, 131.42, 129.91, 129.16, 129.16(2C), 129.08, 128.80, 126.38(2C), 116.31, 116.10, 102.61, 66.17(2C), 61.83, 49.04(2C), 47.03, 32.48. Purity: 95.14% by HPLC (80:20 MeOH/H2O).
(R)-2-(5-(3,4-Dichlorophenyl)-3-phenyl-4,5-dihydro-1H-pyrazol-1-yl)-4-morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidine 6,6-dioxide (8e). A yellow solid. Yield: 57%. m.p. 245–249 °C. ESI-MS m/z: [M + H]+ 588.5; 1H-NMR(400 MHz, DMSO) δ 7.85–7.77 (m, 2H, Ar-H), 7.55 (d, J = 6.6 Hz, 2H, Ar-H), 7.28 (t, J = 8.4 Hz, 2H,Ar-H), 7.18 (d, J = 8.1 Hz, 2H, Ar-H), 5.63 (dd, J = 11.9, 5.7 Hz, 1H, pyrazoline ), 4.12 (dd, J = 39.3, 15.5 Hz, 2H, thiopyrano), 3.87 (dd, J = 17.7,12.4 Hz, 1H, pyrazoline), 3.56 (s, 2H, morpholine), 3.51 (s, 2H, morpholine), 3.43 (s, 2H, thiopyrano), 3.25–3.15 (m, 3H, morpholine and pyrazoline hydrogen), 3.08 (s, 2H, morpholine), 2.97 (s, 2H, thiopyrano). Purity: 96.53% by HPLC (80:20 MeOH/H2O).
(R)-2-(5-(3,4-Dichlorophenyl)-3-(4-methoxyphenyl)-4,5-dihydro-1H-pyrazol-1-yl)-4-morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidine 6,6-dioxide (8f). A pale yellow solid. Yield: 55%. m.p. 203–205 °C. ESI-MS m/z: [M + H]+ 558.4; 1H-NMR (400 MHz, DMSO) δ 7.70 (d, J = 8.0 Hz, 2H, Ar-H), 7.53 (d, J = 10.9 Hz, 2H, Ar-H), 7.17 (d, J = 8.0 Hz, 1H, Ar-H), 6.99 (d, J = 8.0 Hz, 2H, Ar-H), 5.59 (dd, J = 11.4, 5.3 Hz, 1H, pyrazoline), 4.11 (dd, J = 35.3, 15.4 Hz, 2H, thiopyrano), 3.89–3.82 (m, 1H, pyrazoline), 3.79 (s, 3H, –OCH3), 3.54 (d, J = 17.8 Hz, 4H, morpholine), 3.42 (s, 2H, thiopyrano), 3.19 (d, J = 5.6 Hz, 2H, morpholine), 3.15–3.05 (m, 3H, morpholine and pyrazoline hydrogen), 2.97 (s, 2H, thiopyrano). Purity: 98.66% by HPLC (80:20 MeOH/H2O).
(R)-2-(3,5-bis(4-Fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-4-morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidine 6,6-dioxide (8g). A yellow solid. Yield: 53%. m.p. 246–249 °C. ESI-MS m/z: [M + H]+ 525.5; 1H-NMR (400 MHz, DMSO) δ 7.71 (d, J = 8.0 Hz, 2H, Ar-H), 7.23 (s, 2H, Ar-H), 7.11 (t, J = 8.1 Hz, 2H, Ar-H), 7.00 (d, J = 8.0 Hz, 2H, Ar-H), 5.62 (d, J = 7.0 Hz, 1H, pyrazoline), 4.12 (dd, J = 39.3, 15.5 Hz, 2H, thiopyrano), 3.87 (dd, J = 17.7, 12.4 Hz, 1H, pyrazoline), 3.56 (s, 2H, morpholine), 3.51 (s, 2H, morpholine), 3.43 (s, 2H, thiopyrano), 3.25–3.15 (m, 3H, morpholine and pyrazoline hydrogen), 3.08 (s, 2H, morpholine), 2.97 (s, 2H, thiopyrano). Purity: 98.38% by HPLC (80:20 MeOH/H2O).
(R)-2-(5-(4-Fluorophenyl)-3-(4-methoxyphenyl)-4,5-dihydro-1H-pyrazol-1-yl)-4-morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidine 6,6-dioxide (8h). A yellow solid. Yield: 50%. m.p. 140–142 °C. ESI-MS m/z: [M + H]+ 537.6; 1H-NMR (400 MHz, DMSO) δ 7.71 (d, J = 8.0 Hz, 2H,Ar-H), 7.23 (s, 2H, Ar-H), 7.11 (t, J = 8.1 Hz, 2H,Ar-H), 7.00 (d, J = 8.0 Hz, 2H, Ar-H), 5.62 (d, J = 7.0 Hz, 1H, pyrazolin), 4.16 (d, J = 15.4 Hz, 1H, thiopyrano), 4.04 (d, J = 15.5 Hz, 1H, thiopyrano), 3.85 (d, J = 17.6 Hz, 1H, pyrazolin), 3.79 (s, 3H, –OCH3), 3.57 (s, 2H, morpholine), 3.51 (s, 2H, morpholine), 3.43 (s, 2H, thiopyrano), 3.18 (s, 2H, morpholine), 3.08 (d, J = 17.9 Hz, 3H, morpholine and pyrazoline hydrogen), 2.95 (s, 2H, thiopyrano). 13C-NMR (100 MHz, DMSO) δ 165.82, 162.76, 162.43, 160.91, 160.35, 156.45, 152.00, 140.51, 128.43, 128.06, 127.99, 124.91, 115.84, 115.63(2C), 114.65(2C), 102.07, 66.16(2C), 61.81, 55.79, 49.29, 49.05, 47.11, 42.47, 32.46. Purity: 99.04% by HPLC (80:20 MeOH/H2O).
(R)-2-(5-(4-Bromophenyl)-3-(p-tolyl)-4,5-dihydro-1H-pyrazol-1-yl)-4-morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidine 6,6-dioxide (8i). A yellow solid. Yield: 48%. m.p. 282–283 °C. ESI-MS m/z: [M + H]+ 507.5; 1H-NMR (400 MHz, DMSO) δ 7.65 (d, J = 7.9 Hz, 2H, Ar-H), 7.47 (d, J = 8.2 Hz, 2H, Ar-H), 7.24 (d, J = 7.9 Hz, 2H, Ar-H), 7.16 (d, J = 8.3 Hz, 2H, Ar-H), 5.60 (dd, J = 11.9, 5.4 Hz, 1H, pyrazolin), 4.17 (d, J = 15.5 Hz, 1H, thiopyrano), 4.05 (d, J = 15.5 Hz, 1H, thiopyrano), 3.84 (dd, J = 17.7, 12.2 Hz, 1H, pyrazolin), 3.56 (s, 2H, morpholine), 3.51 (d, J = 3.4 Hz, 2H, morpholine), 3.42 (d, J = 5.2 Hz, 2H, thiopyrano), 3.19 (d, J = 5.1 Hz, 2H, morpholine), 3.11–3.03 (m, 3H, morpholine and pyrazoline hydrogen), 2.98 (s, 2H, thiopyrano), 2.32 (s, 3H, –CH3). Purity: 98.63% by HPLC (80:20 MeOH/H2O).
(R)-2-(5-(4-Bromophenyl)-3-(4-fluorophenyl)-4,5-dihydro-1H-pyrazol-1-yl)-4-morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidine 6,6-dioxide (8j). A yellow solid. Yield: 49%. m.p. 158–163 °C. ESI-MS m/z: [M + H]+ 582.5; 1H-NMR (400 MHz, DMSO) δ 7.71 (d, J = 8.0 Hz, 2H, Ar-H), 7.23 (s, 2H, Ar-H), 7.11 (t, J = 8.1 Hz, 2H, Ar-H), 7.00 (d, J = 8.0 Hz, 2H, Ar-H), 5.62 (d, J = 7.0 Hz, 1H, pyrazoline),4.12 (dd, J = 39.3, 15.5 Hz, 2H, thiopyrano), 3.87 (dd, J = 17.7, 12.4 Hz, 1H, pyrazoline), 3.56 (s, 2H, morpholine), 3.51 (s, 2H, morpholine), 3.43 (s, 2H, thiopyrano), 3.25–3.15 (m, 3H, morpholine and pyrazoline hydrogen), 3.08 (s, 2H, morpholine), 2.97 (s, 2H, thiopyrano). Purity: 98.37% by HPLC (80:20 MeOH/H2O).
(R)-2-(5-(4-Bromophenyl)-3-phenyl-4,5-dihydro-1H-pyrazol-1-yl)-4-morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidine 6,6-dioxide (8k). A pale yellow solid. Yield: 52%. m.p. 163–167 °C. ESI-MS m/z: [M + H]+ 568.4; 1H-NMR (400 MHz, DMSO) δ 7.76 (d, J = 6.8 Hz, 2H, Ar-H ), 7.45 (dd, J = 21.0, 6.9 Hz, 5H, Ar-H), 7.17 (d, J = 7.4 Hz, 2H, Ar-H), 5.62 (dd, J = 11.7, 5.1 Hz, 1H, pyrazoline), 4.17 (d, J = 15.4 Hz, 1H,thiopyrano), 4.05 (d, J = 15.5 Hz, 1H, thiopyrano), 3.87 (dd, J = 17.4, 12.4 Hz, 1H, pyrazoline), 3.57 (s, 2H, morpholine), 3.49 (d, J = 11.4 Hz, 4H, morpholine and thiopyrano hydrogen), 3.22–3.12 (m, 3H, morpholine and pyrazoline hydrogen), 3.11–3.04 (m, 2H, morpholine), 2.97 (s, 2H, thiopyrano).13C-NMR (100 MHz, DMSO) δ 165.75, 162.41, 156.34, 152.17, 143.60, 132.24, 131.93(2C), 130.04, 129.17(2C), 128.39(2C), 126.82(2C), 120.35, 102.38, 66.16(2C), 62.09, 49.29(2C), 49.01, 47.03, 42.17, 32.44. Purity: 96.29% by HPLC (80:20 MeOH/H2O).
(R)-2-(3-(4-Methoxyphenyl)-5-phenyl-4,5-dihydro-1H-pyrazol-1-yl)-4-morpholino-7,8-dihydro-5H-thiopyrano[4,3-d]pyrimidine 6,6-dioxide (8l). A pale yellow solid. Yield: 50%. m.p. 109–114 °C. ESI-MS m/z: [M + H]+ 519.6; 1H-NMR (400 MHz, DMSO) δ 7.71 (d, J = 8.7 Hz, 2H, Ar-H), 7.31–7.24 (m, 2H, Ar-H ), 7.19 (d, J = 6.9 Hz, 3H, Ar-H), 6.99 (d, J = 8.8 Hz, 2H, Ar-H), 5.59 (dd, J = 11.9, 5.4 Hz, 1H, pyrazoline), 4.15 (d, J = 15.5 Hz, 1H, thiopyrano), 4.02 (d, J = 15.4 Hz, 1H, thiopyrano), 3.89–3.82 (m, 1H, pyrazoline), 3.78 (s, 3H, –OCH3), 3.53 (d, J = 5.8 Hz, 2H, morpholine), 3.49–3.38 (m, 4H, morpholine and thiopyrano hydrogen), 3.22–3.15 (m, 2H, morpholine), 3.12–3.00 (m, 3H, morpholine and pyrazoline hydrogen), 2.93 (s, 2H, thiopyrano). Purity: 98.22% by HPLC (80:20 MeOH/H2O).