Co-Immobilization of Enzymes and Magnetic Nanoparticles by Metal-Nucleotide Hydrogelnanofibers for Improving Stability and Recycling
Abstract
:1. Introduction
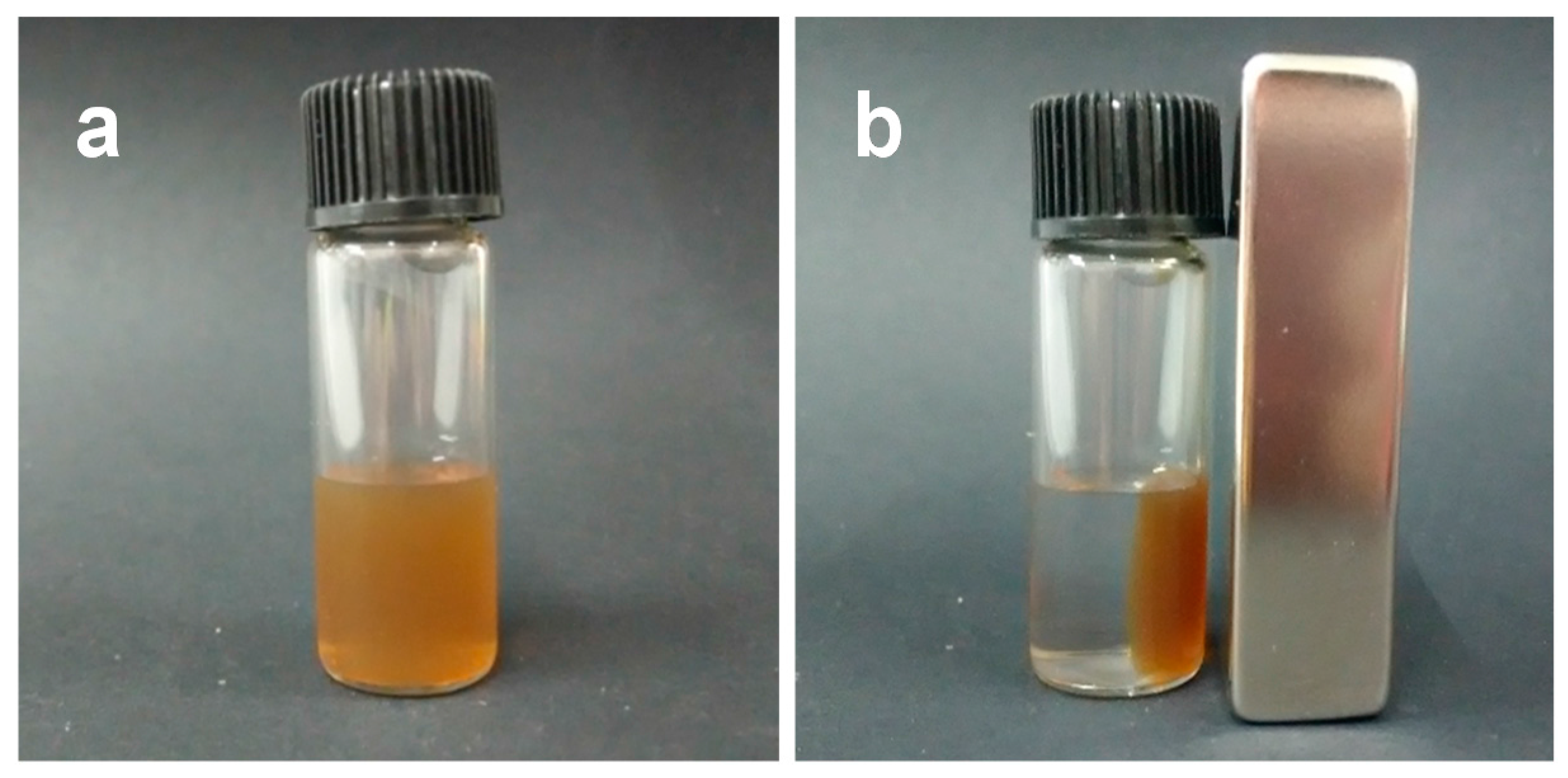
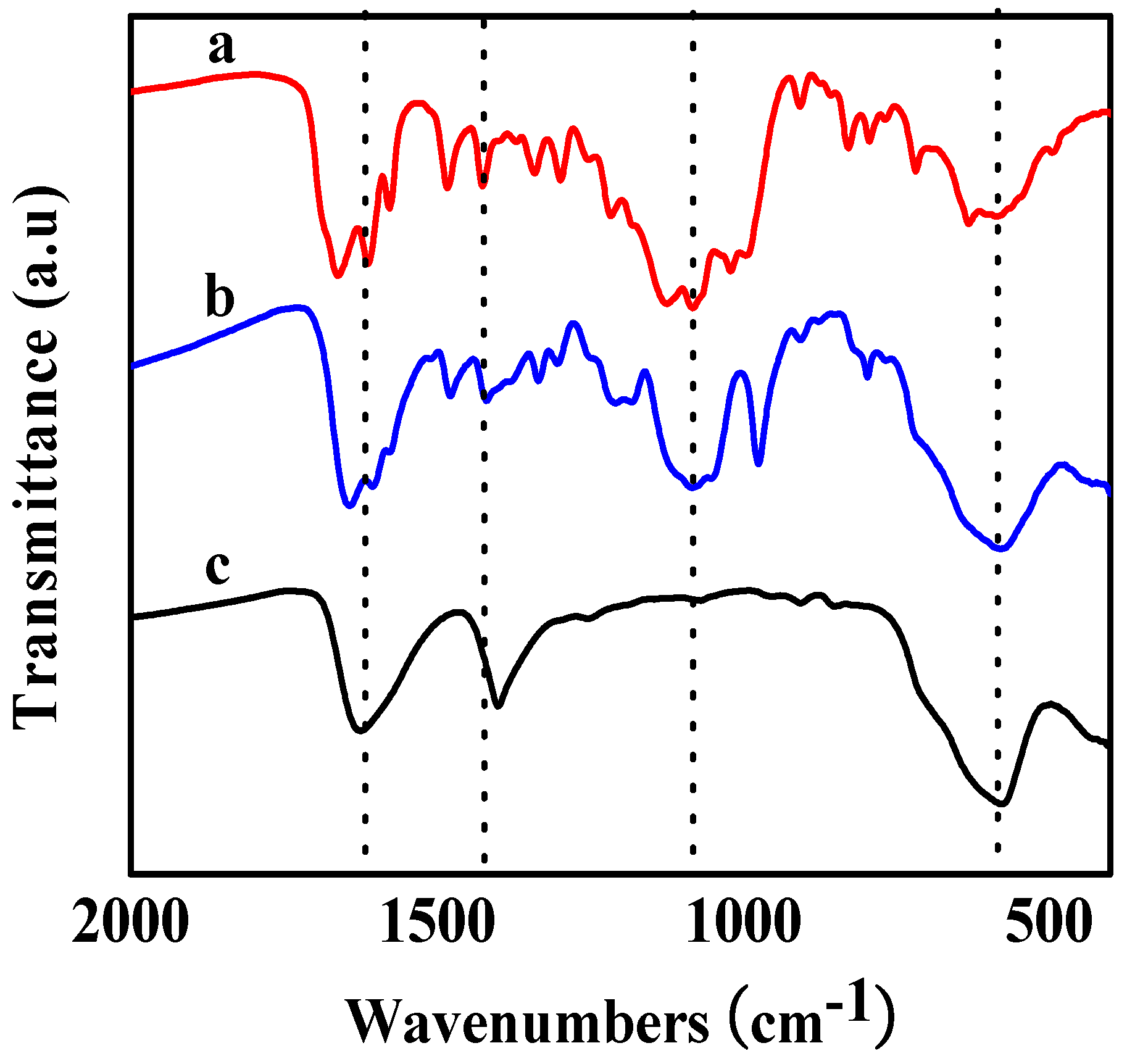
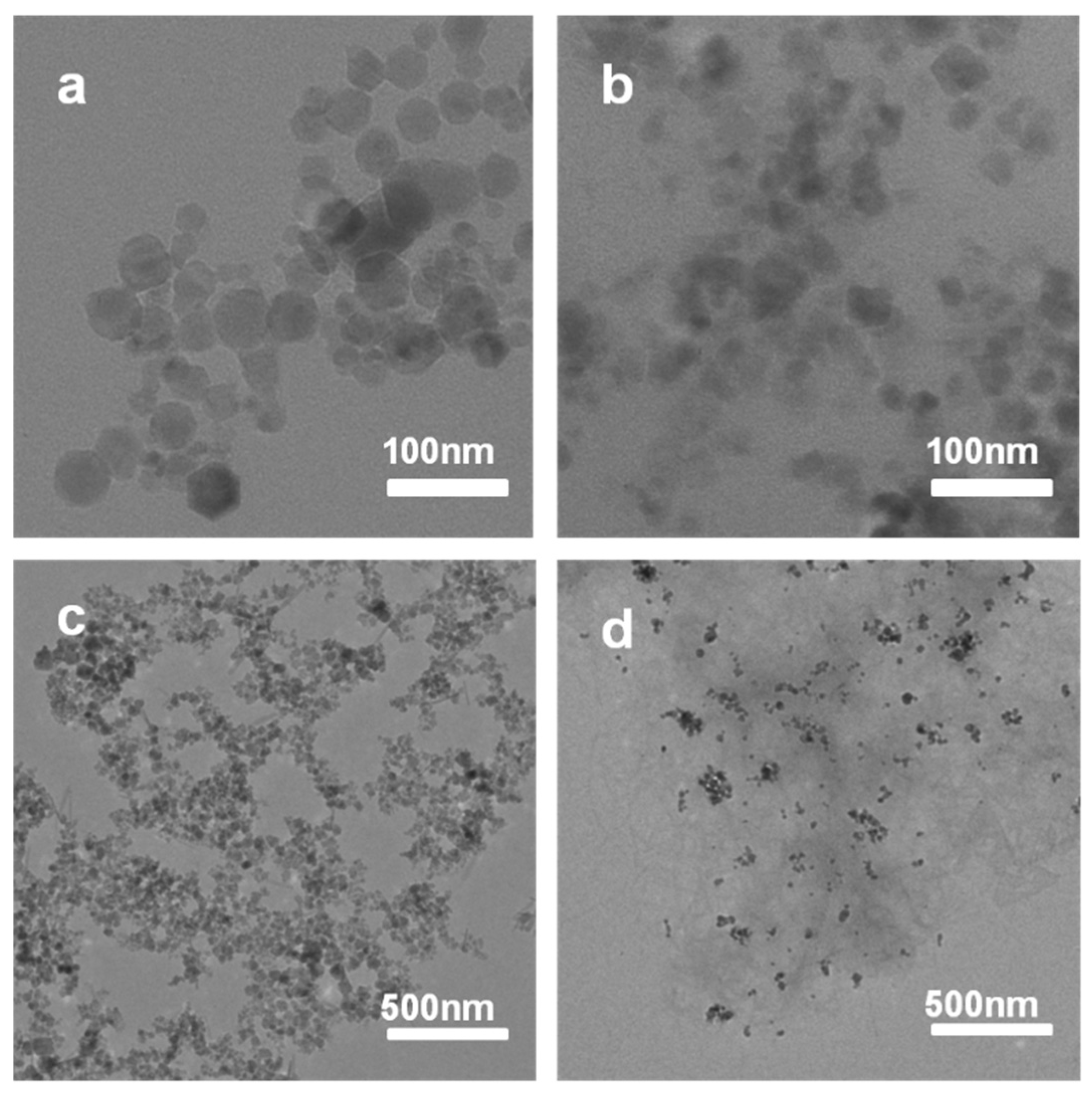
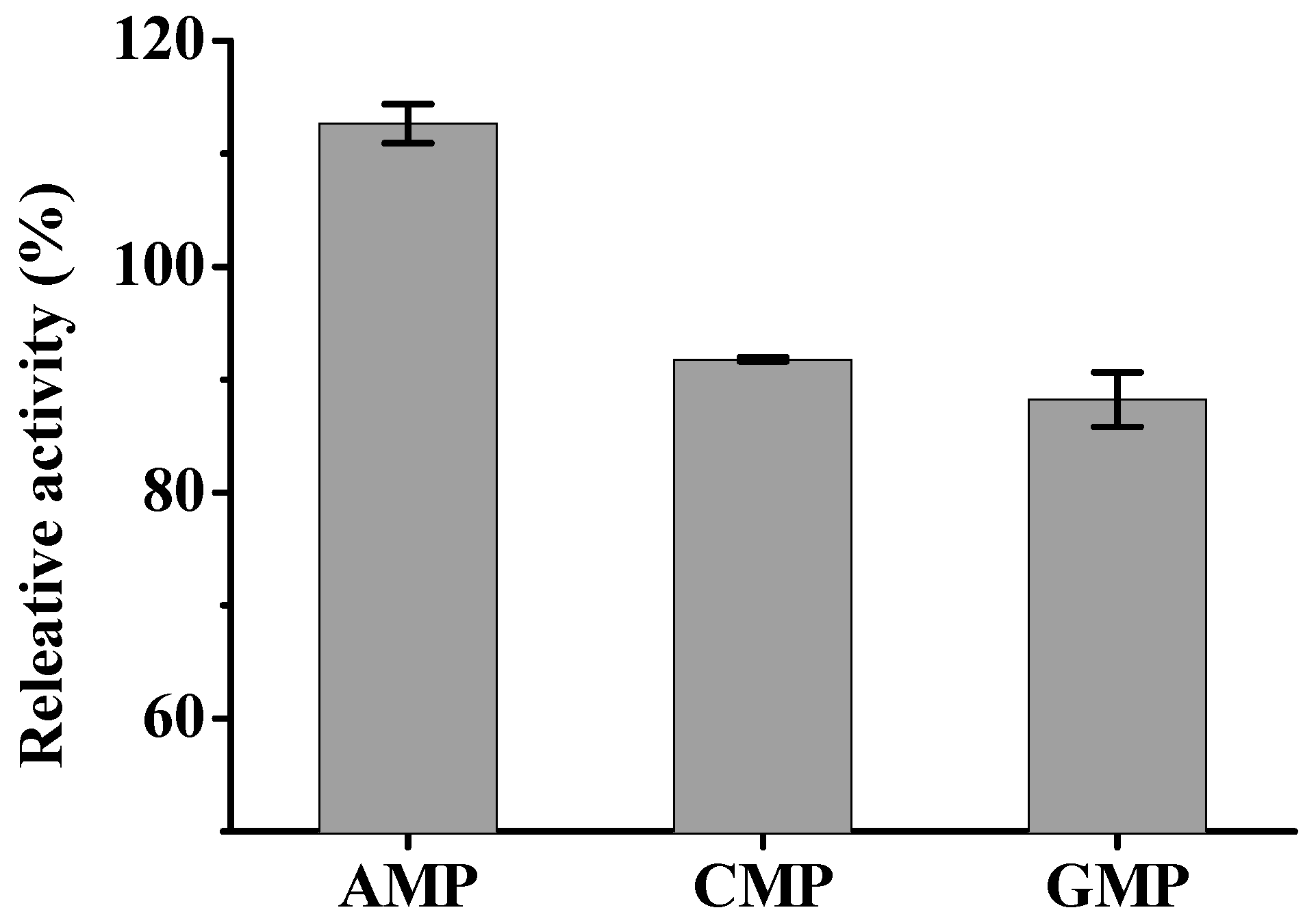
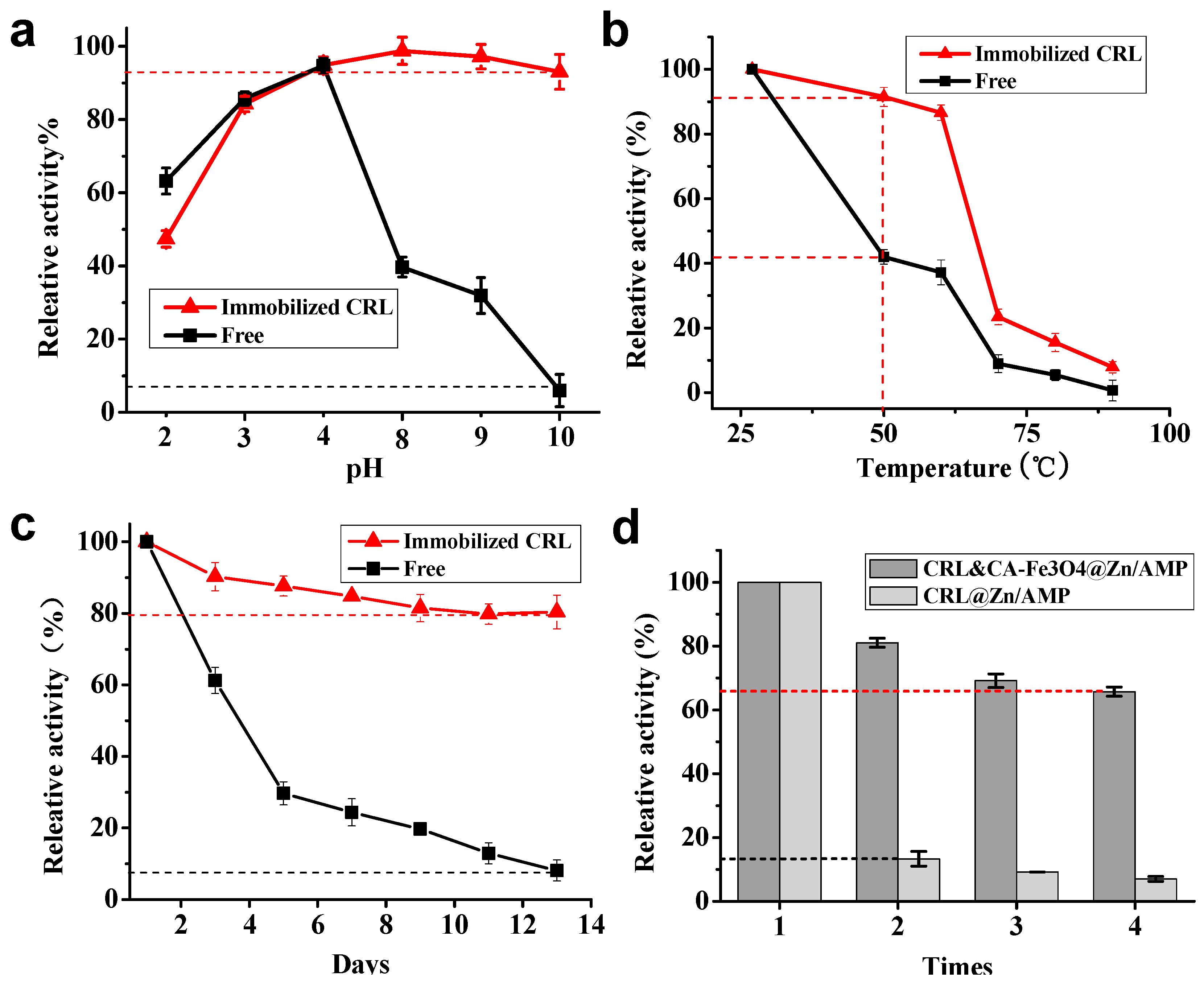
2. Results and Discussion
3. Materials and Methods
3.1. General Remarks
3.2. Materials
3.3. Preparation of Nanoparticles
3.3.1. Preparation of Fe3O4 Nanoparticles
3.3.2. Preparation of Citric Acid Encapsulated Fe3O4 Nanoparticles
3.3.3. Preparation of Magnetic CPs
3.4. Enzyme Immobilization
3.5. Protein Concentration Assay
3.6. Activity Assay of Enzymes
3.7. Enzyme Stability Test
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, D.-H.; Yuwen, L.-X.; Xie, Y.-L.; Li, W.; Li, X.-B. Improving immobilization of lipase onto magnetic microspheres with moderate hydrophobicity/hydrophilicity. Colloids Surf. B Biointerfaces 2012, 89, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Xu, G.; Zhou, Q.L.; Wu, J.P.; Yang, L.R. Improvements of lipase performance in high-viscosity system by immobilization onto a novel kind of poly(methylmethacrylate-co-divinylbenzene) encapsulated porous magnetic microsphere carrier. J. Mol. Catal. B Enzym. 2013, 89, 86–92. [Google Scholar] [CrossRef]
- Zhu, Y.T.; Ren, X.Y.; Liu, Y.M.; Wei, Y.; Qing, L.S.; Liao, X. Covalent immobilization of porcine pancreatic lipase on carboxyl-activated magnetic nanoparticles: Characterization and application for enzymatic inhibition assays. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 38, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Xu, D.; Gong, P.; Sun, H.; Dong, L.; Yao, S. Covalent binding of α-chymotrypsin on the magnetic nanogels covered by amino groups. J. Mol. Catal. B Enzym. 2007, 45, 84–90. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Yuan, Q.; Liang, H. Preparation of multi-enzyme co-immobilized nanoparticles by bis-aryl hydrazone bond conjugation. Biotechnol. Appl. Biochem. 2016, 63, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Bankar, S.B.; Bule, M.V.; Singhal, R.S.; Ananthanarayan, L. Glucose oxidase—An overview. Biotechnol. Adv. 2009, 27, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Constantin, M.-A.; Conrad, J.; Beifuss, U. Laccase-catalyzed oxidative phenolic coupling of vanillidene derivatives. Green Chem. 2012, 14, 2375–2379. [Google Scholar] [CrossRef]
- Lei, L.; Bai, Y.; Li, Y.; Yi, L.; Yang, Y.; Xia, C. Study on immobilization of lipase onto magnetic microspheres with epoxy groups. J. Magn. Magn. Mater. 2009, 321, 252–258. [Google Scholar] [CrossRef]
- Li, X.; Zhu, H.; Feng, J.; Zhang, J.; Deng, X.; Zhou, B.; Zhang, H.; Xue, D.; Li, F.; Mellors, N.J.; et al. One-pot polylol synthesis of graphene decorated with size- and density-tunable Fe3O4 nanoparticles for porcine pancreatic lipase immobilization. Carbon 2013, 60, 488–497. [Google Scholar] [CrossRef]
- Hartmann, M.; Jung, D. Biocatalysis with enzymes immobilized on mesoporous hosts: The status quo and future trends. J. Mater. Chem. 2010, 20, 844–857. [Google Scholar] [CrossRef]
- Garcia, J.; Zhang, Y.; Taylor, H.; Cespedes, O.; Webb, M.E.; Zhou, D. Multilayer enzyme-coupled magnetic nanoparticles as efficient, reusable biocatalysts and biosensors. Nanoscale 2011, 3, 3721–3730. [Google Scholar] [CrossRef] [PubMed]
- Mehta, J.; Bhardwaj, N.; Bhardwaj, S.K.; Kim, K.-H.; Deep, A. Recent advances in enzyme immobilization techniques: Metal-organic frameworks as novel substrates. Coord. Chem. Rev. 2016, 322, 30–40. [Google Scholar] [CrossRef]
- Banjanac, K.; Mihailović, M.; Prlainović, N.; Stojanović, M.; Carević, M.; Marinković, A.; Bezbradica, D. Cyanuric chloride functionalized silica nanoparticles for covalent immobilization of lipase. J. Chem. Technol. Biotechnol. 2016, 91, 439–448. [Google Scholar] [CrossRef]
- Li, Q.; Fan, F.; Wang, Y.; Feng, W.; Ji, P. Enzyme Immobilization on Carboxyl-Functionalized Graphene Oxide for Catalysis in Organic Solvent. Ind. Eng. Chem. Res. 2013, 52, 6343–6348. [Google Scholar] [CrossRef]
- Nyari, N.L.D.; Fernandes, I.A.; Bustamante-Vargas, C.E.; Steffens, C.; de Oliveira, D.; Zeni, J.; Rigo, E.; Dallago, R.M. In situ immobilization of Candida antarctica B lipase in polyurethane foam support. J. Mol. Catal. B Enzym. 2016, 124, 52–61. [Google Scholar] [CrossRef]
- Cai, C.; Gao, Y.; Liu, Y.; Zhong, N.; Liu, N. Immobilization of Candida antarctica lipase B onto SBA-15 and their application in glycerolysis for diacylglycerols synthesis. Food Chem. 2016, 212, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, H.; Wang, L.; Wang, S. Stability and catalytic properties of lipase immobilized on chitosan encapsulated magnetic nanoparticles cross-linked with genipin and glutaraldehyde. J. Chem. Technol. Biotechnol. 2016, 91, 1359–1367. [Google Scholar] [CrossRef]
- Sun, L.; Liang, H.; Yuan, Q.; Wang, T.; Zhang, H. Study on a carboxyl-activated carrier and its properties for papain immobilization. J. Chem. Technol. Biotechnol. 2012, 87, 1083–1088. [Google Scholar] [CrossRef]
- Esmaeilnejad-Ahranjani, P.; Kazemeini, M.; Singh, G.; Arpanaei, A. Amine-functionalized magnetic nanocomposite particles for efficient immobilization of lipase: Effects of functional molecule size on properties of the immobilized lipase. RSC Adv. 2015, 5, 33313–33327. [Google Scholar] [CrossRef]
- Liu, J.; Wang, W.; Liu, H.; Zhou, Y.; Zhang, H.; Zhou, X. Penicillium expansum lipase-coated magnetic Fe3O4–polymer hybrid hollow nanoparticles: A highly recoverable and magnetically separable catalyst for the synthesis of 1,3-dibutylurea. RSC Adv. 2014, 4, 25983–25992. [Google Scholar] [CrossRef]
- Palocci, C.; Venditti, C.I. Lipolytic Enzymes with Improved Activity and Selectivity upon Adsorption on Polymeric Nanoparticles. Biomacromolecules 2007, 8, 3047–3053. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Guo, Y.; Lin, F.; Zhang, Y.; Yuan, Q.; Liang, H. Rational surface silane modification for immobilizing glucose oxidase. Int. J. Biol. Macromol. 2016, 87, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Hao, R.; Xing, R.; Xu, Z.; Hou, Y.; Gao, S.; Sun, S. Synthesis, functionalization, and biomedical applications of multifunctional magnetic nanoparticles. Adv. Mater. 2010, 22, 2729–2742. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liang, Q.; Li, J.; Zhuang, Y.; He, Y.; Bai, B.; Wang, X. Ni3Si2O5(OH)4 multi-walled nanotubes with tunable magnetic properties and their application as anode materials for lithium batteries. Nano Res. 2011, 4, 882–890. [Google Scholar] [CrossRef]
- Amirkhani, L.; Moghaddas, J.; Jafarizadehmalmiri, H. Candida rugosa lipase immobilization on magnetic silica aerogel nanodispersion. RSC Adv. 2016, 6, 12676–12687. [Google Scholar] [CrossRef]
- Yang, D.; Wang, X.; Shi, J.; Wang, X.; Zhang, S.; Han, P.; Jiang, Z. In situ synthesized rGO–Fe3O4 nanocomposites as enzyme immobilization support for achieving high activity recovery and easy recycling. Biochem. Eng. J. 2016, 105, 273–280. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, X.; Tang, B. Preparation of a magnetically recoverable biocatalyst support on monodisperse Fe3O4 nanoparticles. RSC Adv. 2013, 3, 9924–9931. [Google Scholar] [CrossRef]
- Zhu, W.; Li, Y.; Zeng, F.; Yin, H.; Wang, L.; Zhu, H. Superparamagnetic Fe3O4 nanoparticles modified by water-soluble and biocompatible polyethylenimine for lipase immobilization with physical and chemical mechanisms. RSC Adv. 2015, 5, 23039–23045. [Google Scholar] [CrossRef]
- Wang, J.; Ji, F.; Xing, J.; Cui, S.; Bao, Y.; Hao, W. Lipase Immobilization onto the Surface of PGMA-b-PDMAEMA-grafted Magnetic Nanoparticles Prepared via Atom Transfer Radical Polymerization. Chin. J. Chem. Eng. 2014, 22, 1333–1339. [Google Scholar] [CrossRef]
- Hernandez, K.; Fernandez-Lafuente, R. Control of protein immobilization: Coupling immobilization and site-directed mutagenesis to improve biocatalyst or biosensor performance. Enzyme Microb. Technol. 2011, 48, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Vaghari, H.; Jafarizadeh-Malmiri, H.; Mohammadlou, M.; Berenjian, A.; Anarjan, N.; Jafari, N.; Nasiri, S. Application of magnetic nanoparticles in smart enzyme immobilization. Biotechnol. Lett. 2016, 38, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Cantone, S.; Ferrario, V.; Corici, L.; Ebert, C.; Fattor, D.; Spizzo, P.; Gardossi, L. Efficient immobilisation of industrial biocatalysts: Criteria and constraints for the selection of organic polymeric carriers and immobilisation methods. Chem. Soc. Rev. 2013, 42, 6262–6276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Fernandez-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- An, J.; Geib, S.J.; Rosi, N.L. Cation-triggered drug release from a porous zinc-adeninate metal-organic framework. J. Am. Chem. Soc. 2009, 131, 8376–8377. [Google Scholar] [CrossRef] [PubMed]
- Nishiyabu, R.; Hashimoto, N.; Cho, T.; Watanabe, K.; Yasunaga, T.; Endo, A.; Kaneko, K.; Niidome, T.; Murata, M.; Adachi, C. Nanoparticles of Adaptive Supramolecular Networks Self-Assembled from Nucleotides and Lanthanide Ions. J. Am. Chem. Soc. 2009, 131, 2151–2158. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, B.; Huang, P.J.; Liu, J. Rationally designed nucleobase and nucleotide coordinated nanoparticles for selective DNA adsorption and detection. Anal. Chem. 2013, 85, 12144–12151. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Li, B.; Du, Y.; Dong, S.; Wang, E. Nucleobase−Metal Hybrid Materials: Preparation of Submicrometer-Scale, Spherical Colloidal Particles of Adenine−Gold(III) via a Supramolecular Hierarchical Self-Assembly Approach. Chem. Mater. 2007, 21, 2987–2993. [Google Scholar] [CrossRef]
- Purohit, C.S.; Verma, S. A luminescent silver-adenine metallamacrocyclic quartet. J. Am. Chem. Soc. 2006, 128, 400–401. [Google Scholar] [CrossRef] [PubMed]
- Sukul, P.K.; Malik, S. Supramolecular hydrogels of adenine: Morphological, structural and rheological investigations. Soft Matter 2011, 7, 4234–4241. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, Z.; Yuan, Q.; Liu, J. Self-healing metal-coordinated hydrogels using nucleotide ligands. Chem. Commun. 2015, 51, 15196–15199. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Jiang, S.; Yuan, Q.; Li, G.; Wang, F.; Zhang, Z.; Liu, J. Co-immobilization of multiple enzymes by metal coordinated nucleotide hydrogel nanofibers: Improved stability and an enzyme cascade for glucose detection. Nanoscale 2016, 8, 6071–6078. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kanwar, S.S. Synthesis of ethyl ferulate in organic medium using celite-immobilized lipase. Bioresour. Technol. 2011, 102, 2162–2167. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Wang, Y.; Ding, Q.; Jiang, L.; Li, M.; Zhu, W.; Pan, D.; Zhu, H.; Liu, M. Facile synthesis of enzyme-embedded magnetic metal-organic frameworks as a reusable mimic multi-enzyme system: Mimetic peroxidase properties and colorimetric sensor. Nanoscale 2015, 7, 18770–18779. [Google Scholar] [CrossRef] [PubMed]
- Mahto, T.K.; Chowdhuri, A.R.; Sahoo, B.; Sahu, S.K. Polyaniline-functionalized magnetic mesoporous nanocomposite: A smart material for the immobilization of lipase. Polym. Compos. 2016, 37, 1152–1160. [Google Scholar] [CrossRef]
- Liang, H.; Liu, B.; Yuan, Q.; Liu, J. Magnetic Iron Oxide Nanoparticle Seeded Growth of Nucleotide Coordinated Polymers. ACS Appl. Mater. Interfaces 2016, 8, 15615–15622. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xu, W.; Jin, L.; Zha, J.; Tao, T.; Lin, Y.; Wang, Z. Synthesis of amine-functionalized Fe3O4@C nanoparticles for lipase immobilization. J. Mater. Chem. A 2014, 2, 18339–18344. [Google Scholar] [CrossRef]
- Xie, W.; Ning, M. Immobilized Lipase on Fe3O4 Nanoparticles as Biocatalyst for Biodiesel Production. Energy Fuels 2009, 23, 1347–1353. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, M.; Zhao, C.; Lin, Y.; Yang, R.; Wang, Z. Shape-controlled synthesis of Fe3O4/CeO2 hybrid octahedra for lipase immobilization. Crystengcomm 2015, 17, 2536–2543. [Google Scholar] [CrossRef]
- Zhang, Q.; Kang, J.; Yang, B.; Zhao, L.; Hou, Z.; Tang, B. Immobilized cellulase on Fe3O4 nanoparticles as a magnetically recoverable biocatalyst for the decomposition of corncob. Chin. J. Catal. 2016, 37, 389–397. [Google Scholar] [CrossRef]
- Li, S.-F.; Chen, J.-P.; Wu, W.-T. Electrospun polyacrylonitrile nanofibrous membranes for lipase immobilization. J. Mol. Catal. B Enzym. 2007, 47, 117–124. [Google Scholar] [CrossRef]
- Li, Y.; Liang, H.; Sun, L.; Wu, J.; Yuan, Q. Nanoparticle-tethered NAD(+) with in situ cofactor regeneration. Biotechnol. Lett. 2013, 35, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Wang, Y.; Zhu, H.; Zhou, L. Formulation of robust organic-inorganic hybrid magnetic microcapsules through hard-template mediated method for efficient enzyme immobilization. J. Mater. Chem. B 2015, 3, 2883–2891. [Google Scholar] [CrossRef]
- Zevin, S.; Schaner, M.E.; Illsley, N.P.; Giacomini, K.M. Guanidine Transport in a Human Choriocarcinoma Cell Line (JAR). Pharm. Res. 1997, 14, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Velascolozano, S.; Lópezgallego, F.; Vázquezduhalt, R.; Mateosdíaz, J.C.; Guisán, J.M.; Favelatorres, E. Carrier-free immobilization of lipase from Candida rugosa with polyethyleneimines by carboxyl-activated cross-linking. Biomacromolecules 2014, 15, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds Zn/AMP, CA-Fe3O4@Zn/AMP, CRL&CA-Fe3O4@Zn/AMP are available from the authors.

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Jiang, S.; Zhao, X.; Liang, H. Co-Immobilization of Enzymes and Magnetic Nanoparticles by Metal-Nucleotide Hydrogelnanofibers for Improving Stability and Recycling. Molecules 2017, 22, 179. https://doi.org/10.3390/molecules22010179
Li C, Jiang S, Zhao X, Liang H. Co-Immobilization of Enzymes and Magnetic Nanoparticles by Metal-Nucleotide Hydrogelnanofibers for Improving Stability and Recycling. Molecules. 2017; 22(1):179. https://doi.org/10.3390/molecules22010179
Chicago/Turabian StyleLi, Chunfang, Shuhui Jiang, Xinying Zhao, and Hao Liang. 2017. "Co-Immobilization of Enzymes and Magnetic Nanoparticles by Metal-Nucleotide Hydrogelnanofibers for Improving Stability and Recycling" Molecules 22, no. 1: 179. https://doi.org/10.3390/molecules22010179




