In Vitro Evaluation of Third Generation PAMAM Dendrimer Conjugates
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of G3 PAMAM Dendrimer-Naproxen Conjugates
2.2. Determination of Partition Coefficient and Solubility
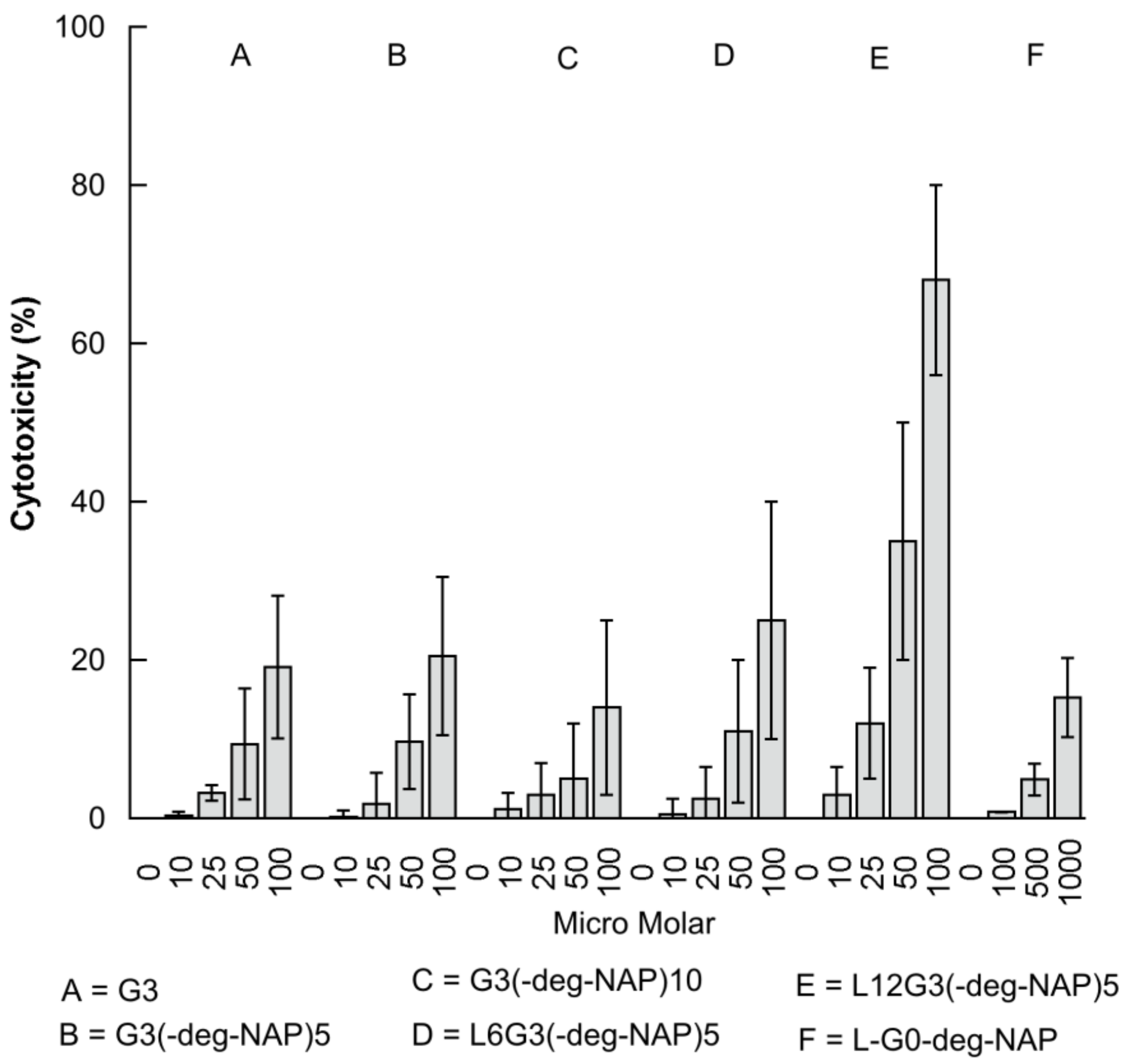
2.3. The Effect of PAMAM Dendrimer Prodrugs on Caco-2 Cell Viability
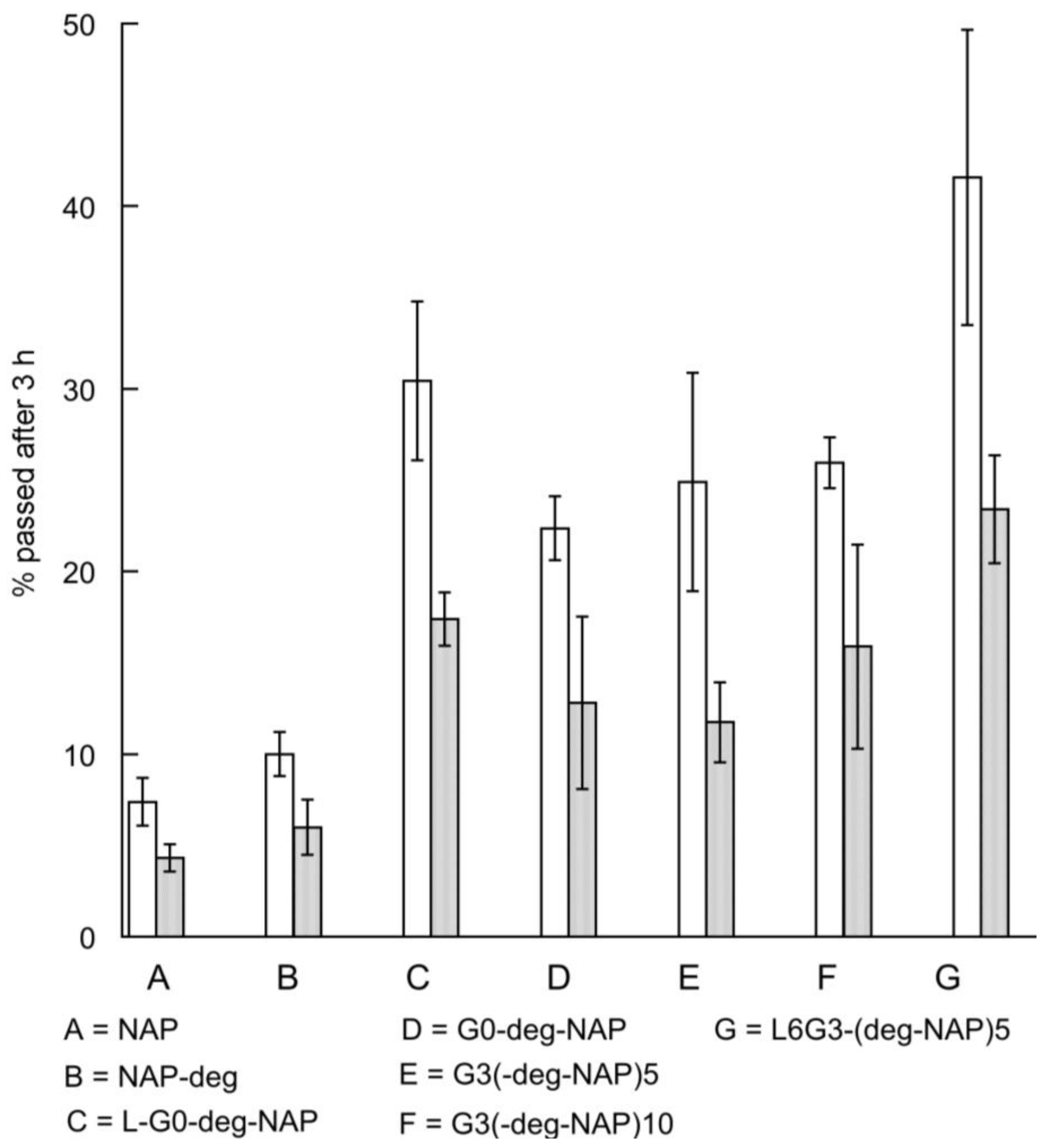
2.4. Transport of Naproxen, G0 PAMAM Dendrimer and Conjugates across Caco-2 Monolayers
3. Materials and Methods
3.1. Materials
3.2. Synthesis of G3-(Diethylene glycol-naproxen)x (G3-(deg-NAPx))
3.3. Synthesis of (Lauroyl)y-G3-(diethylene glycol-naproxen)x (LyG3-(deg-NAP)x)
3.4. Determination of Partition Coefficients and Soluiblities
3.5. Lactate Dehydrogenase Leakage (LDH) Assay
3.6. Transport Studies of G3 PAMAM Dendrimer and Conjugates
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A new class of polymers: Starburst-dendritic macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- D’Emanuele, A.; Attwood, D.; Abu-Rmaileh, R. Dendrimers. In Encyclopedia of Pharmaceutical Technology; Swarbrick, J.B.J.C., Ed.; Marcel Dekker: New York, NY, USA, 2003; pp. 1–21. [Google Scholar]
- Kesharwani, P.; Gothwal, A.; Iyer, A.K.; Jain, K.; Chourasia, M.K.; Gupta, U. Dendrimer nanohybrid carrier systems: An expanding horizon for targeted drug and gene delivery. Drug Discov. Today 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Natfji, A.A.; Osborn, H.M.I.; Greco, F. Feasibility of polymer-drug conjugates for non-cancer applications. Curr. Opin. Colloid Interface Sci. 2017, in press. [Google Scholar] [CrossRef]
- D’Emanuele, A.; Attwood, D. Dendrimer-drug interactions. Adv. Drug Deliv. Rev. 2005, 15, 2147–2162. [Google Scholar] [CrossRef] [PubMed]
- Milhem, O.M.; Myles, C.; McKeown, N.B.; Attwood, D.; D’Emanuele, A. Polyamidoamine starburst dendrimers as solubility enhancers. Int. J. Pharm. 2000, 197, 239–241. [Google Scholar] [CrossRef]
- Yiyun, C.; Tongwen, X. Dendrimers as potential drug carriers. Part I. Solubilization of non-steroidal anti-inflammatory drugs in the presence of polyamidoamine dendrimers. Eur. J. Med. Chem. 2005, 40, 1188–1192. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; Najlah, M.; D’Emanuele, A.; Elhissi, A. Pamam dendrimers as aerosol drug nanocarriers for pulmonary delivery via nebulization. Int. J. Pharm. 2014, 461, 242–250. [Google Scholar] [CrossRef] [PubMed]
- D’Emanuele, A.; Jevprasesphant, R.; Penny, J.; Attwood, D. The use of a dendrimer-propranolol prodrug to bypass efflux transporters and enhance oral bioavailability. J. Control. Release 2004, 95, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Najlah, M.; Freeman, S.; Attwood, D.; D’Emanuele, A. Synthesis, characterization and stability of dendrimer prodrugs. Int. J. Pharm. 2006, 308, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Najlah, M.; Freeman, S.; Attwood, D.; D’Emanuele, A. In vitro evaluation of dendrimer prodrugs for oral drug delivery. Int. J. Pharm. 2006, 336, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Decker, T.; Lohmann-Matthes, M.L. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J. Immunol. Methods 1998, 115, 61–69. [Google Scholar] [CrossRef]
- Jevprasesphant, R.; Penny, J.; Attwood, D.; McKeown, N.B.; D’Emanuele, A. Engineering of dendrimer surfaces to enhance transepithelial transport and reduce cytotoxicity. Pharm. Res. 2003, 20, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Jevprasesphant, R.; Penny, J.; Jalal, R.; Attwood, D.; McKeown, N.B.; D’Emanuele, A. The influence of surface modification on the cytotoxicity of pamam dendrimers. Int. J. Pharm. 2003, 252, 263–266. [Google Scholar] [CrossRef]
- Teow, H.M.; Zhou, Z.; Najlah, M.; Yusof, S.R.; Abbott, N.J.; D’Emanuele, A. Delivery of paclitaxel across cellular barriers using a dendrimer-based nanocarrier. Int. J. Pharm. 2013, 441, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Najlah, M.; D’Emanuele, A. Crossing cellular barriers using dendrimer nanotechnologies. Curr. Opin. Pharmacol. 2006, 6, 522–527. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, M.; Ginski, M.; Rhodes, C.; Ghandehari, H. Transepithelial transport of poly(amidoamine) dendrimers across Caco-2 cell monolayers. J. Control. Release 2002, 81, 355–365. [Google Scholar] [CrossRef]
- Knipp, G.T.; Ho, N.F.H.; Barsuhn, C.L.; Borchardt, R.R. Paracellular diffusion in Caco-2 cell monolayers: Effect of perturbation on the transport of hydrophilic compounds that vary in charge and size. J. Pharm. Sci. 1997, 86, 1105–1110. [Google Scholar] [CrossRef] [PubMed]
- Aungst, B.J. Intestinal permeation enhancers. J. Pharm. Sci. 2000, 89, 429–442. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds not available from the authors. |
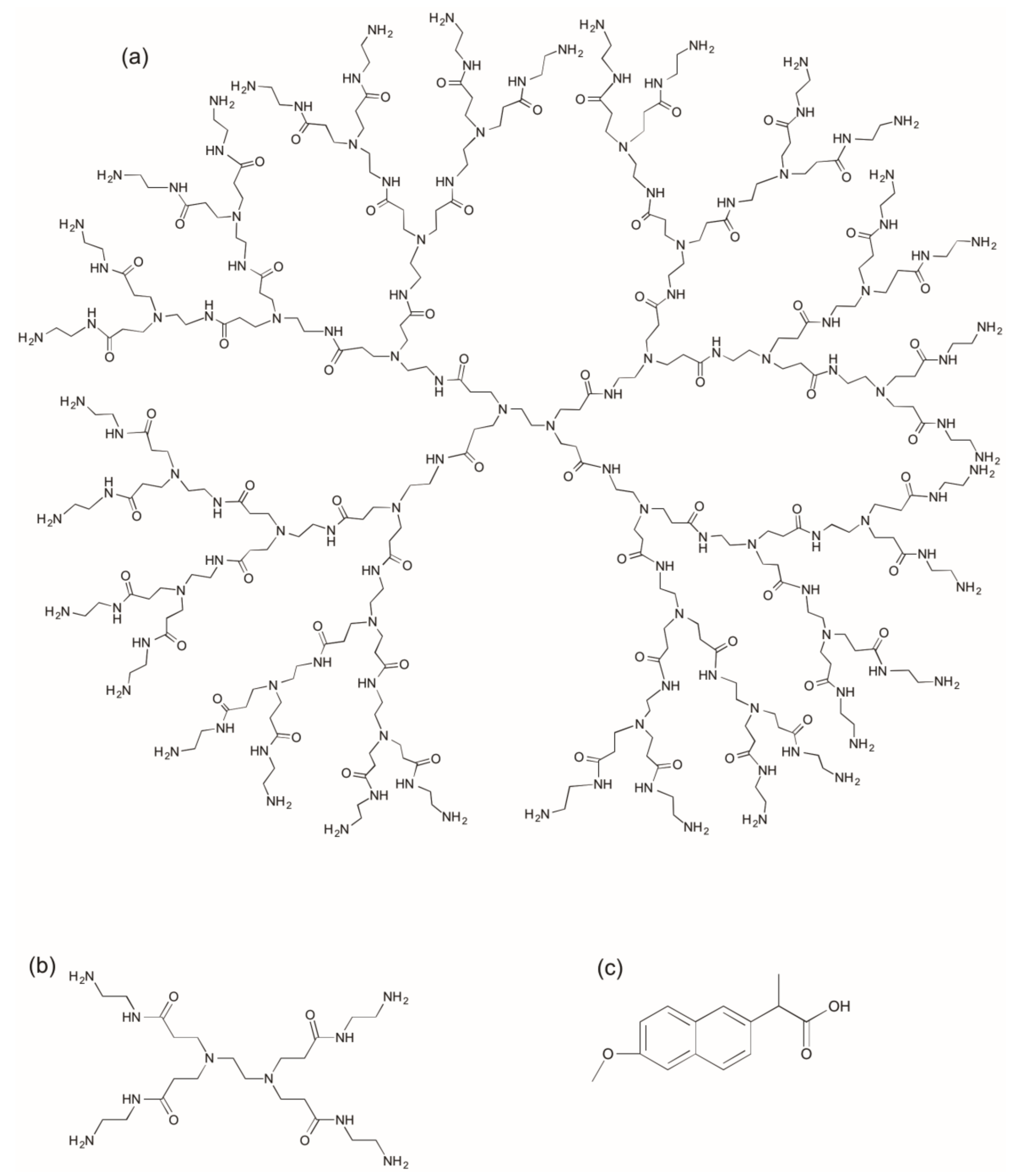


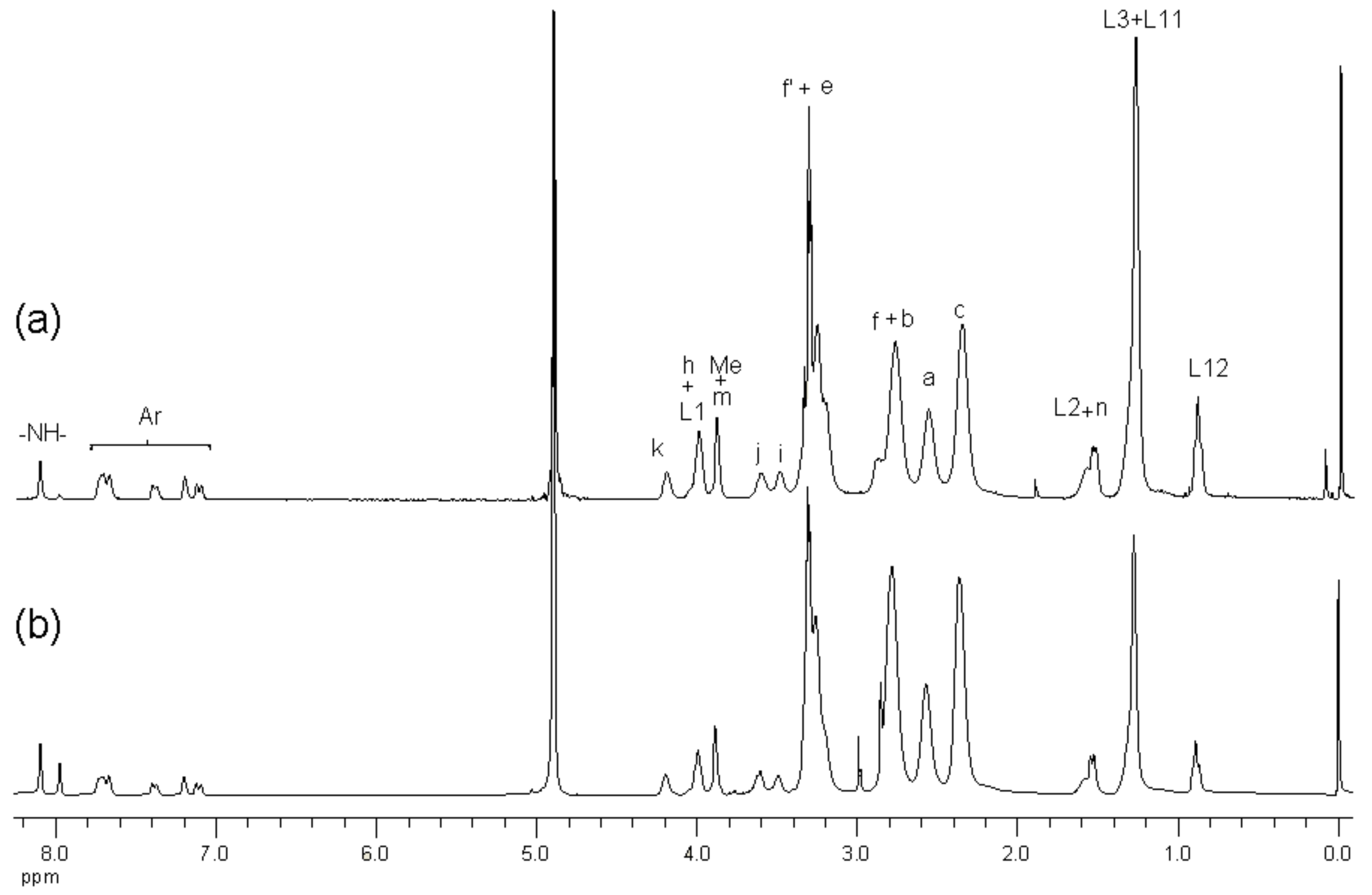
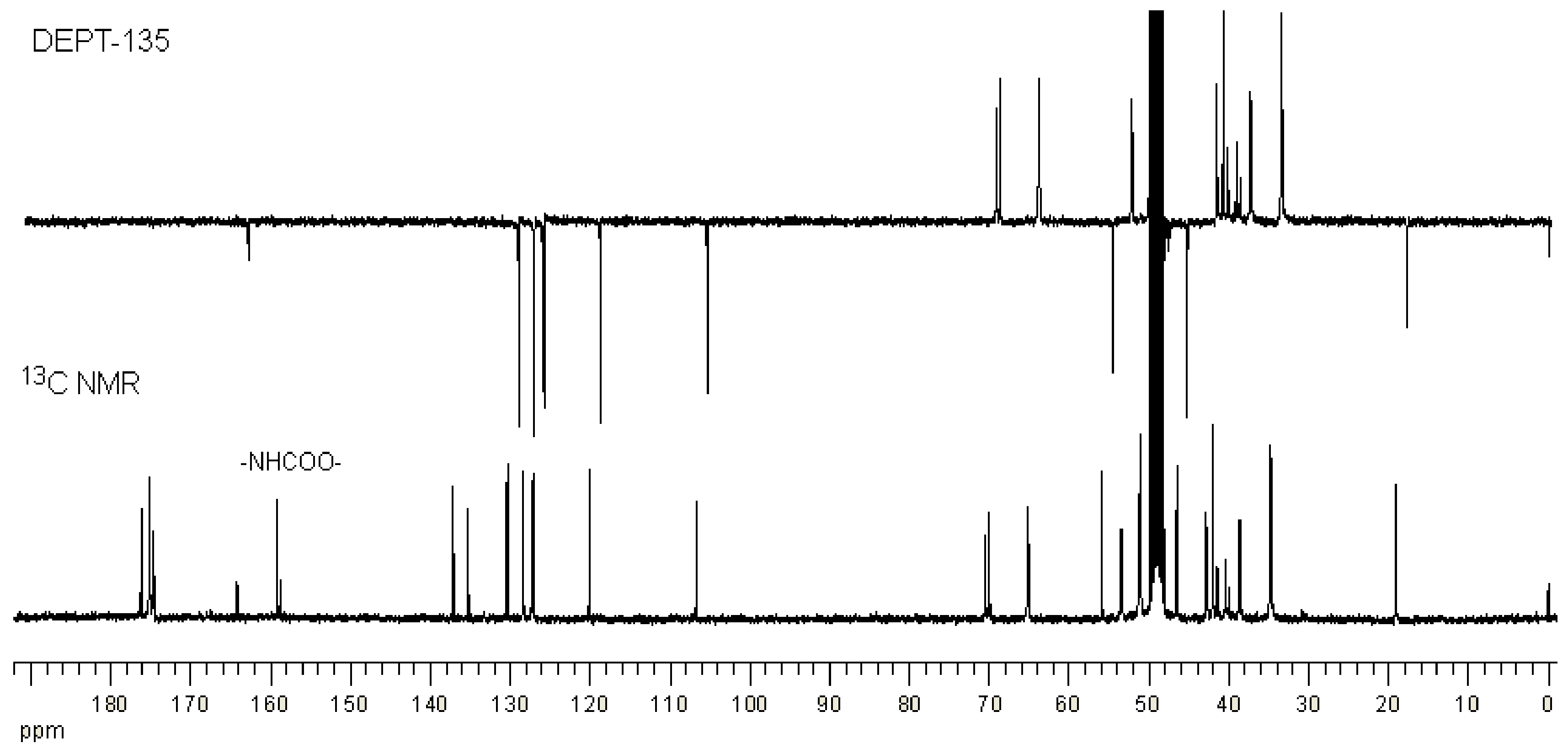
| Conjugation Ratio G3:Naproxen † | Conjugation Ratio G3:Lauroyl Chain † | Average wt/mol (g/mol) | Conjugate Concentration (µM) | Equivalent Concentration of Naproxen (µM) | |
|---|---|---|---|---|---|
| G3 | n/a | n/a | 6909 | n/a | n/a |
| G3-(deg-NAP)5 | 1:5.2 | n/a | 8636 | 19.2 | 99.8 |
| G3-(deg-NAP)10 | 1:10.5 | n/a | 10,363 | 9.5 | 99.8 |
| L6G3-(deg-NAP)5 | 1:4.8 | 1:6.4 | 9916 | 20.8 | 99.8 |
| L12G3-(deg-NAP)5 | 1:5.4 | 1:11.6 | 11,196 | n/a | n/a |
| G0 | n/a | n/a | 517 | n/a | n/a |
| G0-deg-NAP | 1:1 | n/a | 861 | 100.0 | 100.0 |
| L-G0-deg-NAP | 1:1 | 1:1 | 1073 | 100.0 | 100.0 |
| Compound | pH 7.4 | pH 1.2 |
|---|---|---|
| NAP | 0.11 ± 0.01 | 2.8 ± 0.7 |
| G0-deg-NAP | −1.07 ± 0.09 | −1.17 ± 0.1 |
| L-G0-deg-NAP | −0.19 ± 0.07 | −0.18 ± 0.05 |
| G3-(deg-NAP)5 | −0.36 ± 0.12 | −0.35 ± 0.19 |
| G3-(deg-NAP)10 | −0.40 ± 0.13 | −0.37 ± 0.17 |
| L6G3-(deg-NAP)5 | −0.40 ± 0.09 | −0.32 ± 0.11 |
| L12G3-(deg-NAP)5 | −0.18 ± 0.04 | −0.17 ± 0.09 |
| Compound | Solubility mg/mL | Solubility Eq. con. NAP * (mM) |
|---|---|---|
| NAP | 0.06 | 0.26 |
| G0-deg-NAP | >50 | >58 |
| L-G0-deg-NAP | 35 ± 12 | 32 ± 11 |
| G3-(deg-NAP)5 | >50 | >28 |
| G3-(deg-NAP)10 | >50 | >48.2 |
| L6G3-(deg-NAP)5 | 29 ± 17 | 14 ± 8.2 |
| L12G3-(deg-NAP)5 | 18 ± 12 | 8 ± 5.7 |
| Compound | IC50 (µM) |
|---|---|
| G3 | 247 ± 35 |
| G3-(deg-NAP)5 | 225 ± 32 |
| G3-(deg-NAP)10 | 357 ± 57 |
| L6G3-(deg-NAP)5 | 189 ± 28 |
| L12G3-(deg-NAP)5 | 76 ± 15 |
| L-G0-deg-NAP | 3181 ± 455 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najlah, M.; Freeman, S.; Khoder, M.; Attwood, D.; D’Emanuele, A. In Vitro Evaluation of Third Generation PAMAM Dendrimer Conjugates. Molecules 2017, 22, 1661. https://doi.org/10.3390/molecules22101661
Najlah M, Freeman S, Khoder M, Attwood D, D’Emanuele A. In Vitro Evaluation of Third Generation PAMAM Dendrimer Conjugates. Molecules. 2017; 22(10):1661. https://doi.org/10.3390/molecules22101661
Chicago/Turabian StyleNajlah, Mohammad, Sally Freeman, Mouhamad Khoder, David Attwood, and Antony D’Emanuele. 2017. "In Vitro Evaluation of Third Generation PAMAM Dendrimer Conjugates" Molecules 22, no. 10: 1661. https://doi.org/10.3390/molecules22101661






