Studies on the Synthesis, Photophysical and Biological Evaluation of Some Unsymmetrical Meso-Tetrasubstituted Phenyl Porphyrins
Abstract
:1. Introduction
2. Results and Discussion
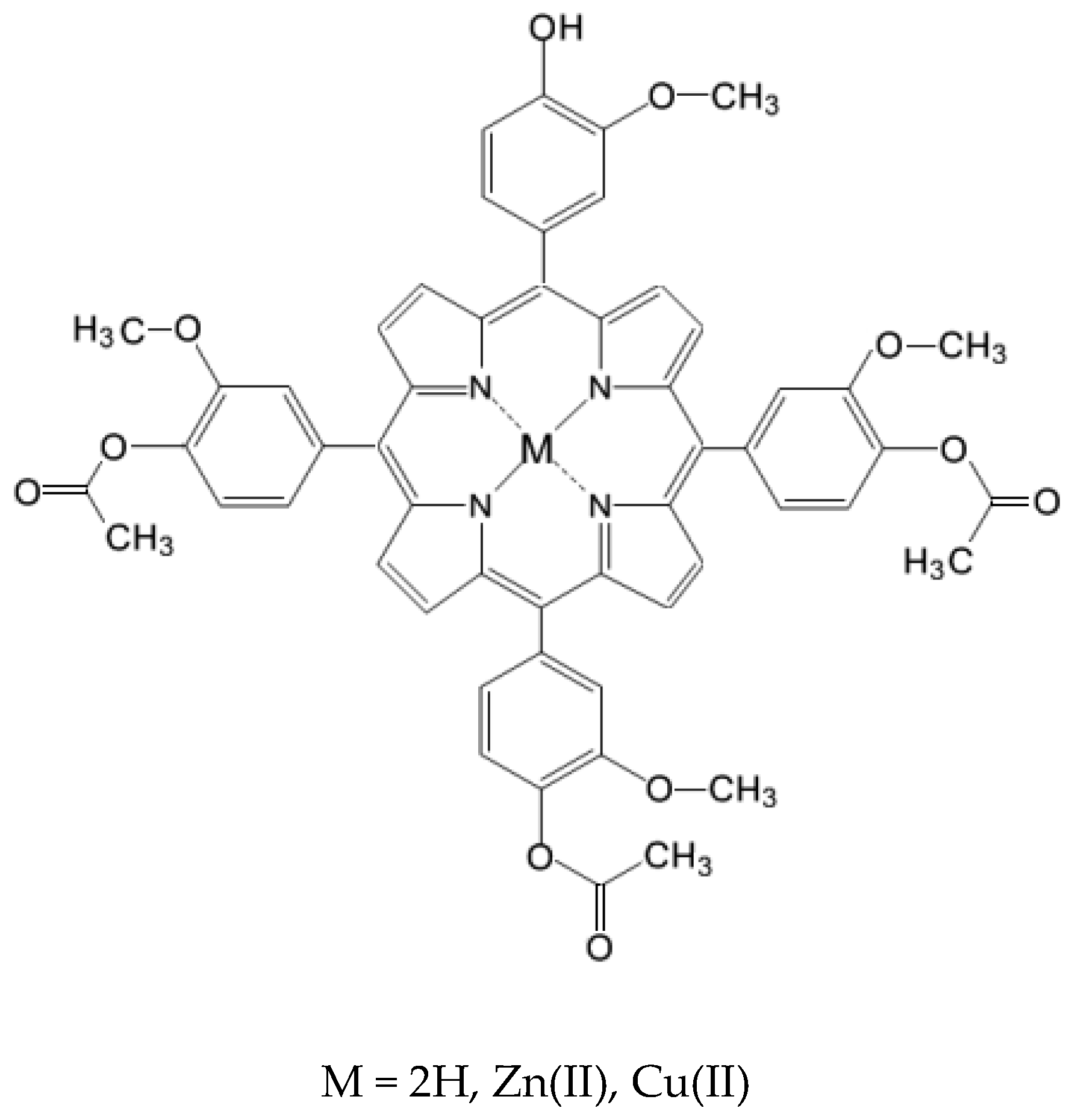
2.1. Chemistry
2.2. Photophysical Characterization of Mesoporphyrinic Compounds
2.2.1. UV-Vis Spectral Characterization
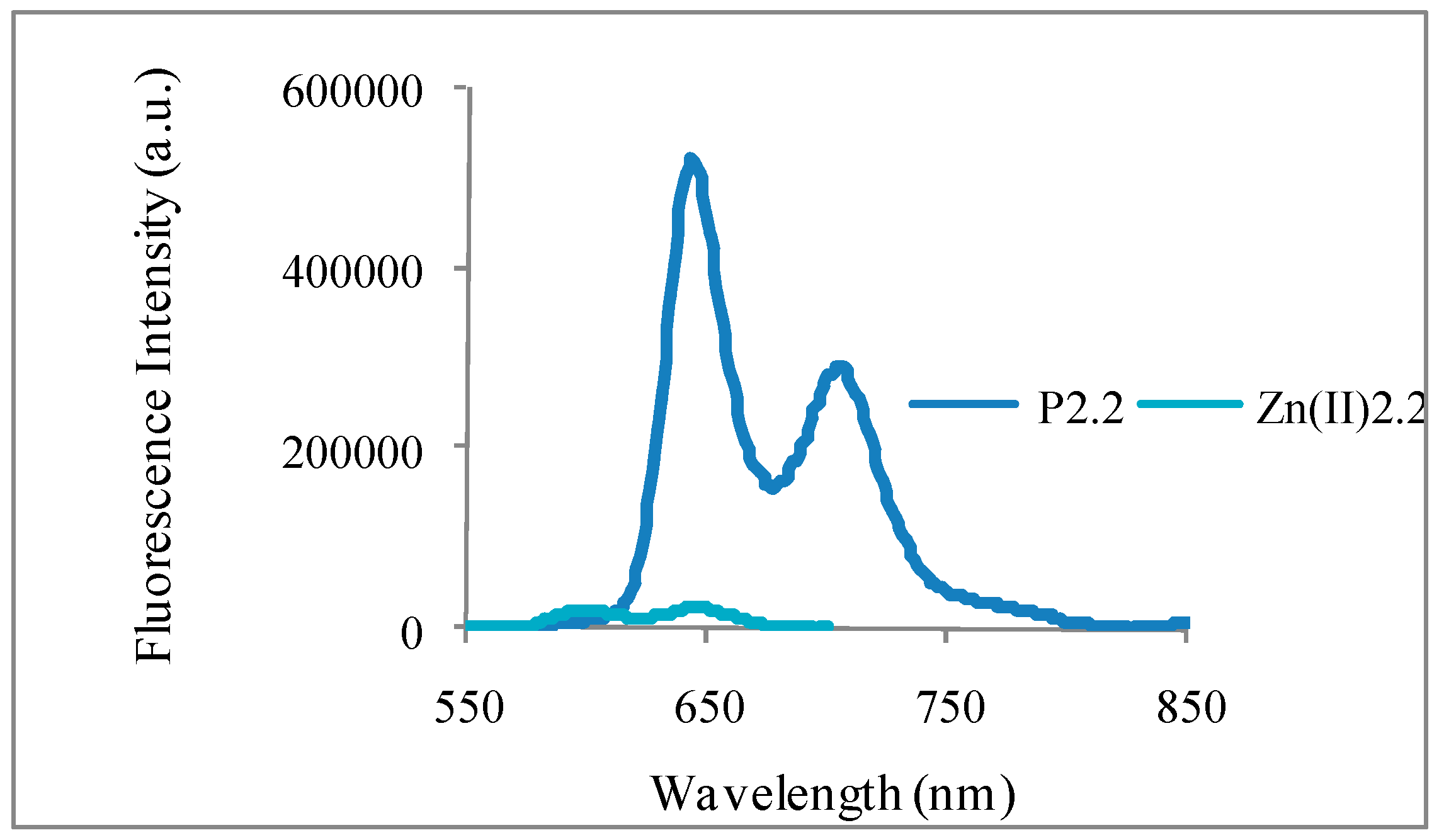
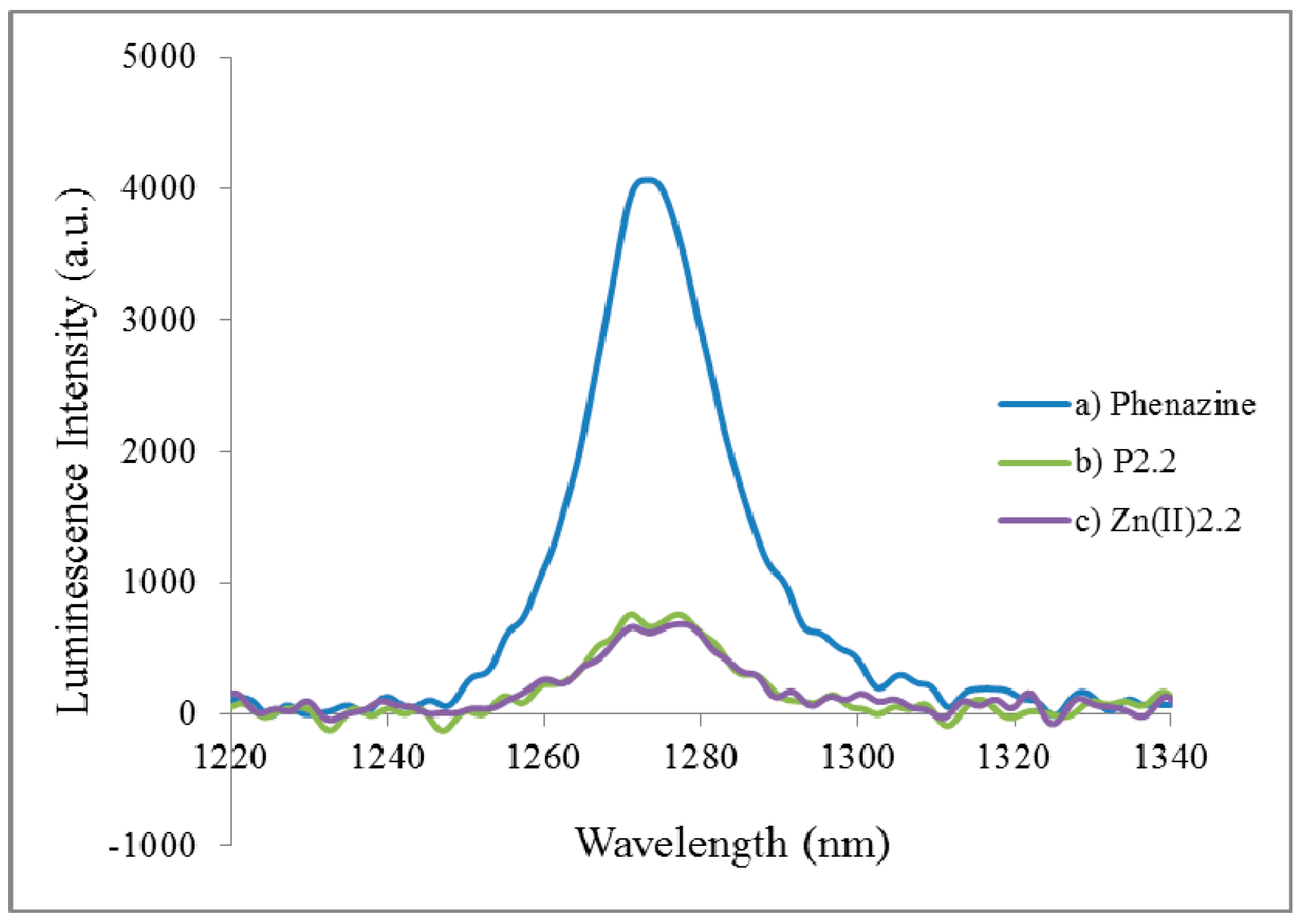
2.2.2. Fluorescence Emission, Lifetime and Singlet Oxygen Formation
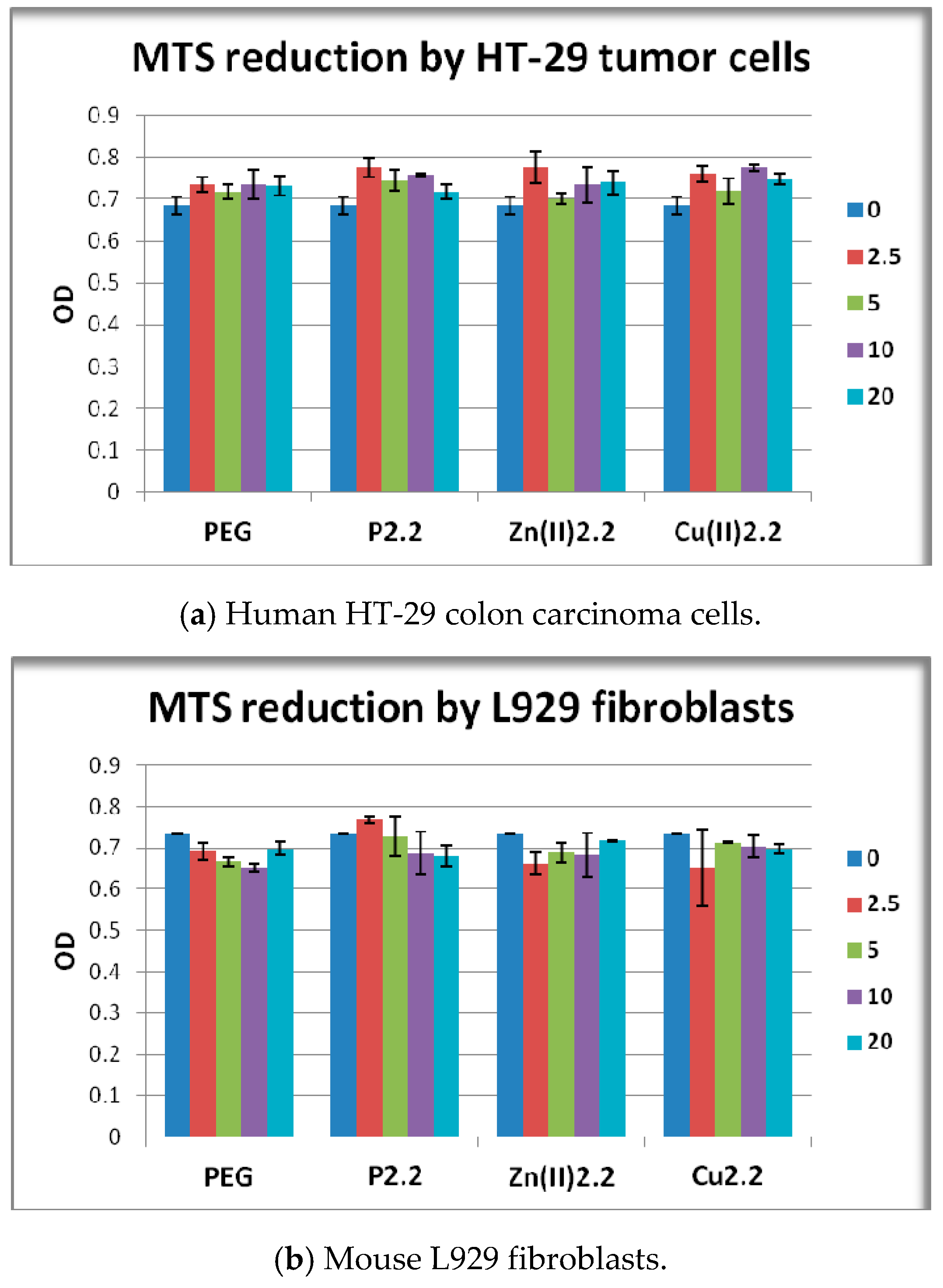
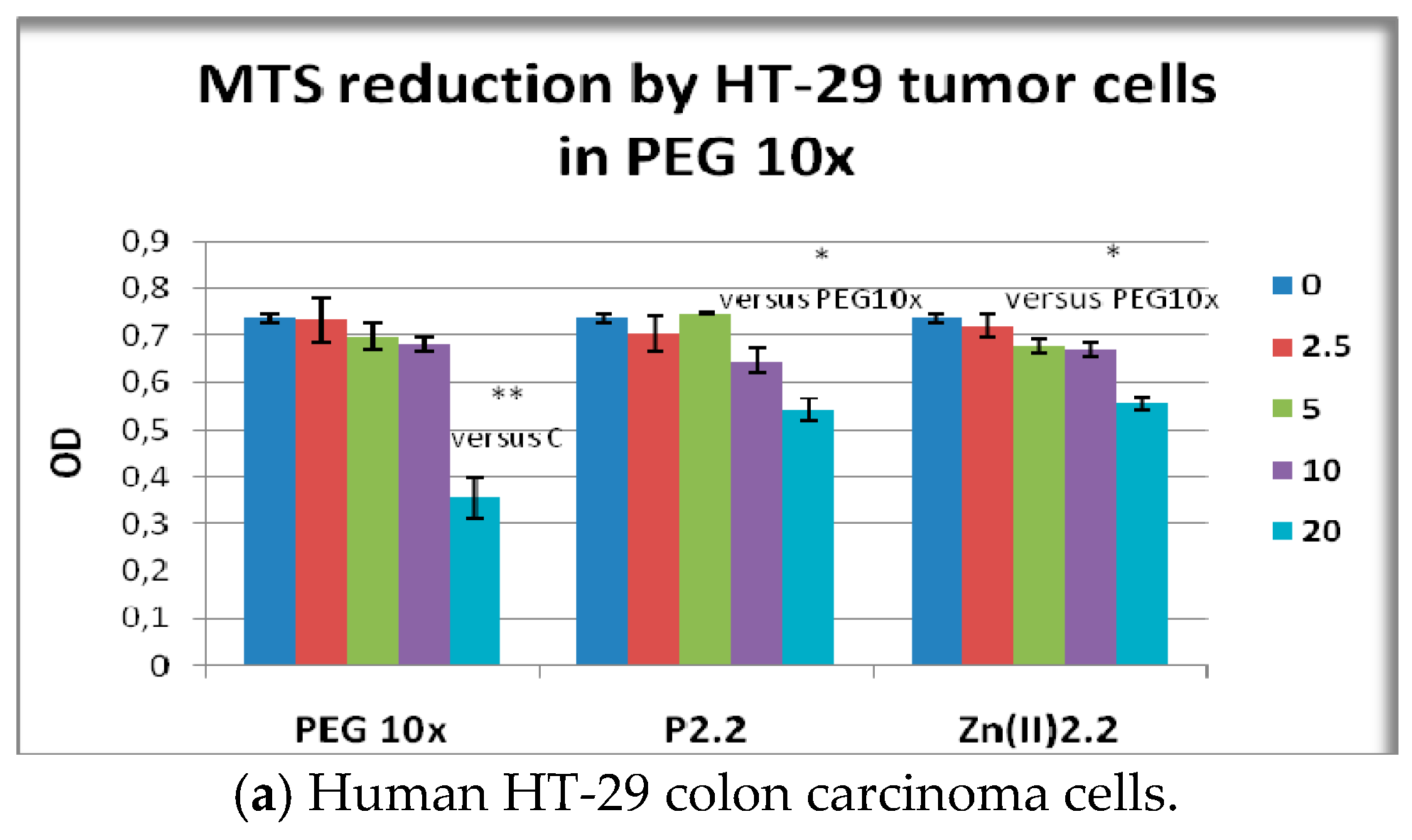
2.3. In Vitro Cytotoxicity Study
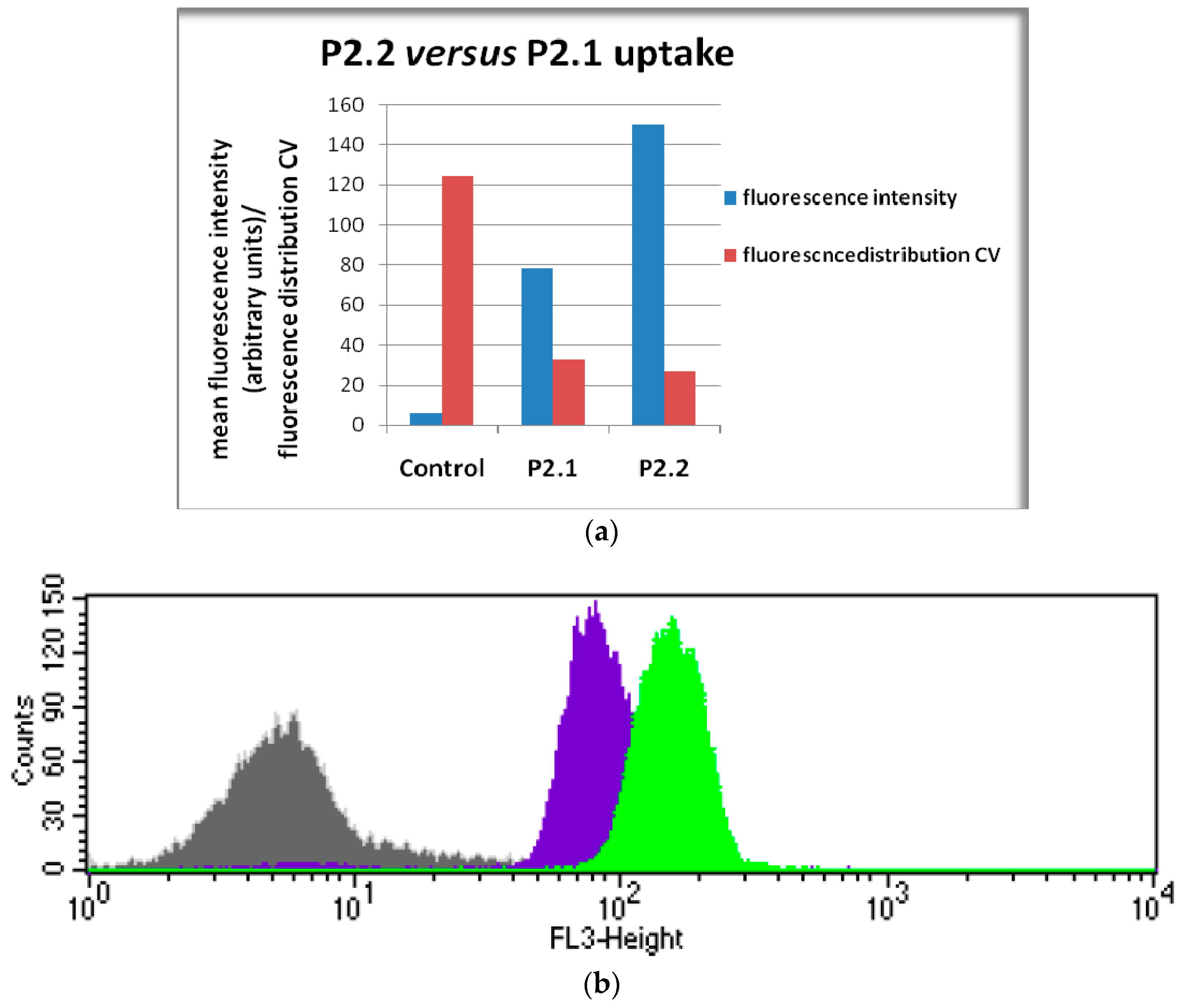
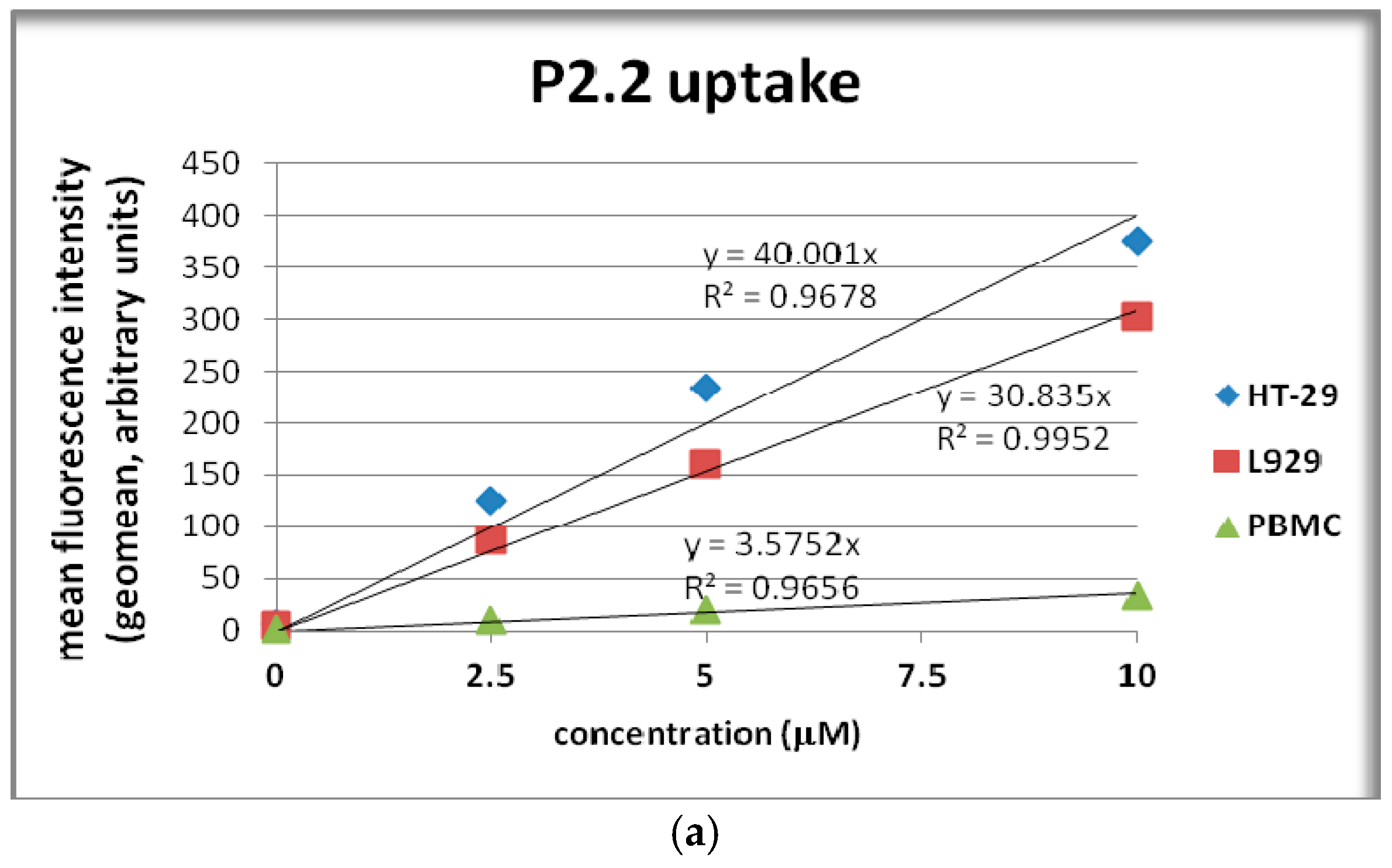
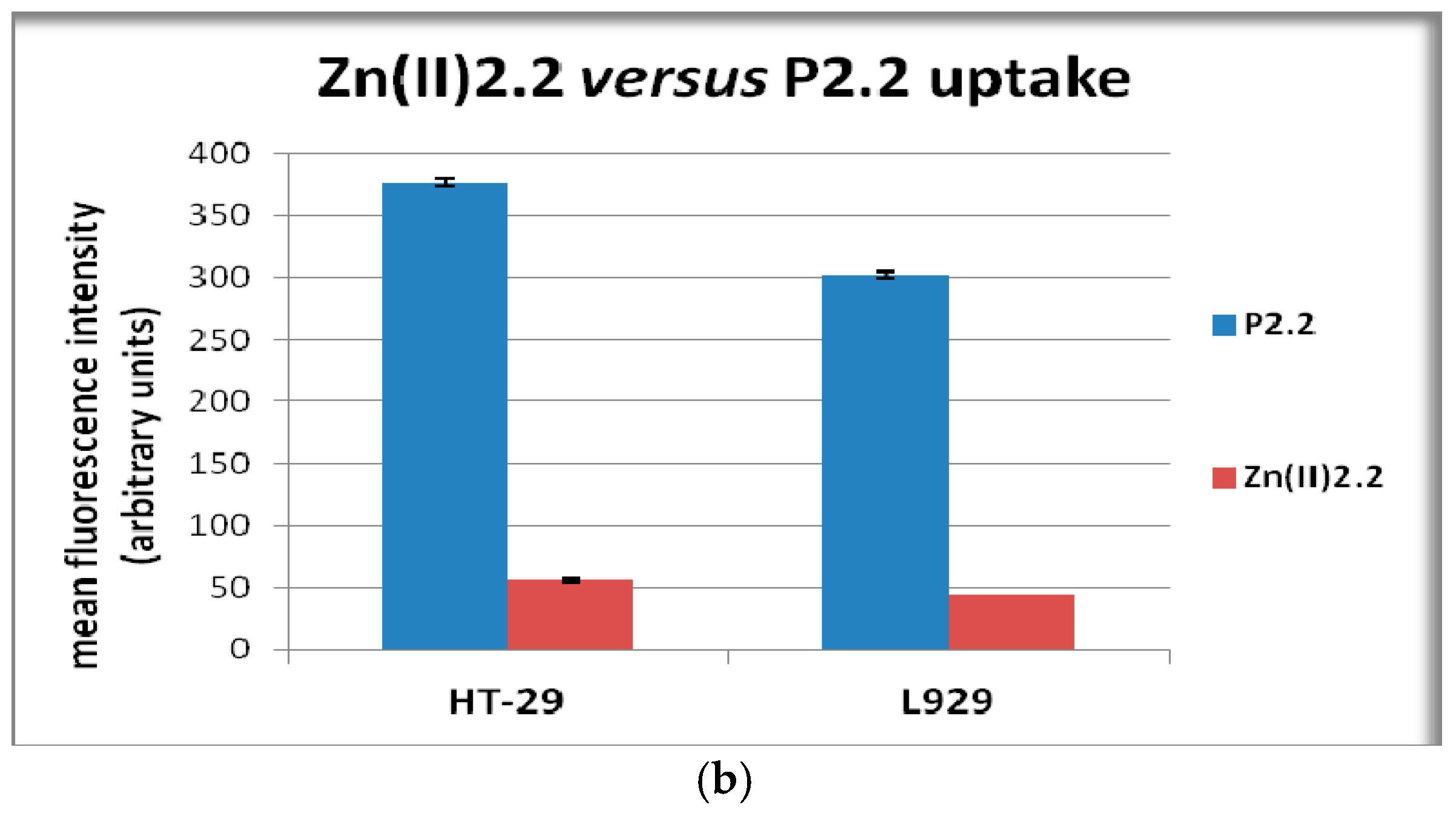
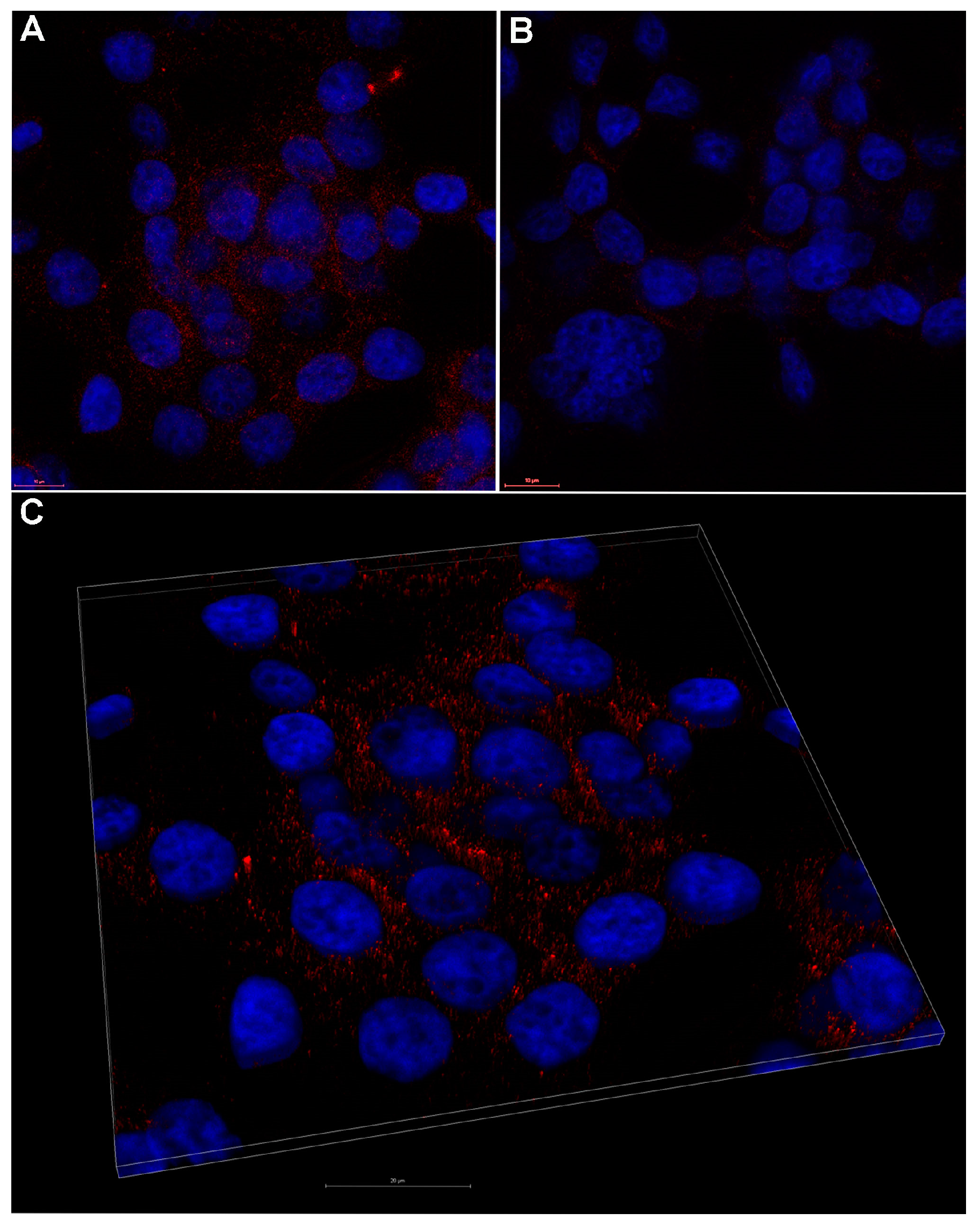
2.4. In Vitro Uptake of Porphyrinic Compounds
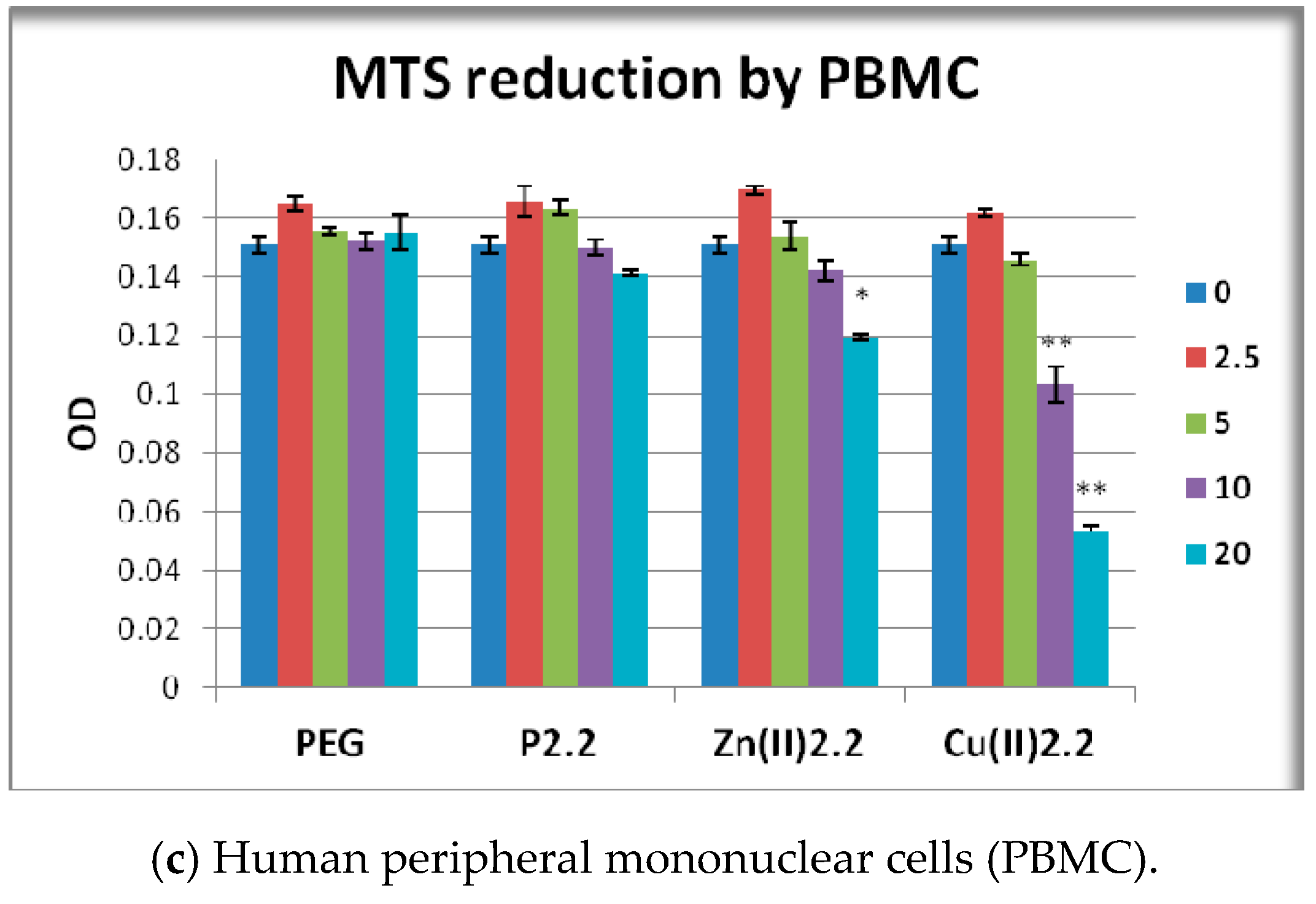
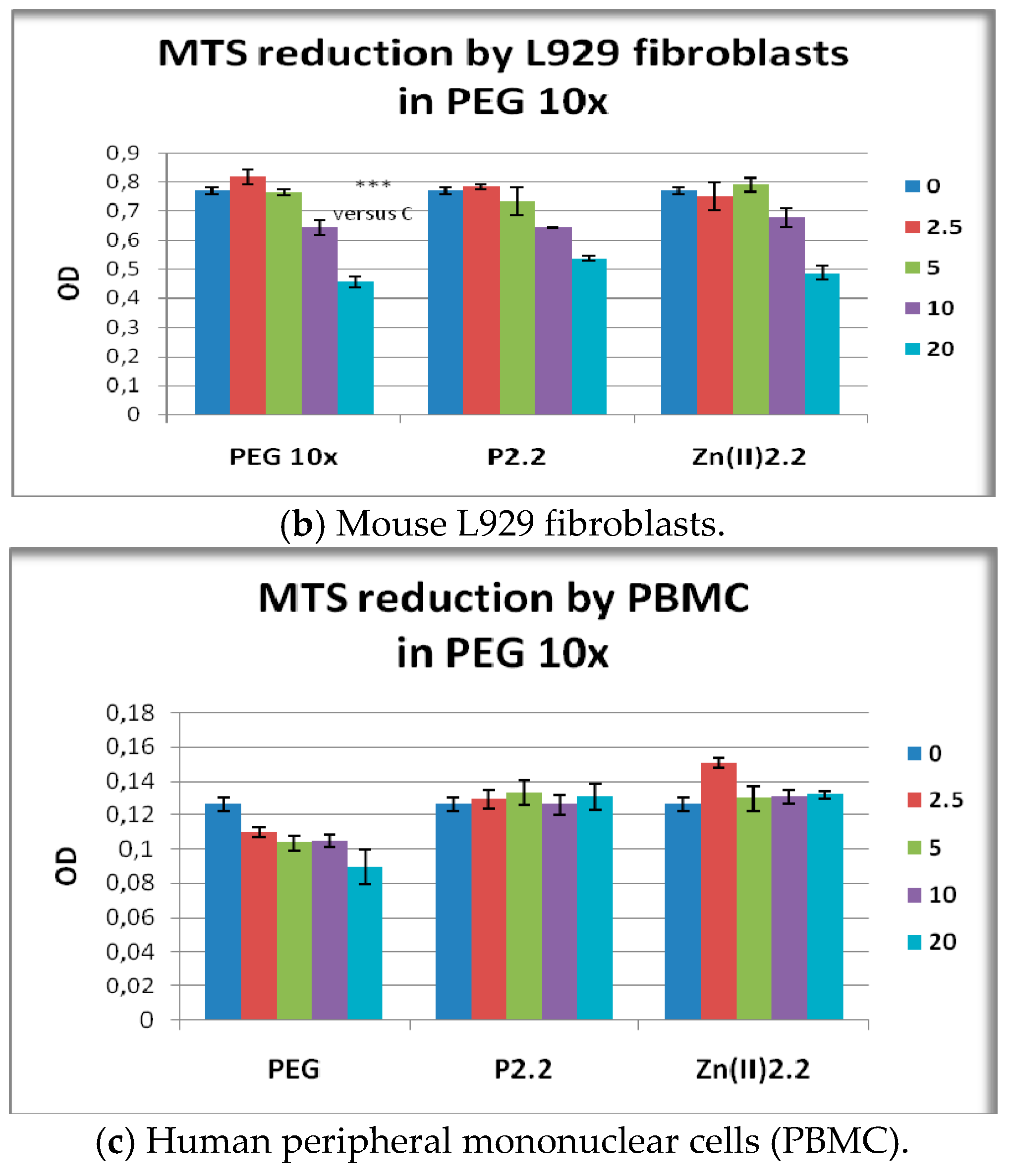
2.5. The In Vitro Effect of Porphyrinic Compounds on Cellular Viability and Proliferation
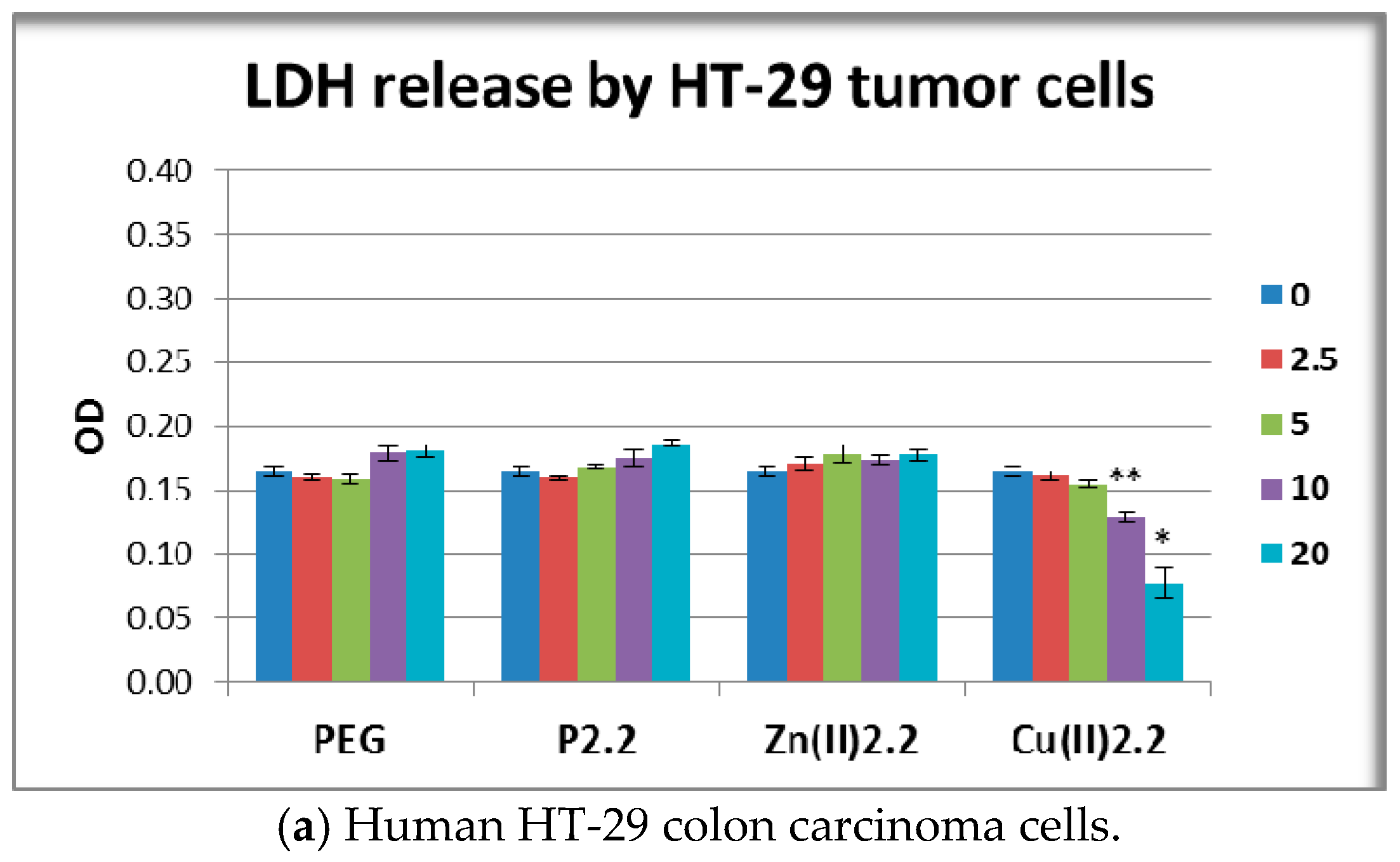
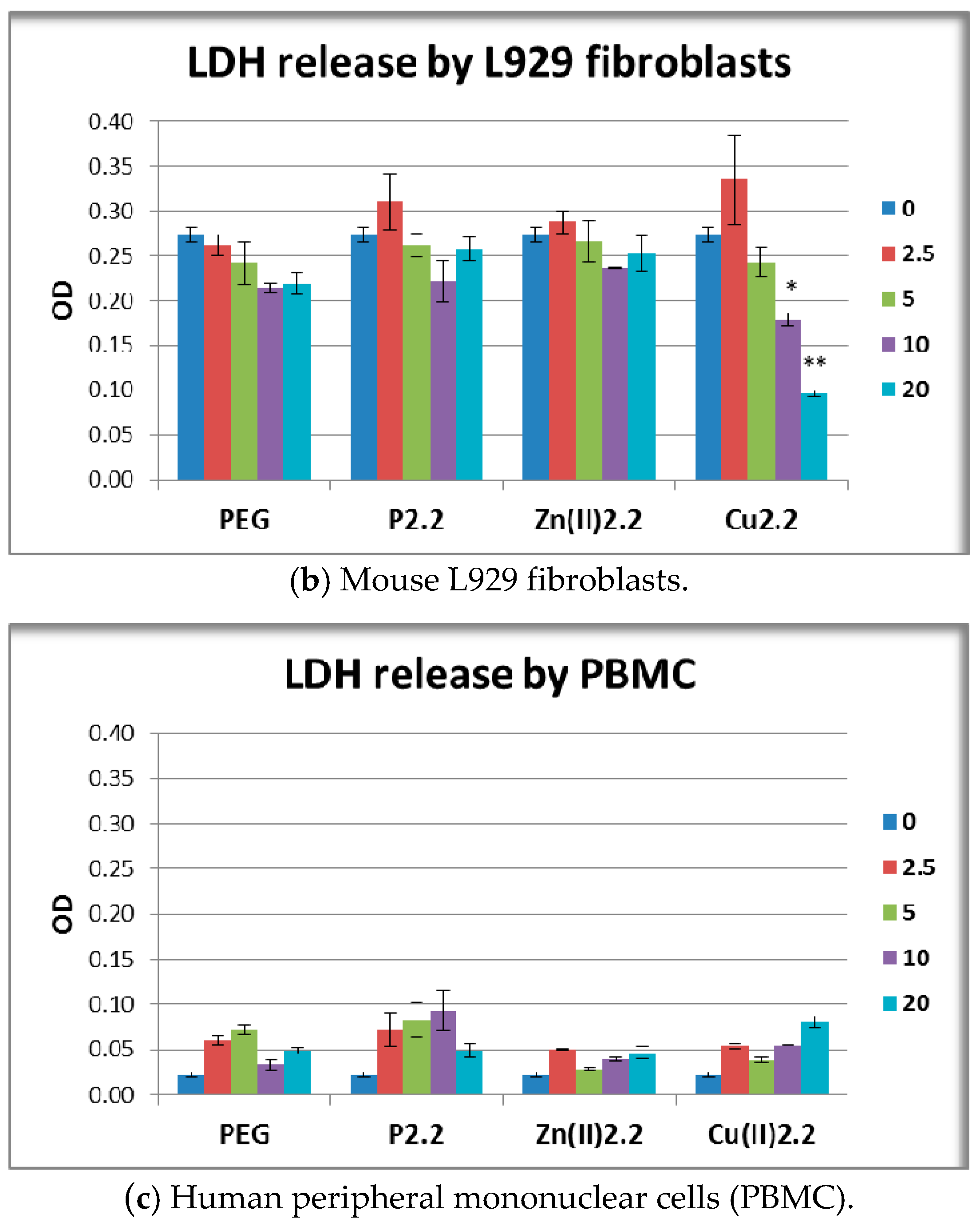
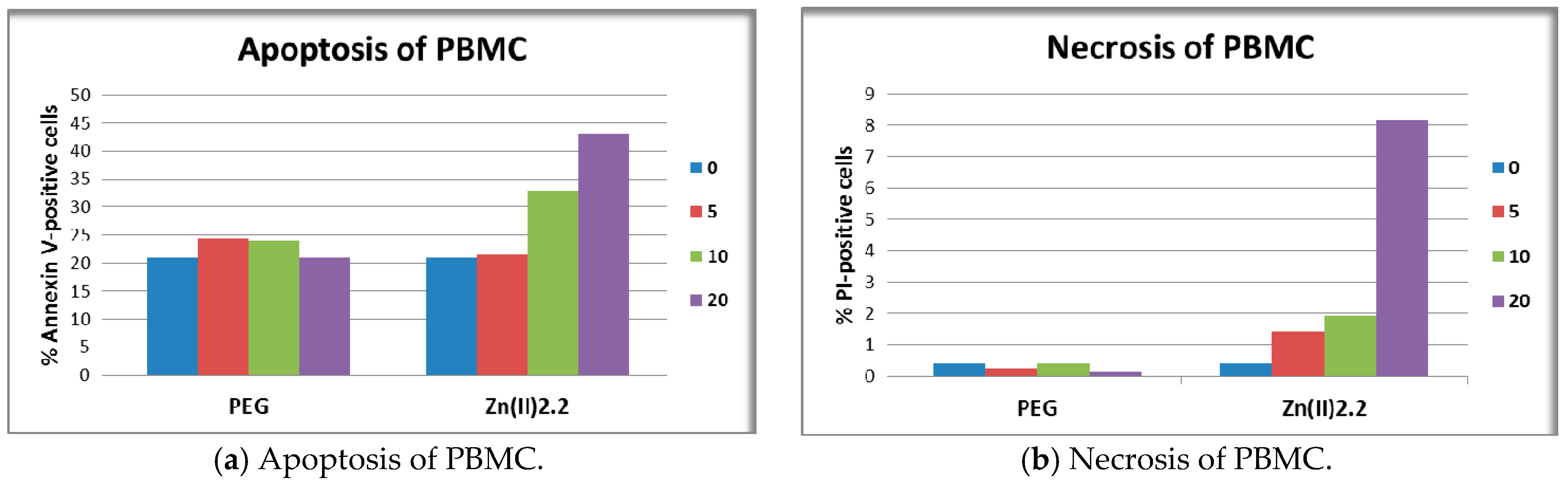
2.6. The In Vitro Effect of Porphyrinic Compounds on Membrane Integrity
2.7. The In Vitro Effect of P2.2 and Zn(II)2.2 in Higher Concentration of PEG 200
3. Experimental Section
3.1. General Information
3.2. Synthesis of 5-(4-Hydroxy-3-methoxyphenyl)-10,15,20-tris-(4-acetoxy-3-methoxyphenyl)porphyrin
3.3. Synthesis of M(II)-5-(4-Hydroxy-3-methoxyphenyl)-10,15,20-tris-(4-acetoxy-3-methoxyphenyl)porphyrins (Zn(II)2.2 and Cu(II)2.2)
3.4. In Vitro “Dark” Cytotoxicity Study
3.5. Cellular Uptake of Porphyrinic Compound
3.6. Cell Viability and Proliferation
3.7. Cell Death
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Josefsen, L.B.; Boyle, R.W. Unique diagnostic and therapeutic roles of porphyrins and phthalocyanines in photodynamic therapy, imaging and theranostics. Theranostics 2012, 2, 916–966. [Google Scholar] [CrossRef] [PubMed]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Mallidi, S.; Zheng, X.; Rahmanzadeh, R.; Mir, Y.; Elrington, S.; Khurshid, A.; Hasan, T. Development and applications of photo-triggered theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1094–1124. [Google Scholar] [CrossRef] [PubMed]
- Manivannan, E.; Nayan, J.P.; Ravindra, K.P. Porphyrin-Based Multifunctional Agents for Tumor-Imaging and Photodynamic Therapy (PDT); Karl, M.K., Kevin, M.S., Roger, G., Eds.; Academic Press: Boston, MA, USA, 2011; Volume 4, pp. 249–323. [Google Scholar]
- Lovell, J.F.; Liu, T.W.B.; Chen, J.; Zheng, G. Activatable Photosensitizers for Imaging and Therapy. Chem. Rev. 2010, 110, 2839–2857. [Google Scholar] [CrossRef] [PubMed]
- Vicente, M.G.H. Porphyrin-based sensitizers in the detection and treatment of cancer: Recent progress. Curr. Med. Chem. 2001, 1, 175–194. [Google Scholar] [CrossRef]
- Simoes, A.V.C.; Adamowicz, A.; Dabrowski, J.M.; Calvete, M.J.F.; Abreu, A.R.; Stochel, G.; Arnaut, L.G.; Pereira, M.M. Amphiphilic meso(sulfonate ester fluoroaryl)porphyrins: Refining the substituents of porphyrin derivatives for phototherapy and diagnostics. Tetrahedron 2012, 68, 8767–8772. [Google Scholar] [CrossRef]
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and nonporphyrin photosensitizers in oncology: Preclinical and clinical advances in photodynamic therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef] [PubMed]
- Ortel, B.; Shea, C.R.; Calzavara-Pinton, P. Molecular mechanisms of photodynamic therapy. Front. Biosci. Landmark 2009, 14, 4157–4172. [Google Scholar] [CrossRef]
- Yano, S.; Hirohara, S.; Obata, M.; Hagiya, Y.; Ogura, S.; Ikeda, A.; Kataoka, H.; Tanaka, M.; Joh, T. Current states and future views in photodynamic therapy. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 46–67. [Google Scholar] [CrossRef]
- Musiol, R.; Serda, M.; Polanski, J. Prodrugs in photodynamic anticancer therapy. Curr. Pharm. Des. 2011, 17, 3548–3559. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.F.F.; Serpa, C.; Dabrowski, J.M.; Monteiro, C.J.P.; Formosinho, S.J.; Stochel, G.; Urbanska, K.; Simoes, S.; Pereira, M.M.; Arnaut, L.G. Mechanisms of singlet-oxygen and superoxide-ion generation by porphyrins and bacteriochlorins and their implications in photodynamic therapy. Chem. Eur. J. 2010, 16, 9273–9286. [Google Scholar] [CrossRef] [PubMed]
- Grancho, J.C.P.; Pereira, M.M.; Miguel, M.D.; Gonsalves, A.M.R.; Burrows, H.D. Synthesis, spectra and photophysics of some free base tetrafluoroalkyl and tetrafluoroaryl porphyrins with potential applications in imaging. Photochem. Photobiol. 2002, 75, 249–256. [Google Scholar] [CrossRef]
- Horiuchi, H.; Kameya, T.; Hosaka, M.; Yoshimura, K.; Kyushin, S.; Matsumoto, H.; Okutsu, T.; Takeuchi, T.; Hiratsuka, H. Silylation enhancement of photodynamic activity of tetraphenylporphyrin derivative. J. Photochem. Photobiol. A 2011, 221, 98–104. [Google Scholar] [CrossRef]
- Pavani, C.; Uchoa, A.F.; Oliveira, C.S.; Iamamoto, Y.; Baptista, M.S. Effect of zinc insertion and hydrophobicity on the membrane interactions and PDT activity of porphyrin photosensitizers. Photochem. Photobiol. Sci. 2009, 8, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Socoteanu, R.; Manda, G.; Boscencu, R.; Vasiliu, G.; Oliveira, A.S. Synthesis, Spectral Analysis and Preliminary in Vitro Evaluation of Some Tetrapyrrolic Complexes with 3d Metal Ions. Molecules 2015, 20, 15488–15499. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.F.V.; Ferreira, D.P.; Oliveira, A.S.; Boscencu, R.; Socoteanu, R.; Ilie, M.; Constantin, C.; Neagu, M. Synthesis, Photophysical and Cytotoxicity Evaluation of A3B Type Mesoporphyrinic Compounds. Dyes Pigment. 2012, 95, 296–303. [Google Scholar] [CrossRef]
- Boscencu, R. Unsymmetrical Mesoporphyrinic Complexes of Copper(II) and Zinc(II). Microwave-Assisted Synthesis, Spectral Characterization and Cytotoxicity Evaluation. Molecules 2011, 16, 5604–5617. [Google Scholar] [CrossRef]
- Boscencu, R. Microwave Synthesis under Solvent-Free Conditions and Spectral Studies of Some Mesoporphyrinic Complexes. Molecules 2012, 17, 5592–5603. [Google Scholar] [CrossRef] [PubMed]
- Boscencu, R.; Oliveira, A.S.; Ferreira, D.P.; Ferreira, L.F.V. Synthesis and spectral evaluation of some unsymmetrical mesoporphyrinic complexes. Int. J. Mol. Sci. 2012, 13, 8112–8125. [Google Scholar] [CrossRef] [PubMed]
- Boscencu, R.; Licsandru, D.; Socoteanu, R.; Oliveira, A.S.; Ferreira, L.F.V. Synthesis and characterization of some mesoporphyrinic compounds unsymetricaly substituted. Rev. Chim. 2007, 58, 498–502. [Google Scholar]
- Socoteanu, R.; Boscencu, R.; Nacea, V.; Oliveira, A.S.; Ferreira, L.F.V. Microwave-assisted synthesis towards unssimetrical tetrapyrrolic compounds. Rev. Chim. 2008, 59, 498–502. [Google Scholar]
- Boscencu, R.; Socoteanu, R.; Ilie, M.; Oliveira, A.S.; Constantin, C.; Ferreira, L.F.V. Synthesis, spectral and biological evaluation of some mesoporphyrinic complexes of Zn(II). Rev. Chim. 2009, 60, 1006–1011. [Google Scholar]
- Oliveira, A.S.; Licsandru, D.; Boscencu, R.; Socoteanu, R.; Nacea, V.; Ferreira, L.F.V. A Singlet Oxygen Photogeneration and Luminescence Study of Unsymmetrically-Substituted Meso-Porphyrinic Compounds. Int. J. Photoenergy 2009. [Google Scholar] [CrossRef]
- Boscencu, R.; Licsandru, D.; Nacea, V. Asymmetrically Substituted Porphyrin Derivative and Process for Obtaining the Same. National Patent 122094 B1/2008, 30 May 2008. [Google Scholar]
- Vasiliu, G.; Boscencu, R.; Socoteanu, R.; Nacea, V. Complex combinations of some transition metals with new unsymmetrical porphyrins. Rev. Chim. 2014, 65, 998–1001. [Google Scholar]
- Boscencu, R.; Ilie, M.; Socoteanu, R.; Oliveira, A.S.; Constantin, C.; Neagu, M.; Manda, M.; Ferreira, L.F.V. Microwave Synthesis, Basic Spectral and Biological Evaluation of Some Copper (II) Mesoporphyrinic Complexes. Molecules 2010, 15, 3731–3743. [Google Scholar] [CrossRef] [PubMed]
- Boscencu, R.; Socoteanu, R.; Oliveira, A.S.; Vieira Ferreira, L.F.; Nacea, V.; Patrinoiu, G. Synthesis and characterization of some unsymmetrically-substituted mesoporphyrinic mono-hydroxyphenyl complexes of Copper(II). Pol. J. Chem. 2008, 82, 509–522. [Google Scholar]
- Boscencu, R.; Socoteanu, R.; Oliveira, A.S.; Ferreira, L.F.V. Studies on Zn(II) monohydroxyphenyl mesoporphyrinic complexes. Synthesis and characterization. J. Serb. Chem. Soc. 2008, 73, 713–726. [Google Scholar] [CrossRef]
- Adler, A.D.; Longo, F.R.; Finarelli, J.D.; Goldmacher, J.; Assour, J.; Korsakoff, L. A simplified synthesis for mesotetraphenylporphine. J. Org. Chem. 1967, 32, 476–490. [Google Scholar] [CrossRef]
- Little, R.A.; Anton, J.A.; Loach, P.A.; Ibers, J.A. The synthesis of some substituted tetraarylporphyrins. J. Heter.Chem. 1974, 12, 343–349. [Google Scholar] [CrossRef]
- Lin, W.C. Electron Spin Resonance and Electronic Structure of Metalloporphyrins. In The Porphyrins; Dolphin, D., Ed.; Academic Press: New York, NY, USA, 1978; Volume 4, pp. 358–364. [Google Scholar]
- Manoharan, P.T.; Roger, M.T. ESR Study of Copper(II) and Silver(II) Tetraphenylporphyrin. In Electron Spin Resonance of Metal Complexes; Yen, T.F., Ed.; Plenum Press: New York, NY, USA, 1969; pp. 143–173. [Google Scholar]
- Gouterman, M. Optical Spectra and Electronic Structure of Porphyrins and Related Rings. In The Porphyrins; Dolphin, D., Ed.; Academic Press: New York, NY, USA, 1978; Volume 3, pp. 11–87. [Google Scholar]
- Gouterman, M.; Wagniere, G.H.; Snyder, L.C. Spectra of porphyrins: Part II. Four orbital model. J. Mol. Spectrosc. 1963, 11, 108–127. [Google Scholar] [CrossRef]
- Manda, G.; Isvoranu, G.; Comanescu, M.V.; Manea, A.; Butuner, B.D.; Korkmaz, K.S. The redox biology network in cancer pathophysiology and therapeutics. Redox Boil. 2015, 5, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Murov, S.L.; Carmichael, I.; Hug, G.L. Handbook of Photochemistry, 2nd ed.; CRC Press: New York, NY, USA, 1989. [Google Scholar]
- Wilkinson, F. Triplet quantum yields and singlet-triplet intersystem crossing. In Organic Molecular Photophysics; Birks, J.B., Ed.; John Wiley and Sons: London, UK, 1885. [Google Scholar]
- Eaton, D.F. Luminescence Spectroscopy. In Handbook of Photochemistry, 2nd ed.; CRC Press: New York, NY, USA, 1989. [Google Scholar]
- Rechmond, R.W.; Braslavsky, S.E. NATO Science Series E; Springer: Berlin, Germany, 1988; pp. 93–95. [Google Scholar]
- Karra, N.; Borlak, J. Surface chemistry, functionalization and surface chemistry. In Nanostructured Biomaterials for Overcoming Biological Barriers; Alonso, M.J., Csaba, N.S., Eds.; RCS Publishing: Cambridge, UK, 2012; p. 560. ISBN 978-1-84973-363-2. [Google Scholar]
- Melzer, C.; Von Der Ohe, J.; Lehnert, H.; Ungefroren, H.; Hass, R. Cancer stem cell niche models and contribution by mesenchymal stroma/stem cells. Mol. Cancer 2017, 16, 28. [Google Scholar] [CrossRef] [PubMed]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Moravec, R.A.; Riss, T.L.; Niles, A.L.; Duellman, S.; Benink, H.A.; Tracy, J.; Worzella, T.J.; Minor, L. Cell Viability Assays. In Assay Guidance Manual [Internet]; Sittampalam, G.S., Coussens, N.P., Brimacombe, K., Eds.; Eli Lilly & Company and the National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2004. [Google Scholar]
- Berridge, M.V.; Tan, A.S. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): Subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch. Biochem. Biophys. 1993, 303, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.K.M.; Moriwaki, K.; De Rosa, M.J. Detection of Necrosis by Release of Lactate Dehydrogenase (LDH) Activity. Methods Mol. Biol. 2013, 979, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Pamp, K.; Bramey, T.; Kirsch, M.; De Groot, H.; Petrat, F. NAD(H) enhances the Cu(II)-mediated inactivation of lactate dehydrogenase by increasing the accessibility of sulfhydryl groups. Free Radic Res. 2005, 39, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Branco, T.J.F.; Botelho do Rego, A.M.; Ferreira Machado, I.; Vieira Ferreira, L.F. A luminescence lifetime distributions analysis in heterogeneous systems by the use of Excel’s Solver. J. Phys. Chem. B 2005, 109, 15958–15967. [Google Scholar] [CrossRef] [PubMed]
- Vieira Ferreira, L.F.; Ferreira Machado, I.L. Surface photochemistry: Organic molecules within nanocavities of calixarenes. Curr. Drug Discov. Technol. 2007, 4, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.P.; Conceição, D.S.; Calhelha, R.C.; Sousa, T.; Socoteanu, R.; Ferreira, I.C.F.R.; Vieira Ferreira, L.F. Porphyrin dye into biopolymeric chitosan films for localizedphotodynamic therapy of Cancer. Carbohydr. Polym. 2016, 151, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Boyum, A. Isolation of mononuclear cells and granulocytes from human blood. Scand. J. Clin. Lab. Investig. 1968, 21, 77–89. [Google Scholar]
Sample Availability: Not available. |
| Solvent | Absorption λmax (nm) [lg ε] (L mol−1 cm−1) | ||||
|---|---|---|---|---|---|
| Soret Band | Qy(1,0) | Qy(0,0) | Qx(1,0) | Qx(0,0) | |
| 5-(4-hydroxy-3-methoxyphenyl)-10,15,20-tris-(4-acetoxy-3-methoxyphenyl)porphyrin | |||||
| CHCl3 | 403.2[5.842] | 497.0[4.152] | 532.6[4.022] | 570.8[3.708] | 628.6[3.364] |
| CH2Cl2 | 402.0[5.629] | 497.6[4.340] | 532.8[4.073] | 570.0[3.729] | 626.4[3.482] |
| DMSO | 404.4[5.505] | 498.0[4.492] | 535.2[4.330] | 572.8[4.200] | 629.2[3.980] |
| EtOH | 400.0[5.479] | 495.6[4.542] | 530.8[4.400] | 571.6[4.388] | 627.6[4.371] |
| PEG 200 | 404.4[5.580] | 498.0[4.630] | 534.0[4.358] | 572.4[3.978] | 628.8[3.940] |
| Zn(II)-5-(4-hydroxy-3-methoxyphenyl)-10,15,20-tris-(4-acetoxy-3-methoxyphenyl)porphyrin | |||||
| CHCl3 | 407.2[5.726] | ---------------- | 534.2[4.390] | 572.5[4.270] | --------------- |
| CH2Cl2 | 402.8[5.706] | ---------------- | 527.6[4.459] | 566.4[3.903] | --------------- |
| DMSO | 411.6[5.470] | ---------------- | 542.0[4.429] | 583.0[4.302] | --------------- |
| EtOH | 405.6[5.602] | ---------------- | 537.6[4.450] | 577.6[4.365] | --------------- |
| PEG 200 | 409.6[5.421] | ---------------- | 538.2[3.908] | 579.5[3.602] | --------------- |
| Cu(II)-5-(4-hydroxy-3-methoxyphenyl)-10,15,20-tris-(4-acetoxy-3-methoxyphenyl)porphyrin | |||||
| CHCl3 | 401.5[5.540] | ---------------- | 519.6[4.634] | ---------------- | --------------- |
| CH2Cl2 | 400.4[5.457] | ---------------- | 518.4[4.343] | ---------------- | --------------- |
| DMSO | 402.4[5.436] | ---------------- | 524.6[4.500] | ---------------- | --------------- |
| EtOH | 396.4[5.386] | ---------------- | 516.2[4.360] | ---------------- | --------------- |
| PEG 200 | 400.0[5.440] | ---------------- | 521.2[4.455] | ---------------- | --------------- |
| Compound | ΦF in EtOH | τF (ns) in EtOH | Φ∆ in CHCl3 |
|---|---|---|---|
| Phenazine (*) | - | - | 0.84 |
| TPP (*) | 0.13 | 10.8 ± 0.1 | - |
| P2.1 | 0.06 | 8.7 ± 0.1 | 0.21 |
| P2.2 | 0.04 | 10.1 ± 0.1 | 0.16 |
| Zn(II)2.2 | 0.002 | 1.7 ± 0.05 | 0.17 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boscencu, R.; Manda, G.; Radulea, N.; Socoteanu, R.P.; Ceafalan, L.C.; Neagoe, I.V.; Ferreira Machado, I.; Basaga, S.H.; Vieira Ferreira, L.F. Studies on the Synthesis, Photophysical and Biological Evaluation of Some Unsymmetrical Meso-Tetrasubstituted Phenyl Porphyrins. Molecules 2017, 22, 1815. https://doi.org/10.3390/molecules22111815
Boscencu R, Manda G, Radulea N, Socoteanu RP, Ceafalan LC, Neagoe IV, Ferreira Machado I, Basaga SH, Vieira Ferreira LF. Studies on the Synthesis, Photophysical and Biological Evaluation of Some Unsymmetrical Meso-Tetrasubstituted Phenyl Porphyrins. Molecules. 2017; 22(11):1815. https://doi.org/10.3390/molecules22111815
Chicago/Turabian StyleBoscencu, Rica, Gina Manda, Natalia Radulea, Radu Petre Socoteanu, Laura Cristina Ceafalan, Ionela Victoria Neagoe, Isabel Ferreira Machado, Selma Huveyda Basaga, and Luís Filipe Vieira Ferreira. 2017. "Studies on the Synthesis, Photophysical and Biological Evaluation of Some Unsymmetrical Meso-Tetrasubstituted Phenyl Porphyrins" Molecules 22, no. 11: 1815. https://doi.org/10.3390/molecules22111815
APA StyleBoscencu, R., Manda, G., Radulea, N., Socoteanu, R. P., Ceafalan, L. C., Neagoe, I. V., Ferreira Machado, I., Basaga, S. H., & Vieira Ferreira, L. F. (2017). Studies on the Synthesis, Photophysical and Biological Evaluation of Some Unsymmetrical Meso-Tetrasubstituted Phenyl Porphyrins. Molecules, 22(11), 1815. https://doi.org/10.3390/molecules22111815







