Stereoselective Synthesis of α-Amino-C-phosphinic Acids and Derivatives
Abstract
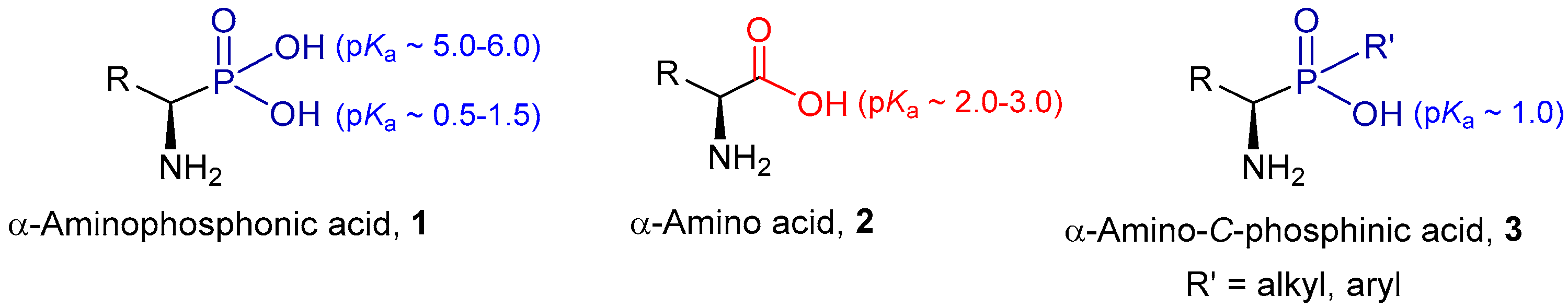
:1. Introduction
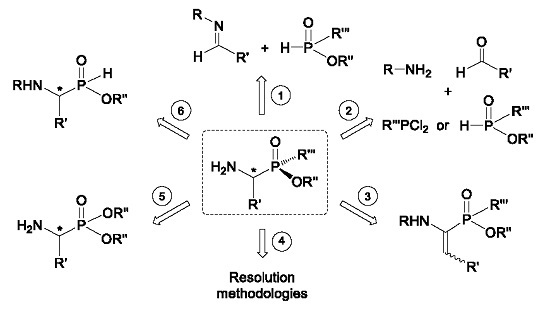
2. Stereoselective Synthesis of Acyclic α-Amino-C-phosphinic Acids and Derivatives
2.1. Stereoselective C-P Bond Formation (Addition of Phosphorus Compounds to Imines)
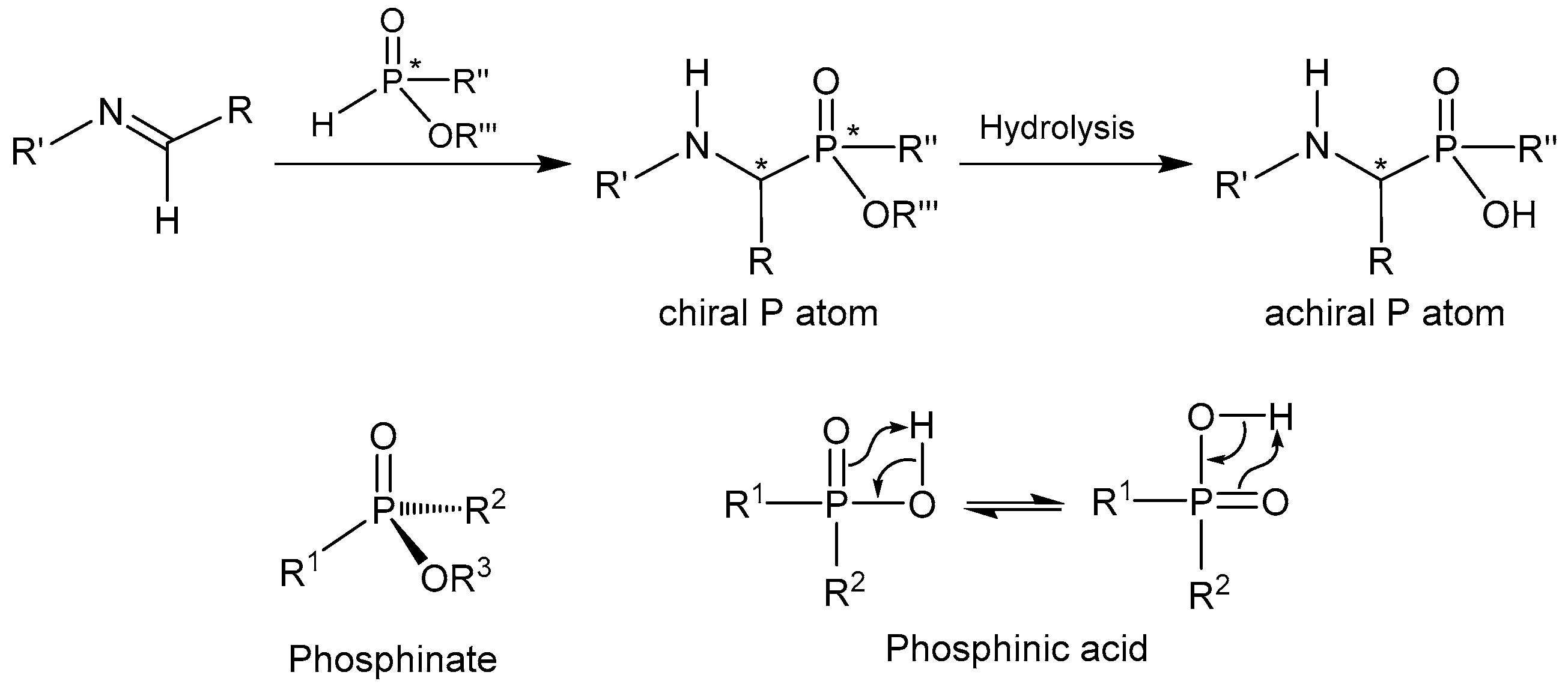
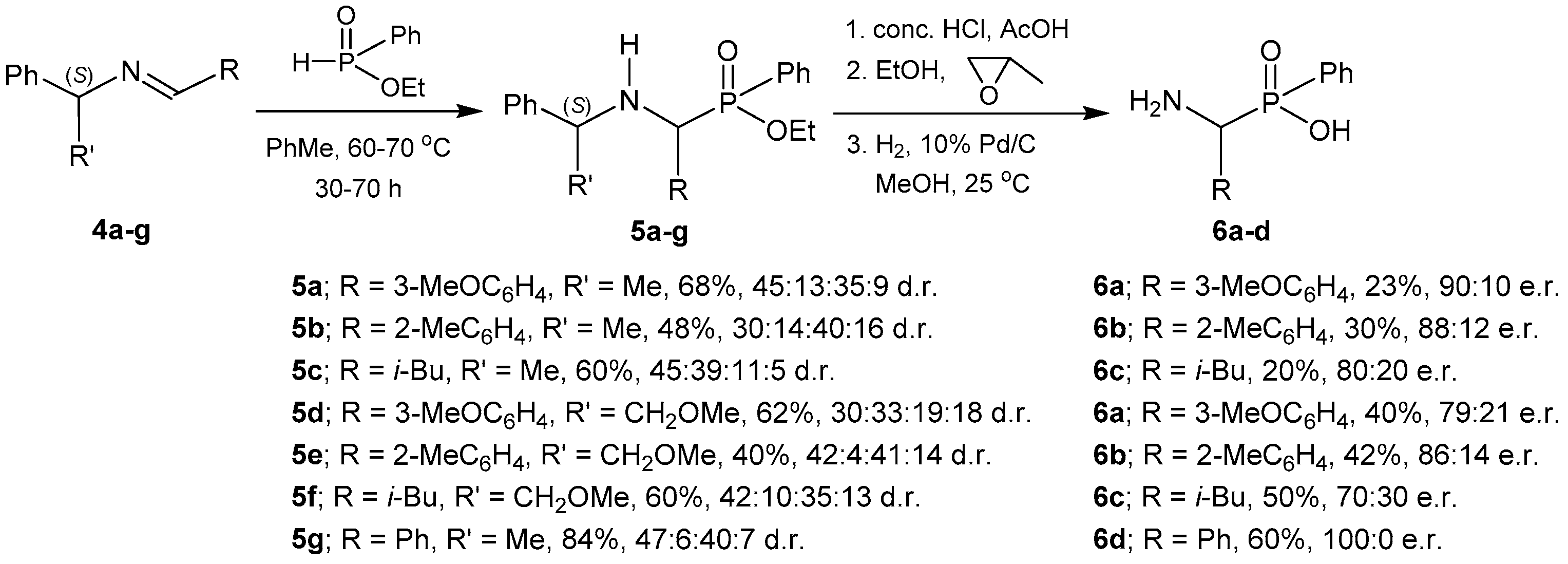
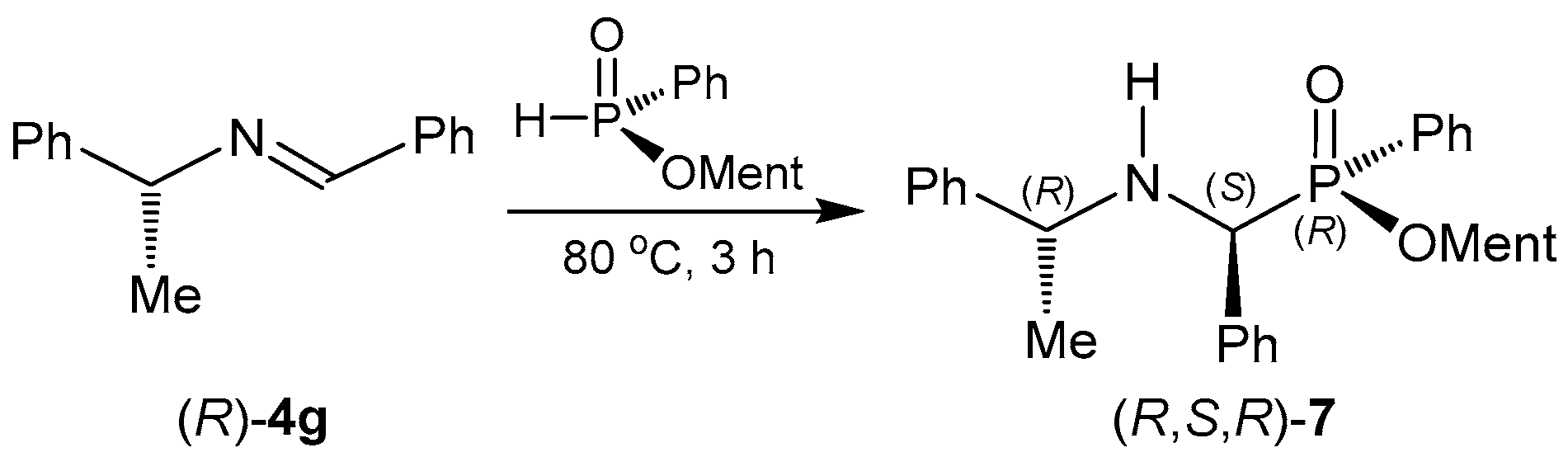
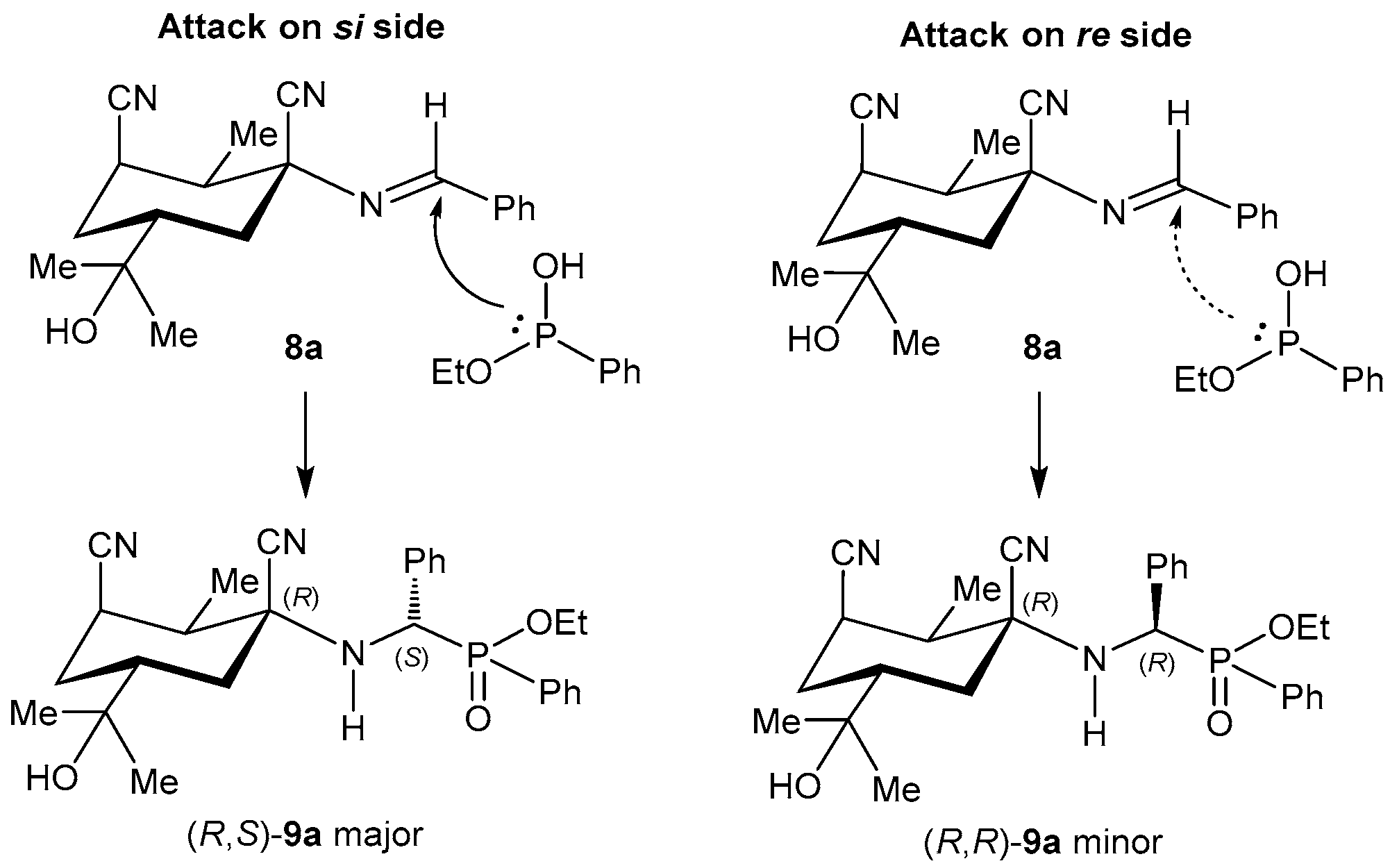
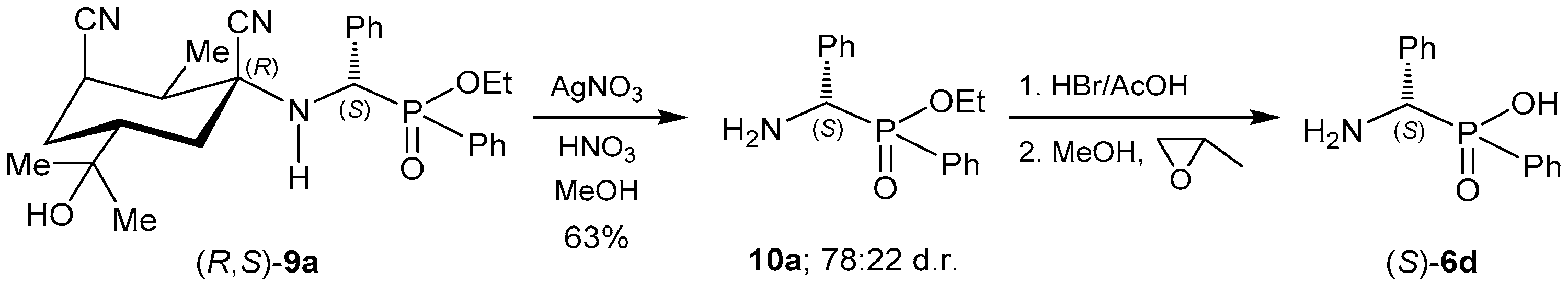
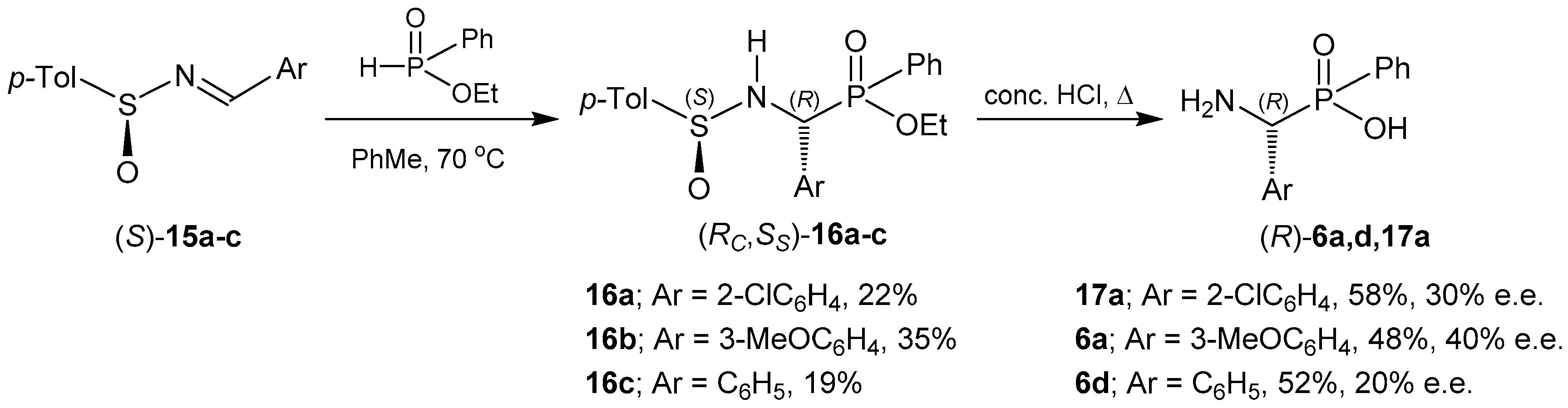
2.1.1. Chiral Imine Compounds
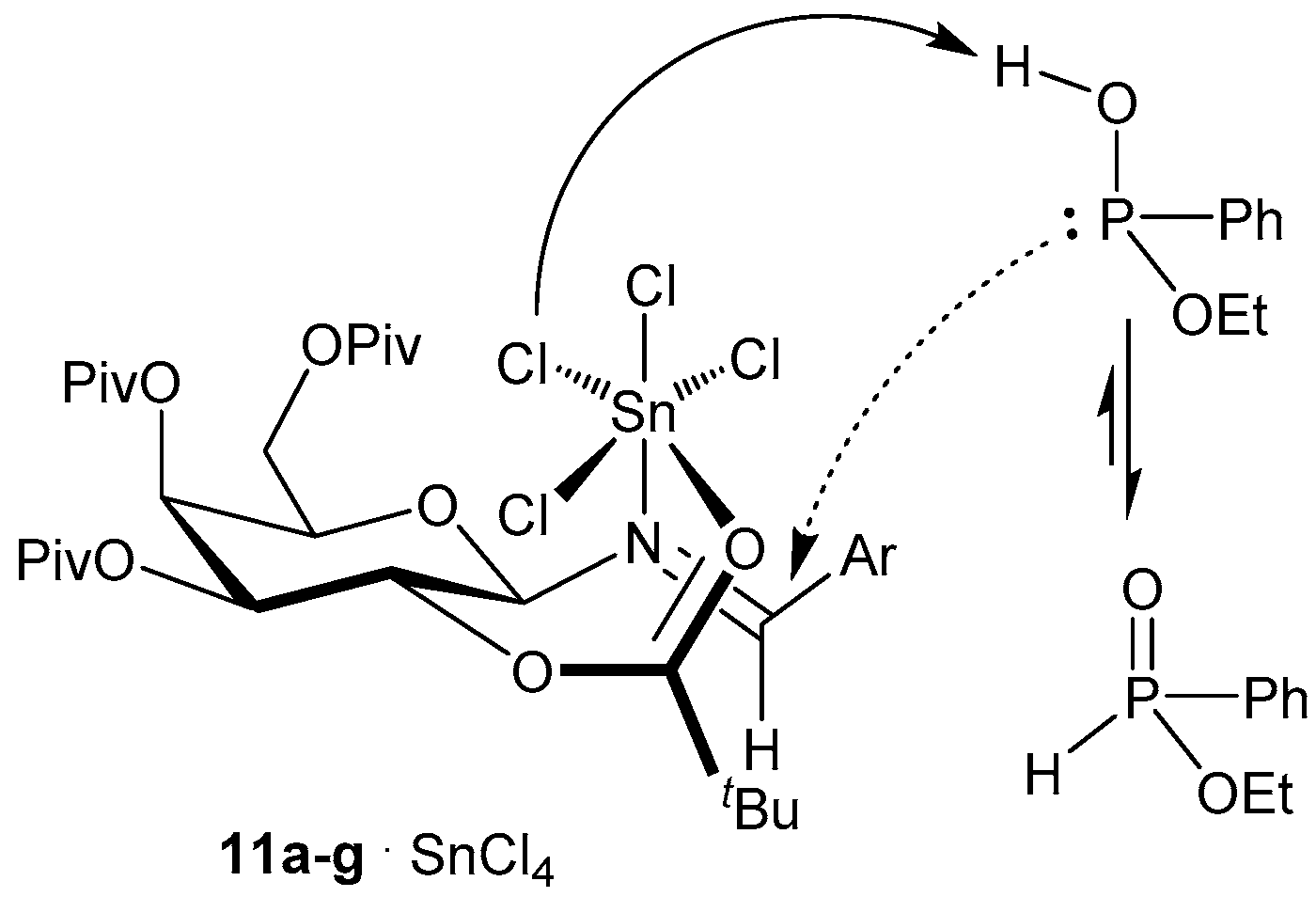
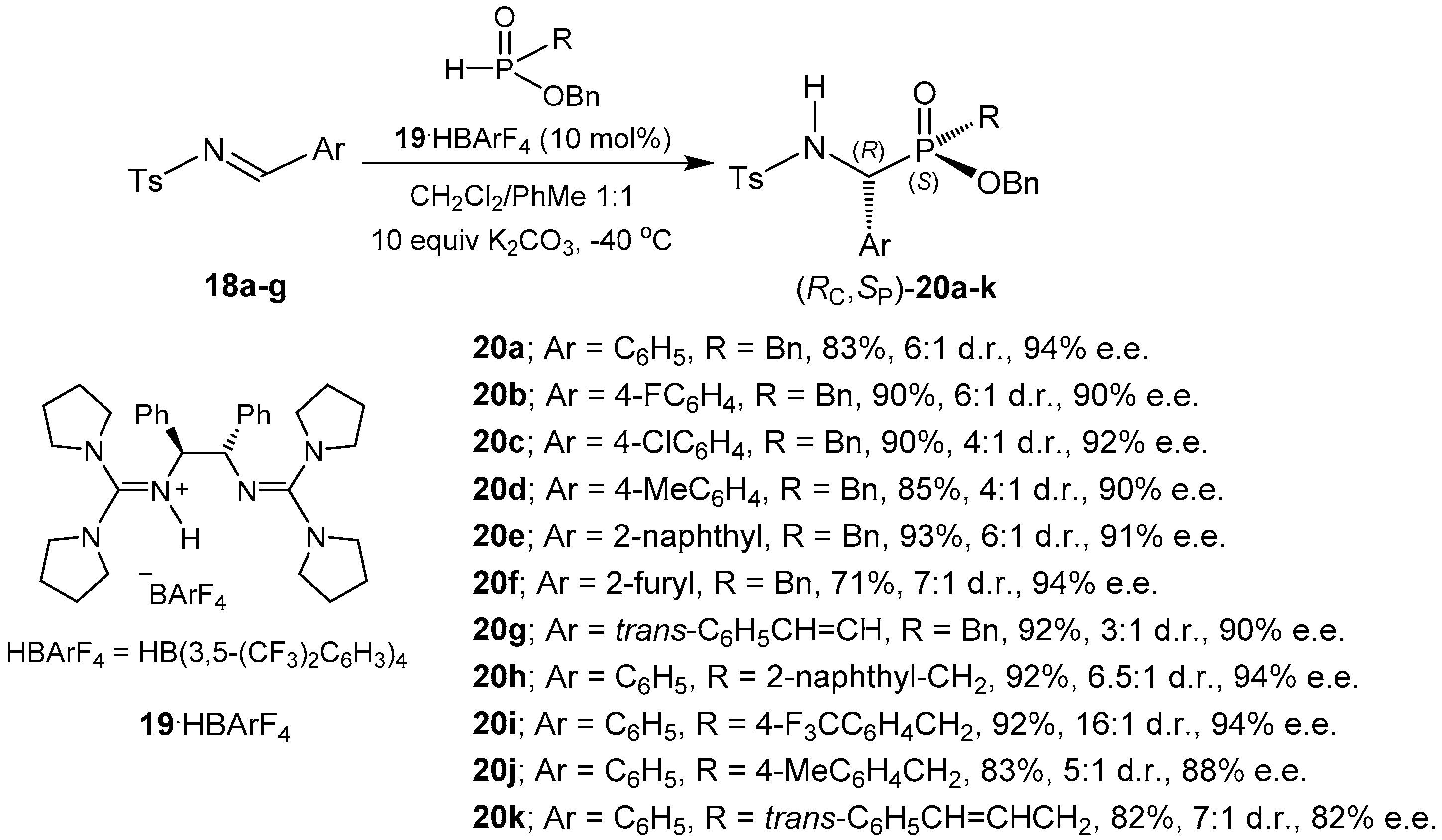
2.1.2. Chiral Catalyst
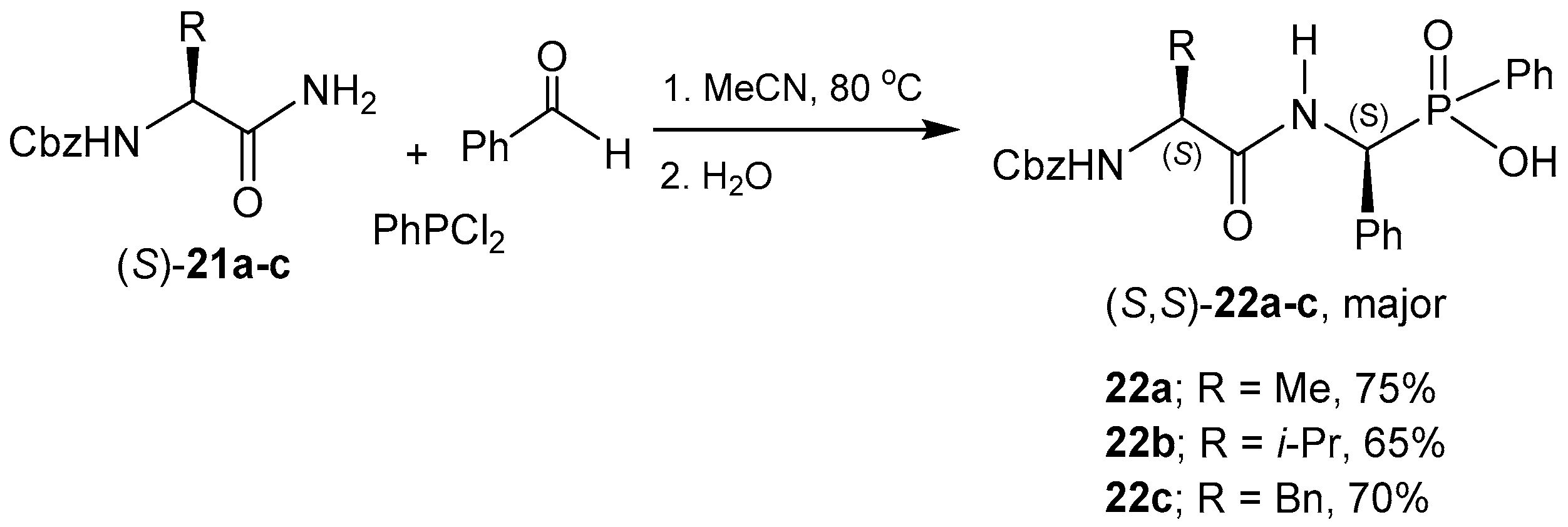
2.2. Stereoselective C-P Bond Formation (One-Pot Three-Component Reaction)
2.2.1. Chiral Amino Compounds
2.3. Stereoselective C-H Bond Formation
2.3.1. Reduction of C=C Bond in α,β-Dehydroaminophosphinates
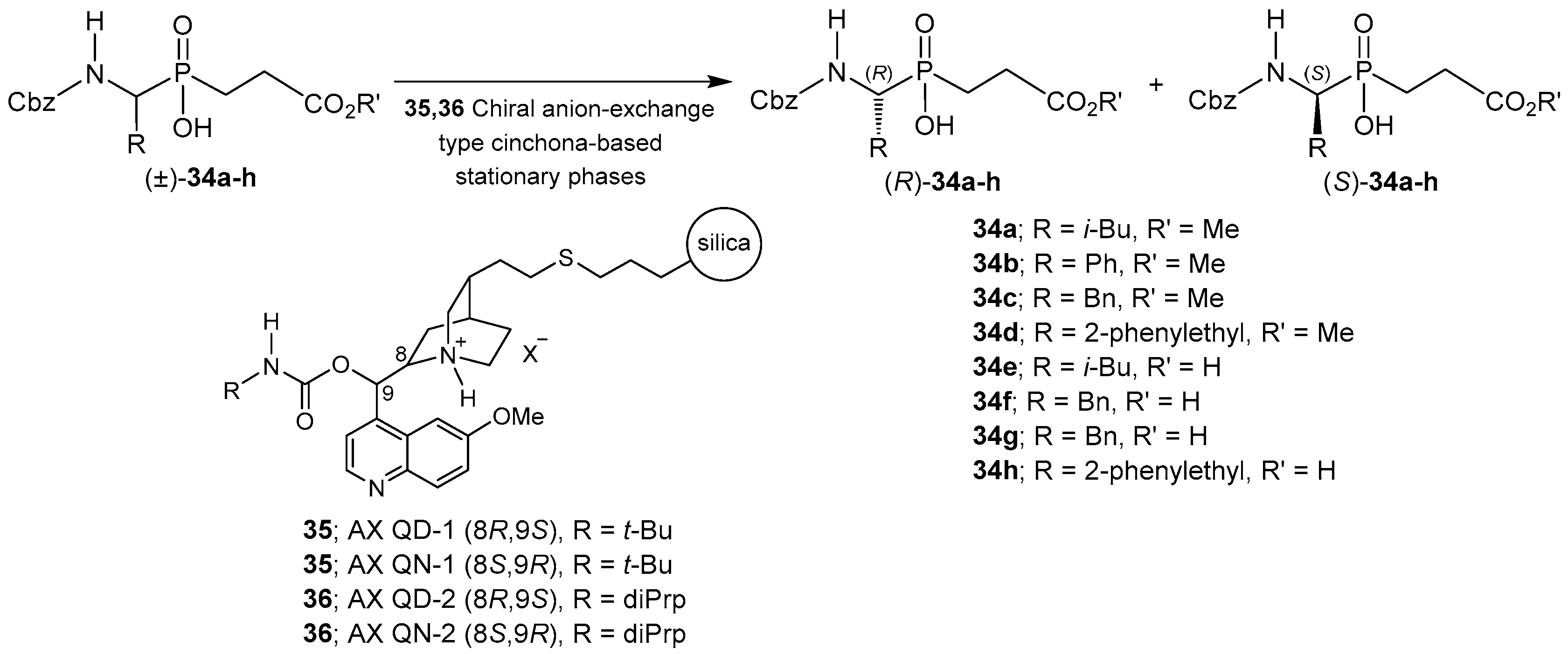
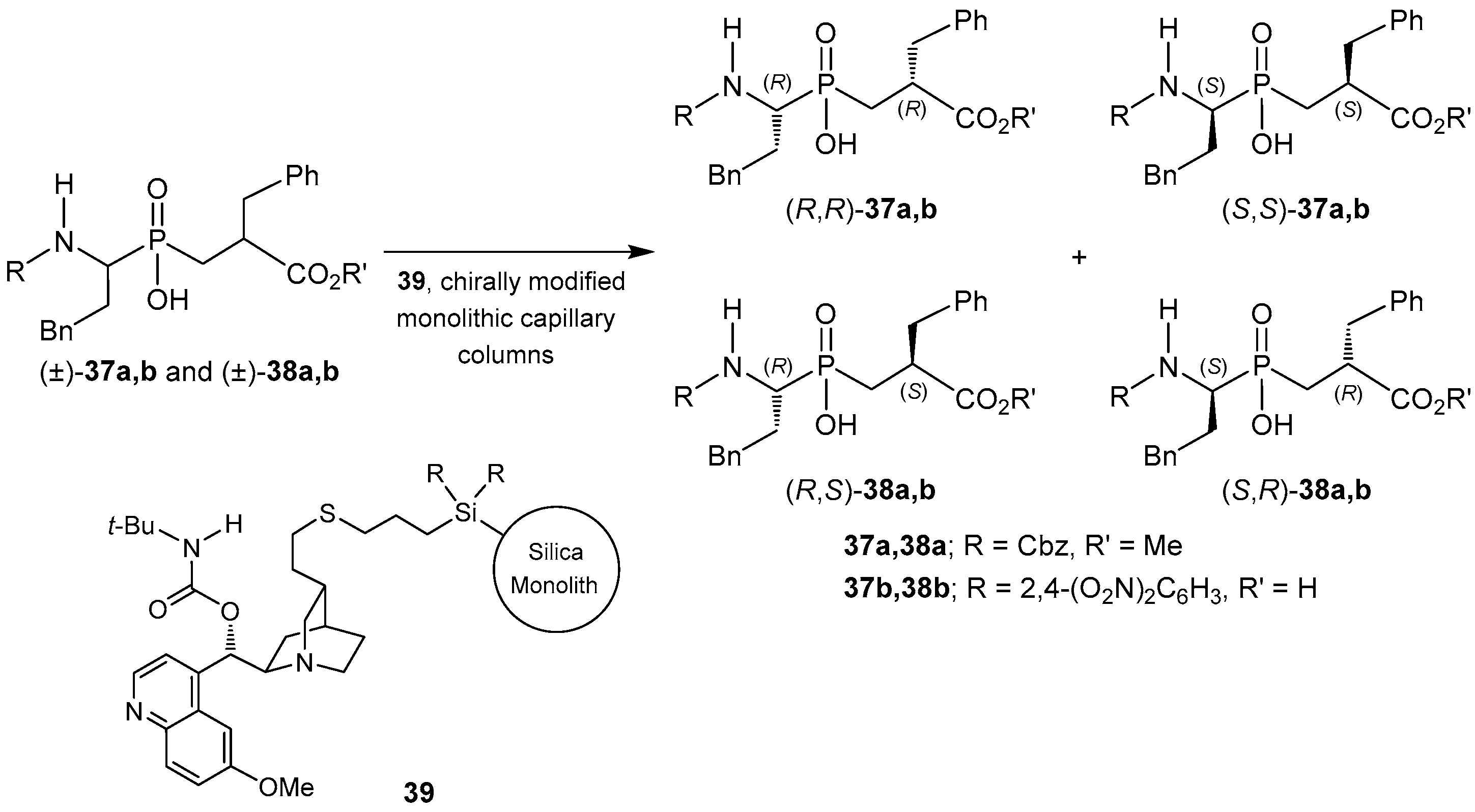
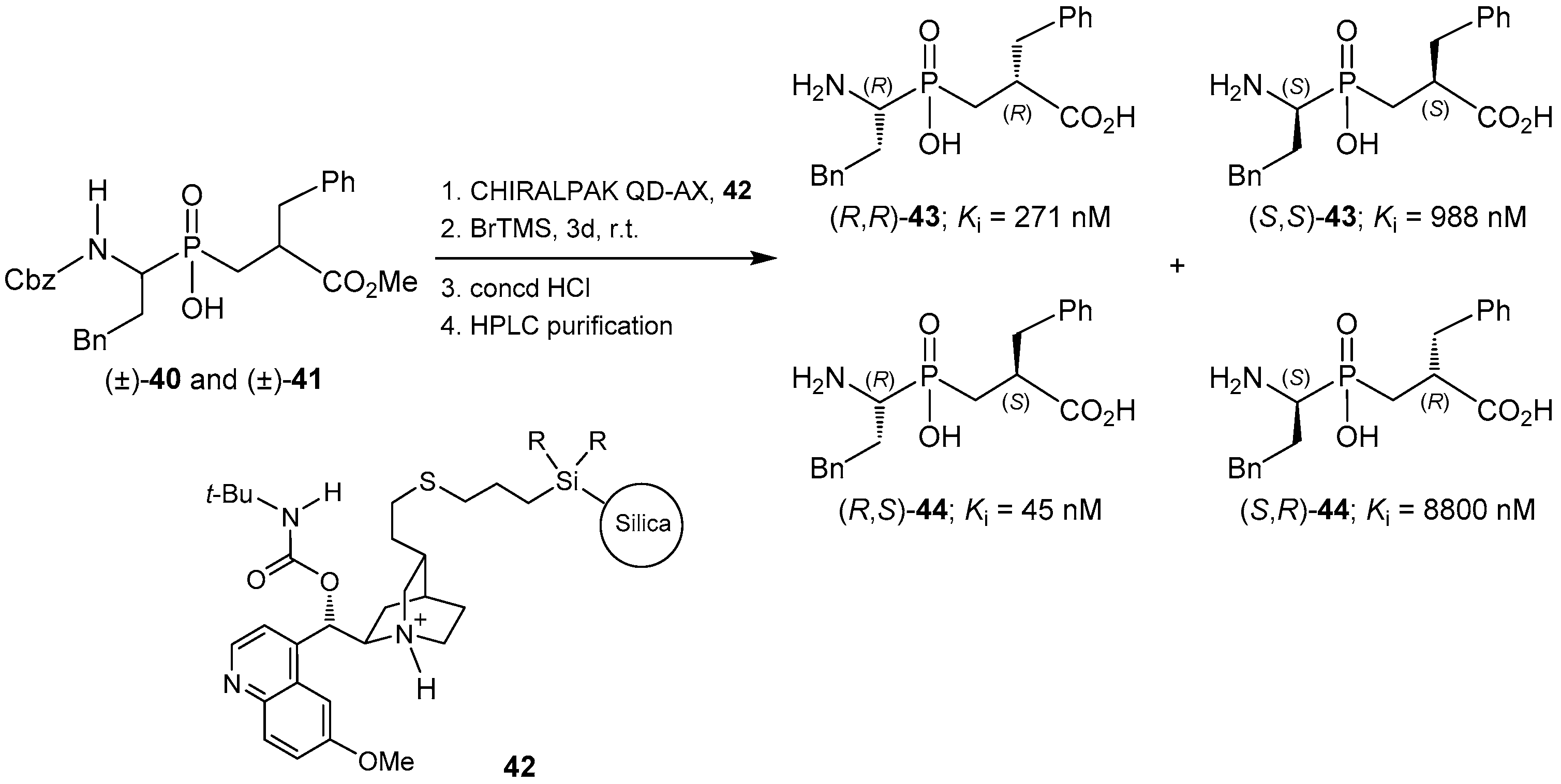
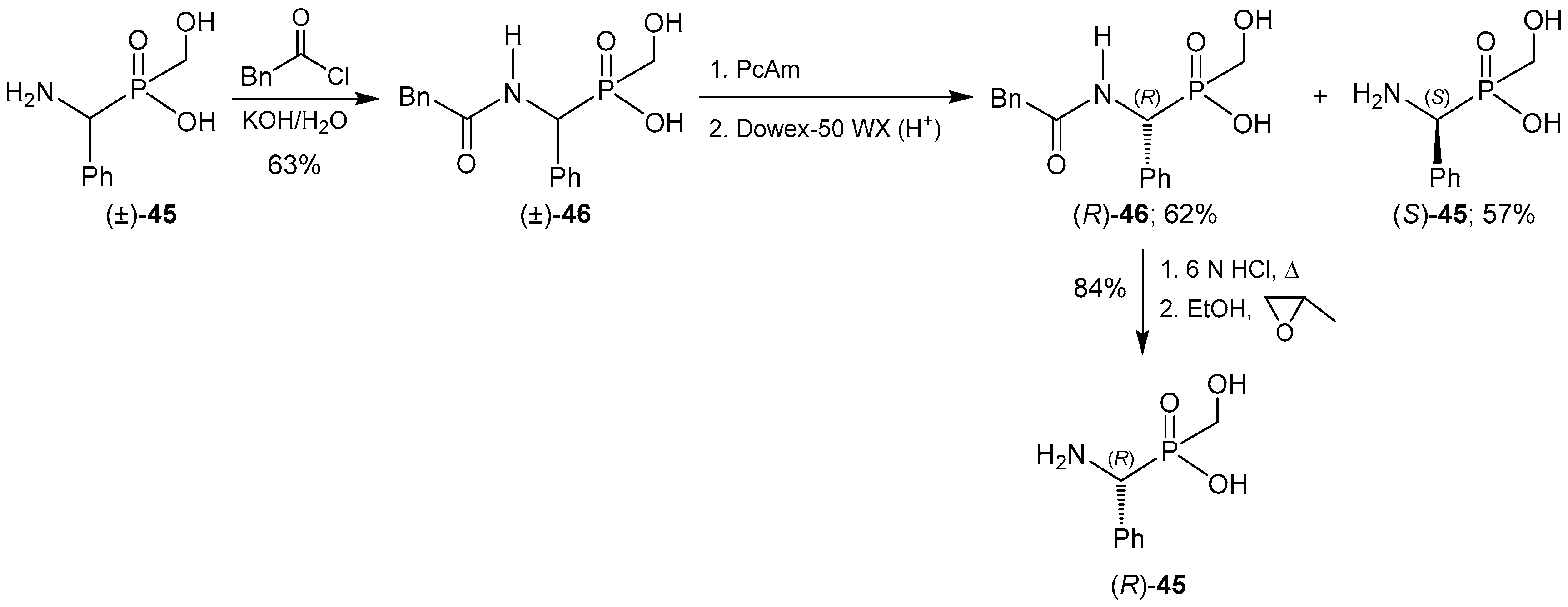
2.4. Resolution Methodologies
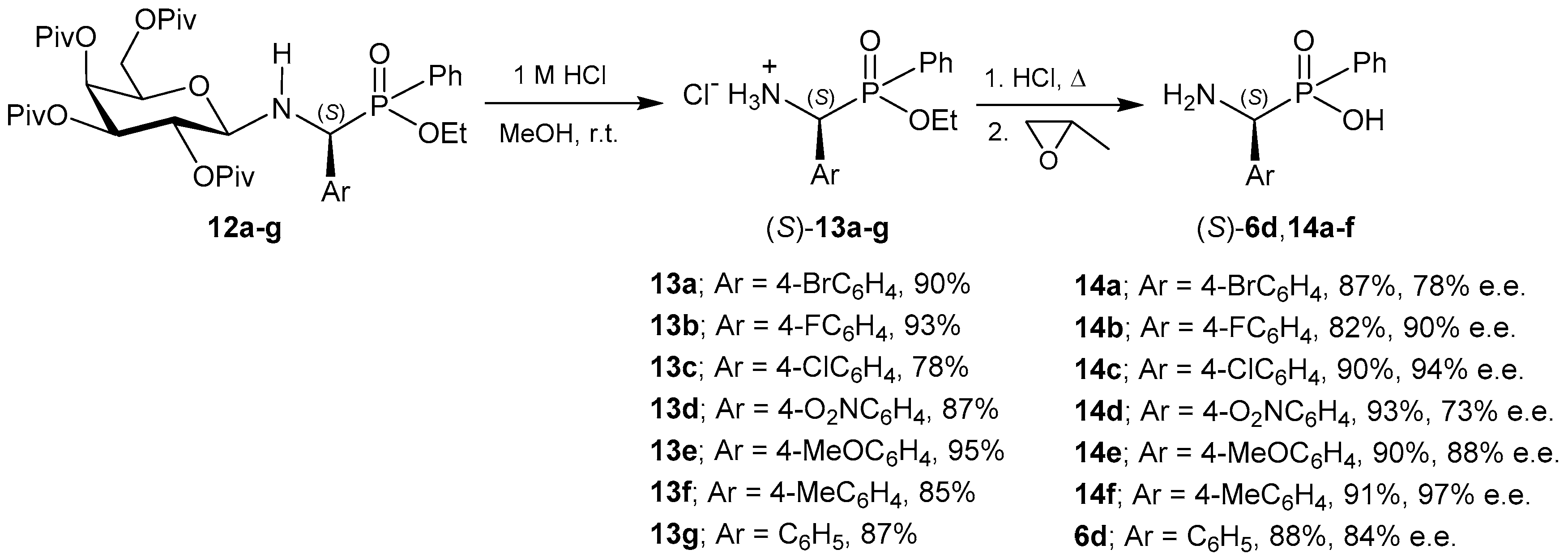
2.5. Conversion from Chiral α-Aminophosphonates
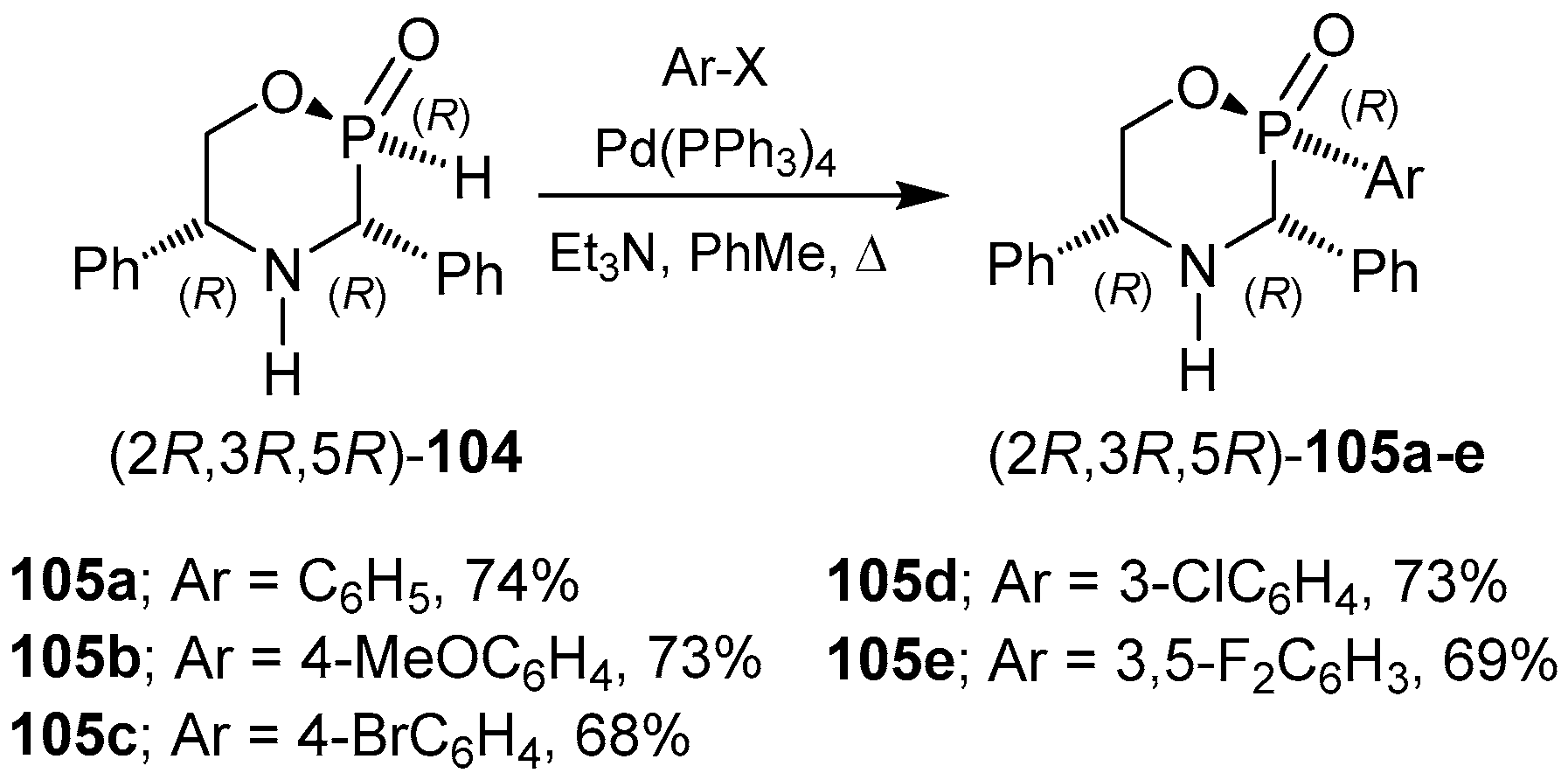
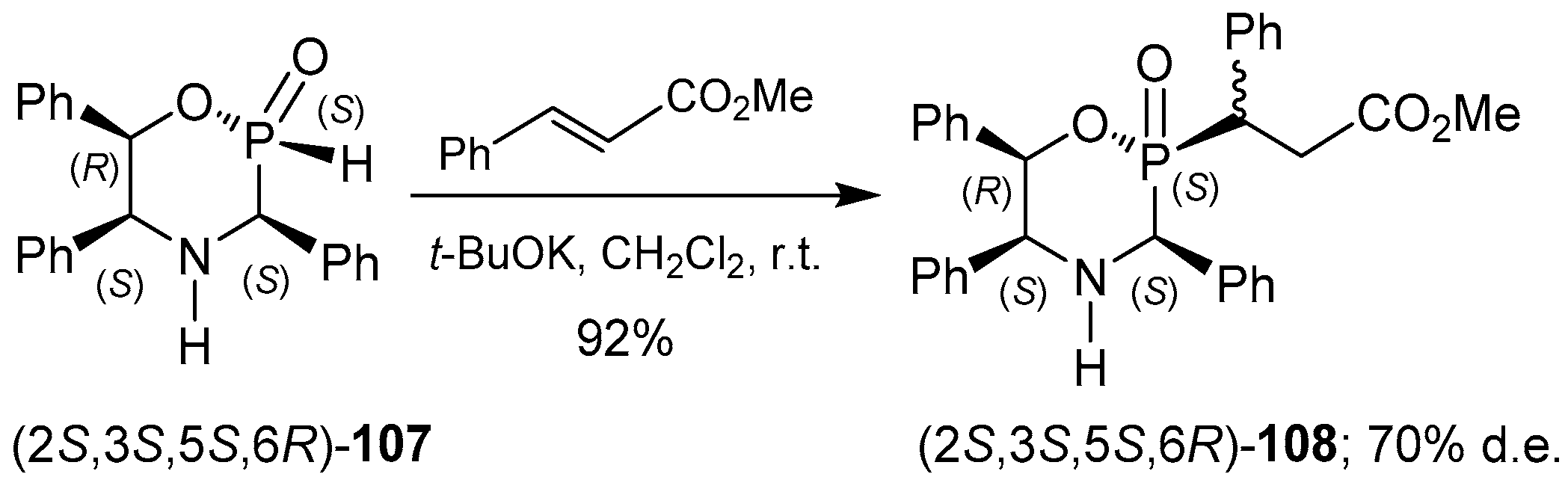
2.6. P-C Bond Formation from Chiral α-Amino-H-phosphinic Derivatives
3. Synthesis of Phosphacyclic α-Amino-C-phosphinates
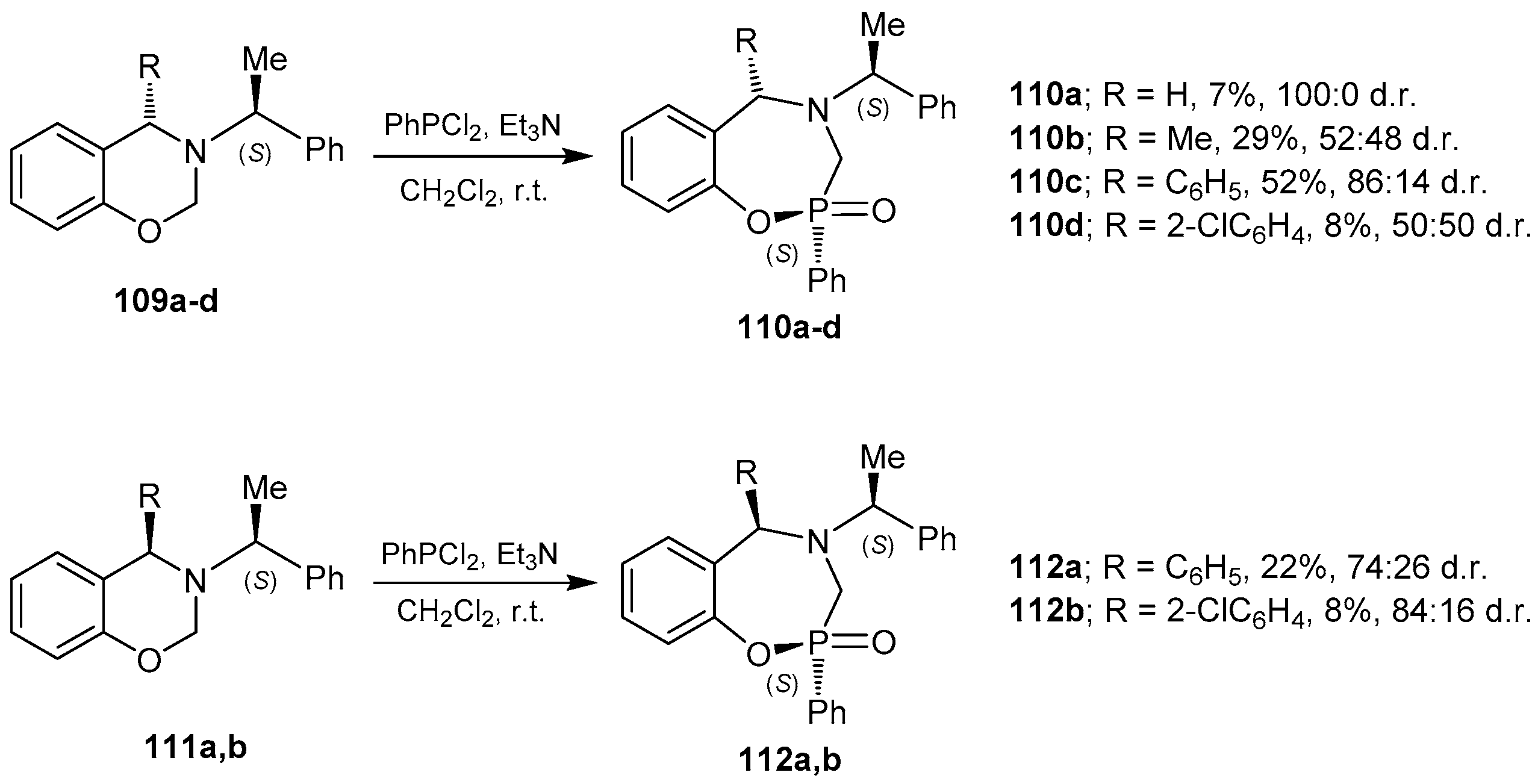
3.1. 1,4,2-Oxazaphosphacycles
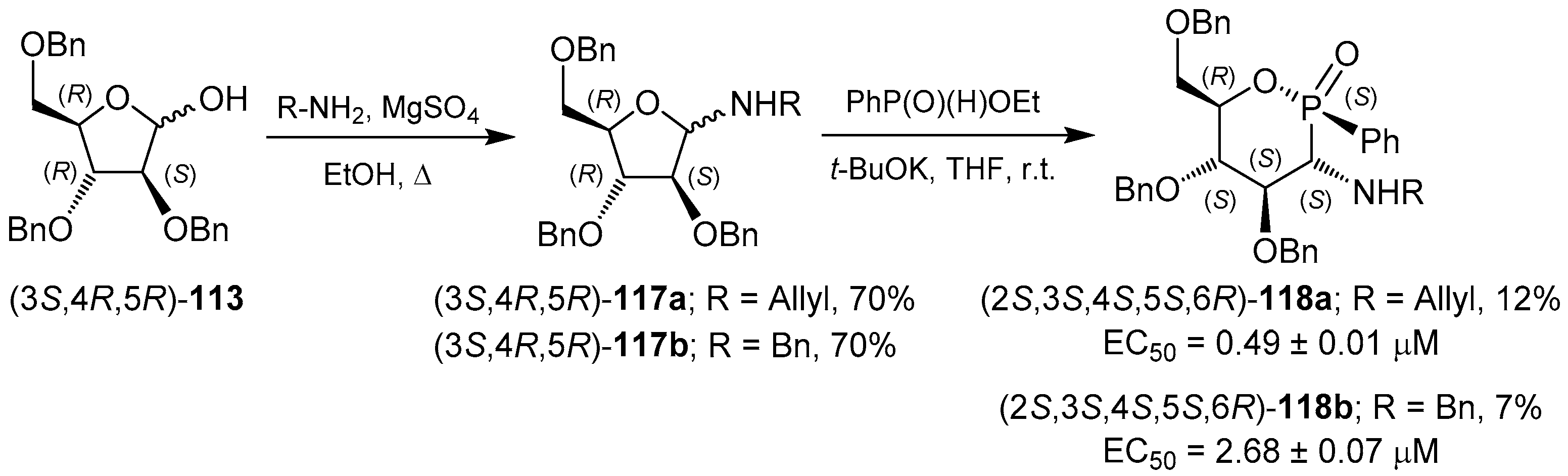
3.2. 1,2-Oxaphosphacycles
4. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Orsini, F.; Sello, G.; Sisti, M. Aminophosphonic acids and derivatives. Synthesis and biological applications. Curr. Med. Chem. 2010, 17, 264–289. [Google Scholar] [CrossRef] [PubMed]
- Naydenova, E.D.; Todorov, P.T.; Troev, K.D. Recent synthesis of aminophosphonic acids as potential biological importance. Amino Acids 2010, 38, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Sienczyk, M.; Oleksyszyn, J. Irreversible inhibition of serine proteases—Design and in vivo activity of diaryl α-aminophosphonate derivatives. Curr. Med. Chem. 2009, 16, 1673–1687. [Google Scholar] [CrossRef] [PubMed]
- Lejczak, B.; Kafarski, P. Biological activity of aminophosphonic acids and their short peptides. Top. Heterocycl. Chem. 2009, 20, 31–63. [Google Scholar]
- Kukhar, V.P.; Hudson, H.R. Aminophosphonic and Aminophospinic Acids: Chemistry and Biological Activity; Wiley: Chichester, UK, 2000. [Google Scholar]
- Ordóñez, M.; Viveros-Ceballos, J.L.; Cativiela, C.; Sayago, F.J. An update on the stereoselective synthesis of α-aminophosphonic acids and derivatives. Tetrahedron 2015, 71, 1745–1784. [Google Scholar] [CrossRef]
- Ordóñez, M.; Sayago, F.J.; Cativiela, C. Synthesis of quaternary α-aminophosphonic acids. Tetrahedron 2012, 68, 6369–6412. [Google Scholar] [CrossRef]
- Ordóñez, M.; Viveros-Ceballos, J.L.; Cativiela, C.; Arizpe, A. Stereoselective synthesis of α-aminophosphonic acids analogs of the 20 Proteinogenic α-amino acids. Curr. Org. Synth. 2012, 9, 310–341. [Google Scholar] [CrossRef]
- Kudzin, Z.H.; Kudzin, M.H.; Drabowicz, J.; Stevens, C.V. Aminophosphonic acids—Phosphorus analogues of natural amino acids. Part 1. Syntheses of α-aminophosphonic acids. Curr. Org. Chem. 2011, 15, 2015–2071. [Google Scholar] [CrossRef]
- Gulyukina, N.S.; Makukhin, N.N.; Beletskaya, I.P. Synthesis methods of (1-aminocyclopropyl)phosphonic acids. Russ. J. Org. Chem. 2011, 47, 633–649. [Google Scholar] [CrossRef]
- Ordóñez, M.; Rojas-Cabrera, H.; Cativiela, C. An overview of stereoselective synthesis of α-aminophosphonic acids and derivatives. Tetrahedron 2009, 65, 17–49. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.J.; van der Marel, G.A.; van Boom, J.H.; Liskamp, R.M.J. Peptides containing the novel methylphosphinamide transition-state isostere. Tetrahedron 1993, 49, 11055–11064. [Google Scholar] [CrossRef]
- Albouy, D.; Brun, A.; Munoz, A.; Etemad-Moghadam, G. New (α-hidroxyalkyl)phosphorus amphiphiles: Synthesis and dissociation constants. J. Org. Chem. 1998, 63, 7223–7230. [Google Scholar] [CrossRef] [PubMed]
- Kukhar, V.P.; Romanenko, V.D.; Hughes, A.B. Chemistry of Aminophosphonic Acids and Phosphonopeptides; Kukhar, V.P., Romanenko, V.D., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2009. [Google Scholar]
- Kraszewski, A.; Stawinski, J. H-Phosphonates: Versatile synthetic precursors to biologically active phosphorus compounds. Pure Appl. Chem. 2007, 79, 2217–2227. [Google Scholar] [CrossRef]
- Mucha, A.; Kafarski, P.; Berlicki, Ł. Remarkable potential of the α-aminophosphonate/phosphinate structural motif in medicinal chemistry. J. Med. Chem. 2011, 54, 5955–5980. [Google Scholar] [CrossRef] [PubMed]
- Dive, V.; Georgiadis, D.; Matziari, M.; Makaritis, A.; Beau, F.; Cuniasse, P.; Yiotakis, A. Phosphinic peptides as zinc metalloproteinase inhibitors. Cell. Mol. Life Sci. 2004, 61, 2010–2019. [Google Scholar] [CrossRef] [PubMed]
- Vassiliou, S.; Grabowiecka, A.; Kosikowska, P.; Yiotakis, A.; Kafarski, P.; Berlicki, L. Design, synthesis, and evaluation of novel organophosphorus inhibitors of bacterial ureases. J. Med. Chem. 2008, 51, 5736–5744. [Google Scholar] [CrossRef] [PubMed]
- Lukas, M.; Vojtisek, P.; Hermann, P.; Rohovec, J.; Lukes, I. Synthesis of phosphinic acid analogues of glycyl-glycine and crystal structure of N-glycyl-aminomethyl-(phenylphosphinic) acid. Synth. Commun. 2002, 32, 79–88. [Google Scholar] [CrossRef]
- Meng, F.-H.; Xu, J.-X. Direct synthesis of phosphinopeptides containing C-terminal α-aminoalkylphosphinic acids. Amino Acids 2010, 39, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Gluza, K.; Kafarski, P. Transition state analogues of enzymatic reaction as potential drugs. In Drug Discovery; El-Shemy, H., Ed.; InTech: Rijeka, Croatia, 2013; pp. 325–372. [Google Scholar]
- Berlicki, L.; Kafarski, P. Computer-aided analysis and design of phosphonic and phosphinic enzyme inhibitors as potential drugs and agrochemicals. Curr. Org. Chem. 2005, 9, 1829–1850. [Google Scholar] [CrossRef]
- Fournié-Zaluski, M.-C.; Poras, H.; Roques, B.P.; Nakajima, Y.; Ito, K.; Yoshimoto, T. Structure of aminopeptidase N from escherichia coli complexed with the transition-state analogue aminophosphinic inhibitor PL250. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 814–822. [Google Scholar] [CrossRef] [PubMed]
- Banegas, I.; Prieto, I.; Vives, F.; Alba, F.; de Gasparo, M.; Segarra, A.B.; Hermoso, F.; Duran, R.; Ramirez, M. Brain aminopeptidases and hypertension. J. Renin Angiotensin Aldosterone Syst. 2006, 7, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Skinner-Adams, T.S.; Stack, C.M.; Trenholme, K.R.; Brown, C.L.; Grembecka, J.; Lowther, J.; Mucha, A.; Drag, M.; Kafarski, P.; McGowan, S.; et al. Plasmodium falciparum neutral aminopeptidases: New targets for anti-malarials. Trends Biochem. Sci. 2010, 35, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Stack, C.M.; Lowther, J.; Cunningham, E.; Donnelly, S.; Gardiner, D.L.; Trenholme, K.R.; Skinner-Adams, T.S.; Teuscher, F.; Grembecka, J.; Mucha, A.; et al. Characterization of the plasmodium falciparum M17 leucyl aminopeptidase: A protease involved in amino acid regulation with potential for antimalarial drug development. J. Biol. Chem. 2007, 282, 2069–2080. [Google Scholar] [CrossRef] [PubMed]
- Yiotakis, A.; Georgiadis, D.; Matziari, M.; Makaritis, A.; Dive, V. Phosphinic peptides: Synthetic approaches and biochemical evaluation as Zn-metalloprotease inhibitors. Curr. Org. Chem. 2004, 8, 1135–1158. [Google Scholar] [CrossRef]
- Matziari, M.; Beau, F.; Cuniasse, P.; Dive, V.; Yiotakis, A. Evaluation of P1′-diversified phosphinic peptides leads to the development of highly selective inhibitors of MMP-11. J. Med. Chem. 2004, 47, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, T. Stereocontrolled synthesis of phosphinyl dipeptide isosteres using an asymmetric center at the phosphorus atom. J. Pharm. Soc. Jpn. 2013, 134, 915–924. [Google Scholar] [CrossRef]
- Drag, M.; Pawelczak, M.; Kafarski, P. Stereoselective synthesis of 1-aminoalkanephosphonic acids with two chiral centers and their activity towards leucine aminopeptidase. Chirality 2003, 15, S104–S107. [Google Scholar] [CrossRef] [PubMed]
- Mucha, A. Synthesis and modifications of phosphinic dipeptide analogues. Molecules 2012, 17, 13530–13568. [Google Scholar] [CrossRef] [PubMed]
- Virieux, D.; Volle, J.N.; Pirat, J.L. Acyclic to cyclic aminophosphonic and phosphinic acids. ARKIVOC-Online J. Org. Chem. 2012, 11, 264–277. [Google Scholar]
- Kobayashi, S.; Ishitani, H. Catalytic enantioselective addition to imines. Chem. Rev. 1999, 99, 1069–1094. [Google Scholar] [CrossRef] [PubMed]
- Gröger, H. Catalytic enantioselective Strecker reactions and analogous syntheses. Chem. Rev. 2003, 103, 2795–2828. [Google Scholar] [CrossRef] [PubMed]
- Pudovik, A.N. Addition of dialkyl phosphites to imines. New method of synthesis of esters of amino phosphonic acids. Dokl. Akad. Nauk SSSR 1952, 83, 865–869. [Google Scholar]
- Tibhe, G.D.; Lagunas-Rivera, S.; Vargas-Díaz, E.; García-Barradas, O.; Ordóñez, M. Uncatalyzed one-pot diastereoselective synthesis of α-amino phosphonates under solvent-free conditions. Eur. J. Org. Chem. 2010, 2010, 6573–6581. [Google Scholar] [CrossRef]
- Hoffmann, R.W. Allylic 1,3-strain as a controlling factor in stereoselective transformations. Chem. Rev. 1989, 89, 1841–1860. [Google Scholar] [CrossRef]
- Afarinkia, K.; Cadogan, J.I.G.; Rees, C.W. Diastereoselectivity in the formation of phosphorus-carbon bonds. Synlett 1992, 1992, 124–125. [Google Scholar] [CrossRef]
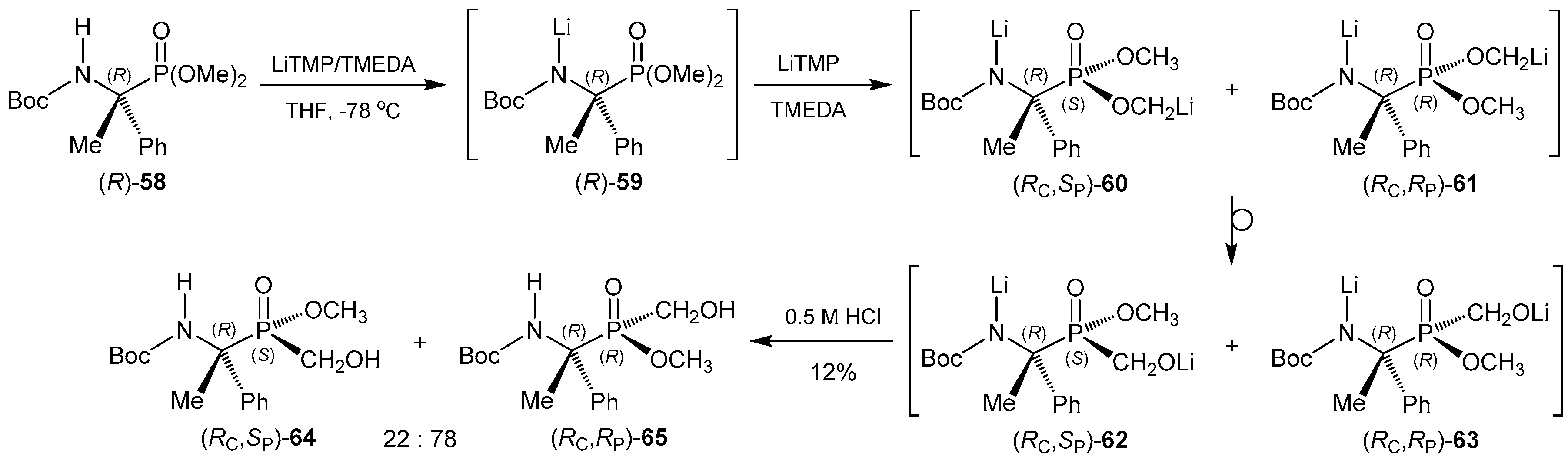
- Szabó, A.; Jászay, Z.M.; Hegedűs, L.; Tőke, L.; Petneházy, I. The first enantioselective synthesis of α-aminophosphinates. Tetrahedron Lett. 2003, 44, 4603–4606. [Google Scholar] [CrossRef]
- Yang, M.; Sun, Y.-M.; Hou, Q.-G.; Zhao, C.-Q. (RP)-5-Methyl-2-(propan-2-yl)cyclohexyl phenyl{phenyl[(1-phenylethyl)amino]methyl}phosphinate. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68. [Google Scholar] [CrossRef] [PubMed]
- Rossi, J.-C.; Marull, M.; Larcher, N.; Taillades, J.; Pascal, R.; van der Lee, A.; Gerbier, P. A recyclable chiral auxiliary for the asymmetric syntheses of α-aminonitriles and α-aminophosphinic derivatives. Tetrahedron Asymmetry 2008, 19, 876–883. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Narindoshvili, T.; Draghici, B.; Angrish, P. Regioselective syntheses of β-N-linked glycoaminoacids and glycopeptides. J. Org. Chem. 2008, 73, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Zheng, W.; Wang, D.; Zhang, P.; Pan, Y. Practical stereo- and regioselective, copper(I)-promoted Strecker synthesis of sugar-modified α,β-unsaturated imines. Helv. Chim. Acta 2006, 89, 520–526. [Google Scholar] [CrossRef]
- Knauer, S.; Kranke, B.; Krause, L.; Kunz, H. Amino sugars and glycosylamines as tools in stereoselective synthesis. Curr. Org. Chem. 2004, 8, 1739–1761. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Yu, J.; Miao, Z.; Chen, R. Stereoselective synthesis of α-amino(phenyl)methyl(phenyl)phosphinic acids with O-pivaloylated d-galactosylamine as chiral auxiliary. Chem. Eur. J. 2009, 15, 9290–9293. [Google Scholar] [CrossRef] [PubMed]
- Edupuganti, R.; Davis, F.A. Synthesis and applications of masked oxo-sulfinamides in asymmetric synthesis. Org. Biomol. Chem. 2012, 10, 5021–5031. [Google Scholar] [CrossRef] [PubMed]
- Szabó, A.; Jászay, Z.M.; Töke, L.; Petneházy, I. Interesting by-products in the synthesis of chiral α-aminophosphinates from enantiopure sulfinimines. Tetrahedron Lett. 2004, 45, 1991–1994. [Google Scholar] [CrossRef]
- Leow, D.; Tan, C.-H. Chiral guanidine catalyzed enantioselective reactions. Chem. Asian J. 2009, 4, 488–507. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Loh, W.; Zhang, Y.; Chen, T.; Ma, T.; Liu, H.; Wang, J.; Tan, C.-H. Chiral guanidinium salt catalyzed enantioselective phospha-Mannich reactions. Angew. Chem. Int. Ed. 2009, 48, 7387–7390. [Google Scholar] [CrossRef] [PubMed]
- Cherkasov, R.A.; Galkin, V.I. The Kabachnik-Fields reaction: Synthetic potential and the problem of the mechanism. Russ. Chem. Rev. 1998, 67, 857–882. [Google Scholar] [CrossRef]
- Fields, E.K. The synthesis of esters of substituted amino phosphonic acids. J. Am. Chem. Soc. 1952, 74, 1528–1531. [Google Scholar] [CrossRef]
- Kabachnik, M.I.; Medved, T.Y. New synthesis of aminophosphonic acids. Dokl. Akad. Nauk SSSR 1952, 83, 689–692. [Google Scholar]
- Meng, F.; Xu, J. Synthesis of phosphinodepsipeptides via the pseudo-four-component condensation reaction. Tetrahedron 2013, 69, 4944–4952. [Google Scholar] [CrossRef]
- Meng, F.; He, F.; Song, X.; Zhang, L.; Hu, W.; Liu, G.; Xu, J. Facile synthesis of hybrid sulfonophosphinodipeptides composing of taurines and 1-aminoalkylphosphinic acids. Amino Acids 2012, 43, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Dwars, T.; Schmidt, U.; Fischer, C.; Grassert, I.; Kempe, R.; Fröhlich, R.; Drauz, K.; Oehme, G. Synthesis of optically active α-aminophosphinic acids by catalytic asymmetric hydrogenation in organic solvents and aqueous micellar media. Angew. Chem. Int. Ed. 1998, 37, 2851–2853. [Google Scholar] [CrossRef]
- Brovarets, V.S.; Zyuz, K.V.; Budnik, L.V.; Solodenko, V.A.; Drach, B.S. New approach to the synthesis of 1-acylaminoalkenylphosphonic acids, their analogs and derivatives. Zh. Obs. Khim. 1993, 63, 1259–1265. [Google Scholar]
- Dwars, T.; Schmidt, U.; Fischer, C.; Grassert, I.; Krause, H.-W.; Michalik, M.; Oehme, G. Synthesis of enantiomerically enriched α-aminophosphonic acid derivatives via asymmetric hydrogenation. Phosphorus Sulfur Silicon Relat. Elem. 2000, 158, 209–240. [Google Scholar] [CrossRef]
- Lefevre, N.; Brayer, J.-L.; Folléas, B.; Darses, S. Chiral α-amino phosphonates via rhodium-catalyzed asymmetric 1,4-addition reactions. Org. Lett. 2013, 15, 4274–4276. [Google Scholar] [CrossRef] [PubMed]
- Zoń, J. A simple preparation of diethyl 1-acylamino-1-ethenephosphonates. Synthesis 1981, 1981, 324–324. [Google Scholar] [CrossRef]
- Lämmerhofer, M.; Hebenstreit, D.; Gavioli, E.; Lindner, W.; Mucha, A.; Kafarski, P.; Wieczorek, P. High-performance liquid chromatographic enantiomer separation and determination of absolute configurations of phosphinic acid analogues of dipeptides and their α-aminophosphinic acid precursors. Tetrahedron Asymmetry 2003, 14, 2557–2565. [Google Scholar] [CrossRef]
- Preinerstorfer, B.; Lubda, D.; Mucha, A.; Kafarski, P.; Lindner, W.; Lämmerhofer, M. Stereoselective separations of chiral phosphinic acid pseudodipeptides by CEC using silica monoliths modified with an anion-exchange-type chiral selector. Electrophoresis 2006, 27, 4312–4320. [Google Scholar] [CrossRef] [PubMed]
- Grembecka, J.; Mucha, A.; Cierpicki, T.; Kafarski, P. The most potent organophosphorus inhibitors of leucine aminopeptidase. Structure-based design, chemistry, and activity. J. Med. Chem. 2003, 46, 2641–2655. [Google Scholar] [CrossRef] [PubMed]
- Mucha, A.; Lämmerhofer, M.; Lindner, W.; Pawełczak, M.; Kafarski, P. Individual stereoisomers of phosphinic dipeptide inhibitor of leucine aminopeptidase. Bioorg. Med. Chem. Lett. 2008, 18, 1550–1554. [Google Scholar] [CrossRef] [PubMed]
- Rozhko, L.F.; Ragulun, V.V. α-Hydroxy-α-aminophosphinic acids: I. Synthesis of a new analog of phenylglycine and its enantiomers. Russ. J. Gen. Chem. 2005, 75, 533–536. [Google Scholar] [CrossRef]
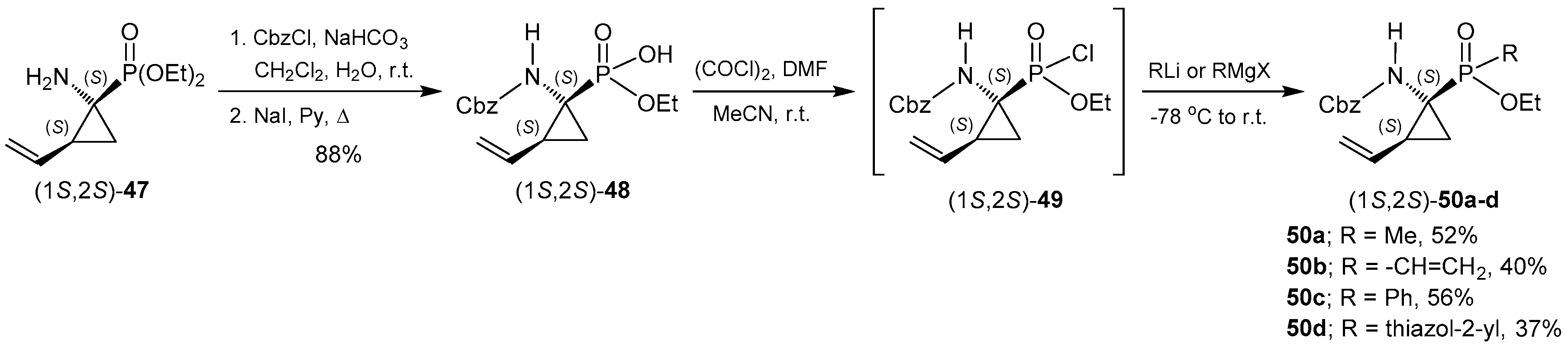
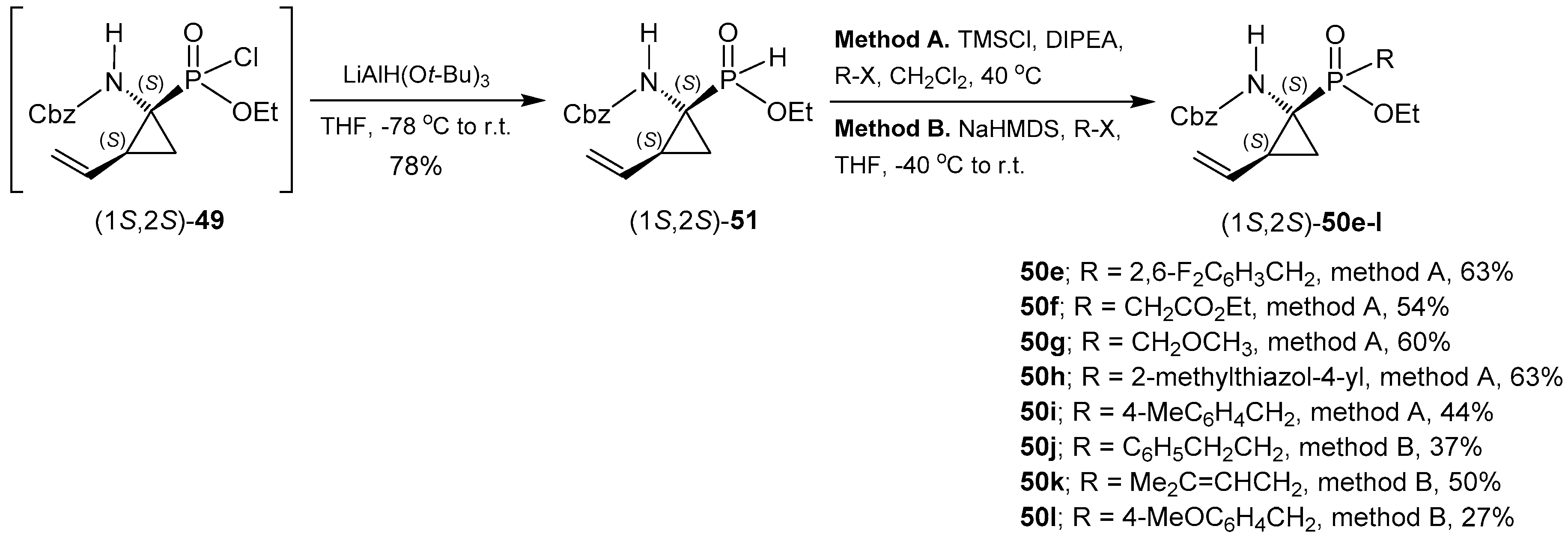
- Pyun, H.; Clarke, M.O.; Cho, A.; Casarez, A.; Ji, M.; Fardis, M.; Pastor, R.; Sheng, X.C.; Kim, C.U. Synthesis of 1-amino-2-vinylcyclopropane-1-phosphinates. Conversion of a phosphonate to phosphinates. Tetrahedron Lett. 2012, 53, 2360–2363. [Google Scholar] [CrossRef]
- Clarke, M.O.; Chen, X.; Cho, A.; Delaney, W.E.; Doerffler, E.; Fardis, M.; Ji, M.; Mertzman, M.; Pakdaman, R.; Pyun, H.; et al. Novel, potent, and orally bioavailable phosphinic acid inhibitors of the hepatitis C virus NS3 protease. Bioorg. Med. Chem. Lett. 2011, 21, 3568–3572. [Google Scholar] [CrossRef] [PubMed]
- Pyun, H.-J.; Chaudhary, K.; Somoza, J.R.; Sheng, X.C.; Kim, C.U. Synthesis and resolution of diethyl (1S,2S)-1-amino-2-vinylcyclopropane-1-phosphonate for HCV NS3 protease inhibitors. Tetrahedron Lett. 2009, 50, 3833–3835. [Google Scholar] [CrossRef]
- Njoroge, F.G.; Venkatraman, S.; Girijavallabhan, V.M. Depeptidized Inhibitors of Hepatitis C Virus NS3 protease. U.S. Patent 2005164921 (A1), 28 July 2005. [Google Scholar]
- Qian, R.; Roller, A.; Hammerschmidt, F. Phosphonate–phosphinate rearrangement. J. Org. Chem. 2015, 80, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Collinsova, M.; Jiracek, J. Phosphinic acid compounds in biochemistry, biology and medicine. Curr. Med. Chem. 2000, 7, 629–647. [Google Scholar] [CrossRef] [PubMed]
- Matziari, M.; Georgiadis, D.; Dive, V.; Yiotakis, A. Convenient synthesis and diversification of dehydroalaninyl phosphinic peptide analogues. Org. Lett. 2001, 3, 659–662. [Google Scholar] [CrossRef] [PubMed]
- Baylis, E.K.; Campbell, C.D.; Dingwall, J.G. 1-Aminoalkylphosphonous acids. Part 1. Isosteres of the protein amino acids. J. Chem. Soc. Perkin Trans. 1 1984, 2845–2853. [Google Scholar] [CrossRef]
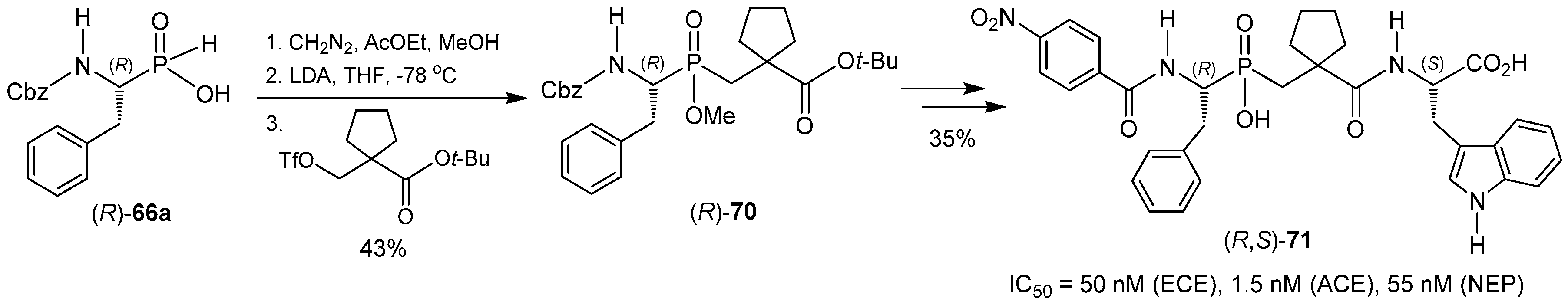
- McKittrick, B.A.; Stamford, A.W.; Weng, X.; Ma, K.; Chackalamannil, S.; Czarniecki, M.; Cleven, R.; Fawzi, A.B. Design and synthesis of phosphinic acids that triply inhibit endothelin converting enzyme, angiotensin converting enzyme and neutral endopeptidase 24.11. Bioorg. Med. Chem. Lett. 1996, 6, 1629–1634. [Google Scholar] [CrossRef]
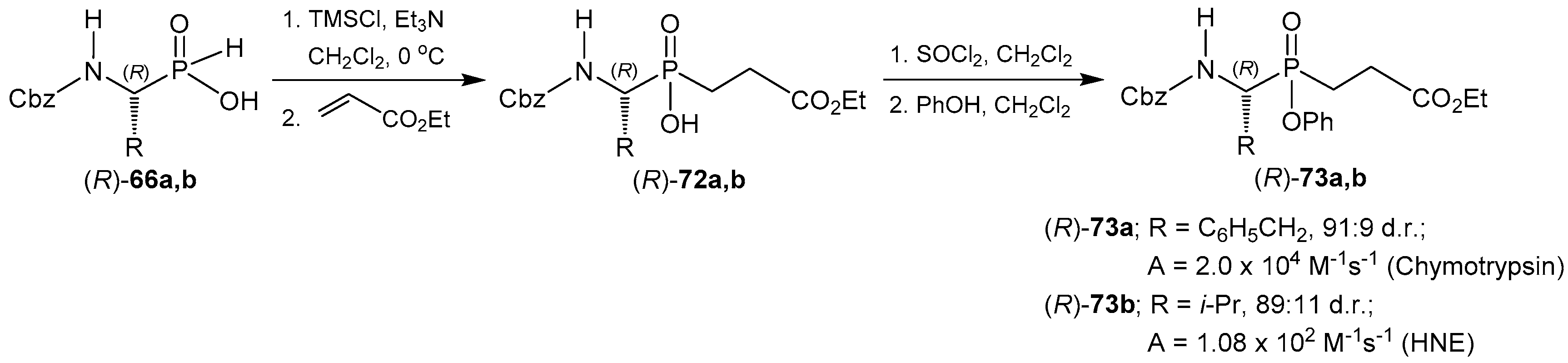
- Hamilton, R.; Wharry, S.; Walker, B.; Walker, B.J. The synthesis of phosphinic acid based proteinase inhibitors. Phosphorus Sulfur Silicon Relat. Elem. 1999, 144, 761–764. [Google Scholar] [CrossRef]
- Lloyd, J.; Schmidt, J.B.; Hunt, J.T.; Barrish, J.C.; Little, D.K.; Tymiak, A.A. Solid phase synthesis of phosphinic acid endothelin converting enzyme inhibitors. Bioorg. Med. Chem. Lett. 1996, 6, 1323–1326. [Google Scholar] [CrossRef]
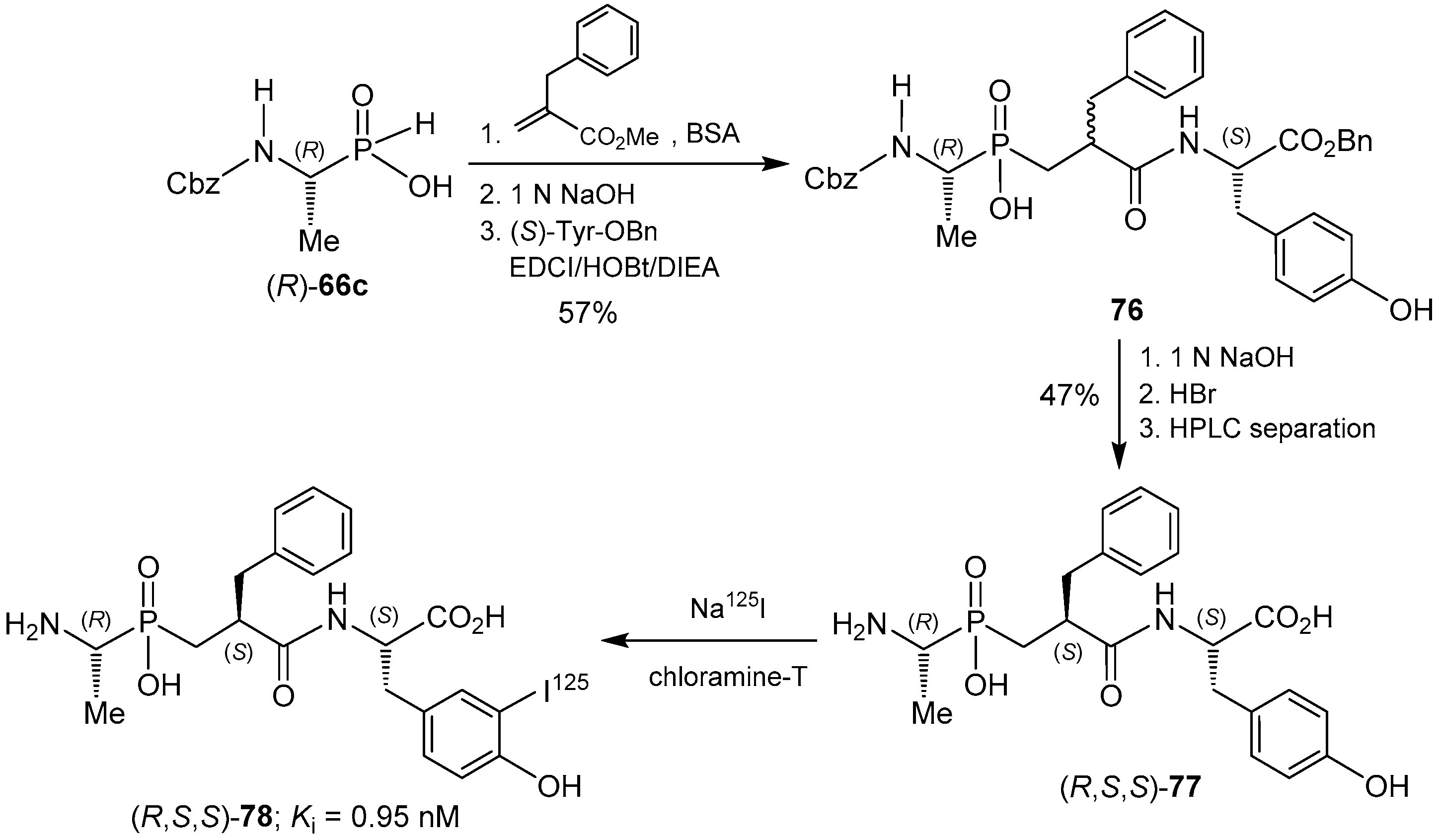
- Chen, H.; Bischoff, L.; Fournie-Zaluski, M.-C.; Roques, B.P. Synthesis of 2(S)-benzyl-3-[hydroxy(1′(R)-amino ethyl)phosphinyl]propanoyl-l-3-[125I]-iodotyrosine: A radiolabelled inhibitor of aminopeptidase N. J. Label. Compd. Radiopharm. 2000, 43, 103–111. [Google Scholar] [CrossRef]
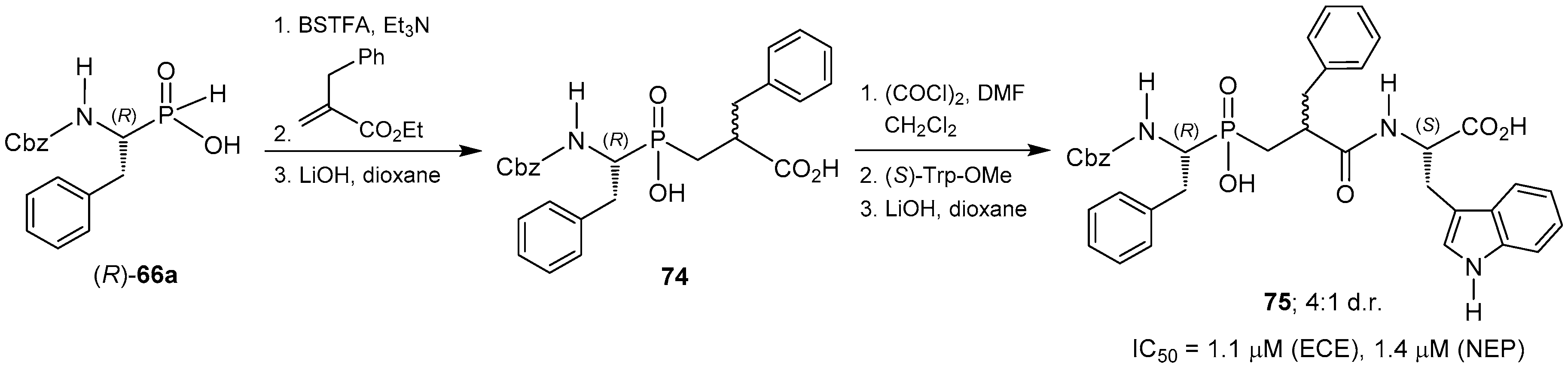
- Chen, H.; Noble, F.; Mothé, A.; Meudal, H.; Coric, P.; Danascimento, S.; Roques, B.P.; George, P.; Fournié-Zaluski, M.-C. Phosphinic derivatives as new dual enkephalin-degrading enzyme inhibitors: Synthesis, biological properties, and antinociceptive activities. J. Med. Chem. 2000, 43, 1398–1408. [Google Scholar] [CrossRef] [PubMed]
- Matziari, M.; Dellis, D.; Dive, V.; Yiotakis, A.; Samios, J. Conformational and solvation studies via computer simulation of the novel large scale diastereoselectively synthesized phosphinic MMP inhibitor RXP03 diluted in selected solvents. J. Phys. Chem. B 2010, 114, 421–428. [Google Scholar] [CrossRef] [PubMed]
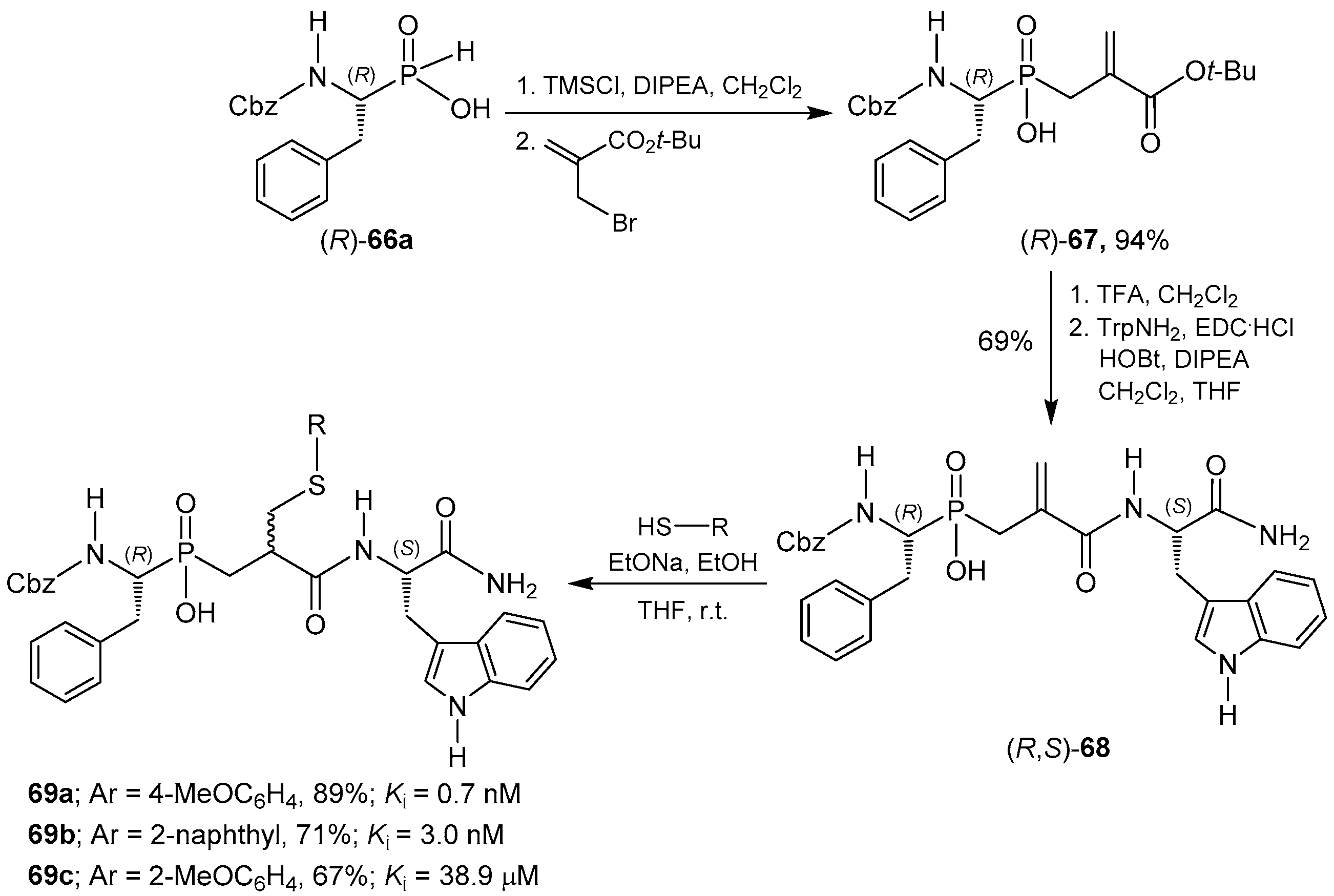
- Vassiliou, S.; Mucha, A.; Cuniasse, P.; Georgiadis, D.; Lucet-Levannier, K.; Beau, F.; Kannan, R.; Murphy, G.; Knäuper, V.; Rio, M.-C.; et al. Phosphinic pseudo-tripeptides as potent inhibitors of matrix metalloproteinases: A structure-activity study. J. Med. Chem. 1999, 42, 2610–2620. [Google Scholar] [CrossRef] [PubMed]
- Dive, V.; Andarawewa, K.L.; Boulay, A.; Matziari, M.; Beau, F.; Guerin, E.; Rousseau, B.; Yiotakis, A.; Rio, M.-C. Dosing and scheduling influence the antitumor afficacy of a phosphinic peptide inhibitor of matrix metalloproteinases. Int. J. Cancer 2005, 113, 775–781. [Google Scholar] [CrossRef] [PubMed]
- David, A.; Steer, D.; Bregant, S.; Devel, L.; Makaritis, A.; Beau, F.; Yiotakis, A.; Dive, V. Cross-linking yield variation of a potent matrix metalloproteinase photoaffinity probe and consequences for functional proteomics. Angew. Chem. Int. Ed. 2007, 46, 3275–3277. [Google Scholar] [CrossRef] [PubMed]
- Makaritis, A.; Georgiadis, D.; Dive, V.; Yiotakis, A. Diastereoselective solution and multipin-based combinatorial array synthesis of a novel class of potent phosphinic metalloprotease inhibitors. Chem. Eur. J. 2003, 9, 2079–2094. [Google Scholar] [CrossRef] [PubMed]
- Jullien, N.; Makritis, A.; Georgiadis, D.; Beau, F.; Yiotakis, A.; Dive, V. Phosphinic tripeptides as dual angiotensin-converting enzyme C-domain and endothelin-converting enzyme-1 inhibitors. J. Med. Chem. 2010, 53, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Zervoudi, E.; Saridakis, E.; Birtley, J.R.; Seregin, S.S.; Reeves, E.; Kokkala, P.; Aldhamen, Y.A.; Amalfitano, A.; Mavridis, I.M.; James, E.; et al. Rationally designed inhibitor targeting antigen-trimming aminopeptidases enhances antigen presentation and cytotoxic T-cell responses. Proc. Natl. Acad. Sci. USA 2013, 110, 19890–19895. [Google Scholar] [CrossRef] [PubMed]
- Matziari, M.; Nasopoulou, M.; Yiotakis, A. Active methylene phosphinic peptides: A new diversification approach. Org. Lett. 2006, 8, 2317–2319. [Google Scholar] [CrossRef] [PubMed]
- Gegnas, L.D.; Waddell, S.T.; Chabin, R.M.; Reddy, S.; Wong, K.K. Inhibitors of the bacterial cell wall biosynthesis enzyme Mur D. Bioorg. Med. Chem. Lett. 1998, 8, 1643–1648. [Google Scholar] [CrossRef]
- Rogakos, V.; Georgiadis, D.; Dive, V.; Yiotakis, A. A modular rearrangement approach toward medicinally relevant phosphinic structures. Org. Lett. 2009, 11, 4696–4699. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, T.; Kinbara, A.; Okubo, N.; Sato, S.; Fukaya, H. Diastereoselective synthesis of Pro-Phe phosphinyl dipeptide isosteres. Tetrahedron Asymmetry 2012, 23, 1633–1639. [Google Scholar] [CrossRef]
- Yamagishi, T.; Mori, J.; Haruki, T.; Yokomatsu, T. A chemo-enzymatic synthesis of optically active 1,1-diethoxyethyl(aminomethyl)phosphinates: Useful chiral building blocks for phosphinyl dipeptide isosteres. Tetrahedron Asymmetry 2011, 22, 1358–1363. [Google Scholar] [CrossRef]
- Berger, O.; Montchamp, J.-L. Phosphinate-containing heterocycles: A mini-review. Beilstein J. Org. Chem. 2014, 10, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Mathey, F. Phosphorus-Carbon Heterocyclic Chemistry: The Rise of a New Domain; Elsevier Health Sciences: Oxford, UK, 2001. [Google Scholar]
- Quin, L.D. A Guide to Organophosphorus Chemistry; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Mathey, F. Chemistry of 3-membered carbon-phosphorus heterocycles. Chem. Rev. 1990, 90, 997–1025. [Google Scholar] [CrossRef]
- Volle, J.-N.; Virieux, D.; Starck, M.; Monbrun, J.; Clarion, L.; Pirat, J.-L. Chiral phosphinyl analogues of 2-C-arylmorpholinols: 2-Aryl-3,5-diphenyl-[1,4,2]-oxazaphosphinanes. Tetrahedron Asymmetry 2006, 17, 1402–1408. [Google Scholar] [CrossRef]
- Kelley, J.L.; Musso, D.L.; Boswell, G.E.; Soroko, F.E.; Cooper, B.R. (2S,3S,5R)-2-(3,5-Difluorophenyl)-3,5-dimethyl-2-morpholinol: A novel antidepressant agent and selective inhibitor of norepinephrine uptake. J. Med. Chem. 1996, 39, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Monbrun, J.; Dayde, B.; Cristau, H.-J.; Volle, J.-N.; Virieux, D.; Pirat, J.-L. Diastereoselective Michael addition of 2H-2-oxo-1,4,2-oxaza phosphinanes to olefins. Tetrahedron 2011, 67, 540–545. [Google Scholar] [CrossRef]
- Salgado-Escobar, O.; Chavelas-Hernández, L.; Domínguez-Mendoza, B.; Linzaga-Elizalde, I.; Ordoñez, M. Synthesis of chiral 1,4,2-oxazaphosphepines. Molecules 2015, 20, 13794–13813. [Google Scholar] [CrossRef] [PubMed]
- Clarion, L.; Jacquard, C.; Sainte-Catherine, O.; Loiseau, S.; Filippini, D.; Hirlemann, M.-H.; Volle, J.-N.; Virieux, D.; Lecouvey, M.; Pirat, J.-L.; et al. Oxaphosphinanes: New therapeutic perspectives for glioblastoma. J. Med. Chem. 2012, 55, 2196–2211. [Google Scholar] [CrossRef] [PubMed]
- Clarion, L.; Jacquard, C.; Sainte-Catherine, O.; Decoux, M.; Loiseau, S.; Rolland, M.; Lecouvey, M.; Hugnot, J.-P.; Volle, J.-N.; Virieux, D.; et al. C-Glycoside mimetics inhibit glioma stem cell proliferation, migration, and invasion. J. Med. Chem. 2014, 57, 8293–8306. [Google Scholar] [CrossRef] [PubMed]






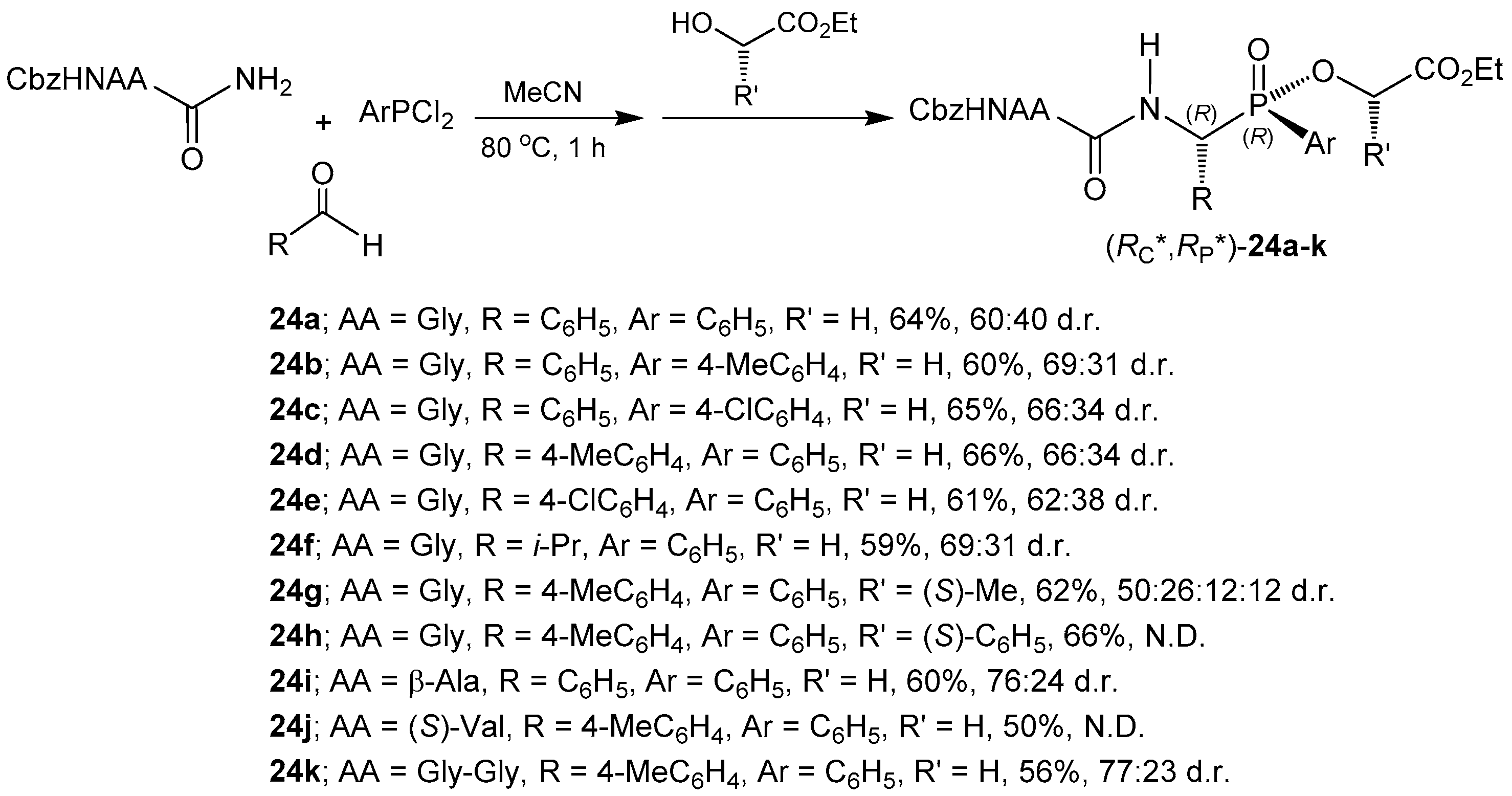
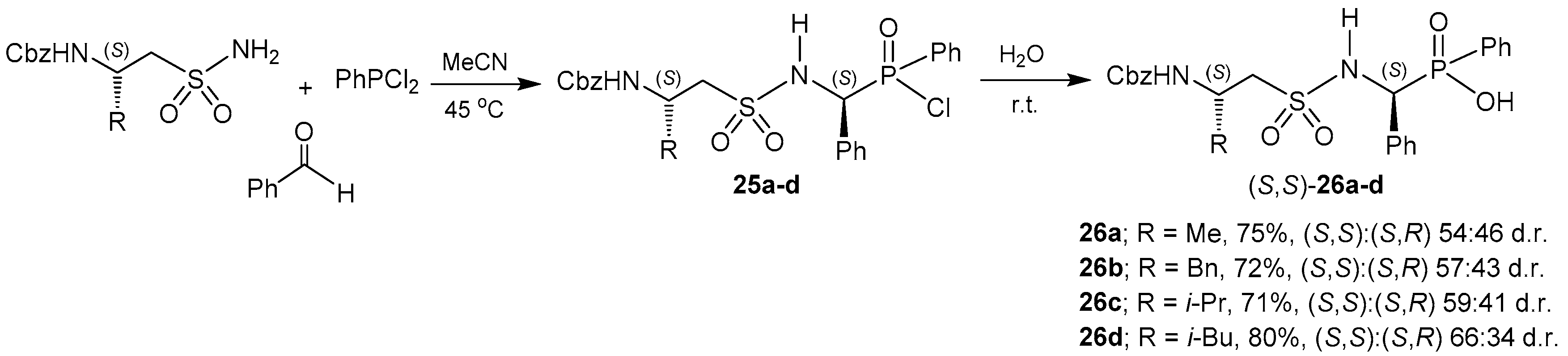
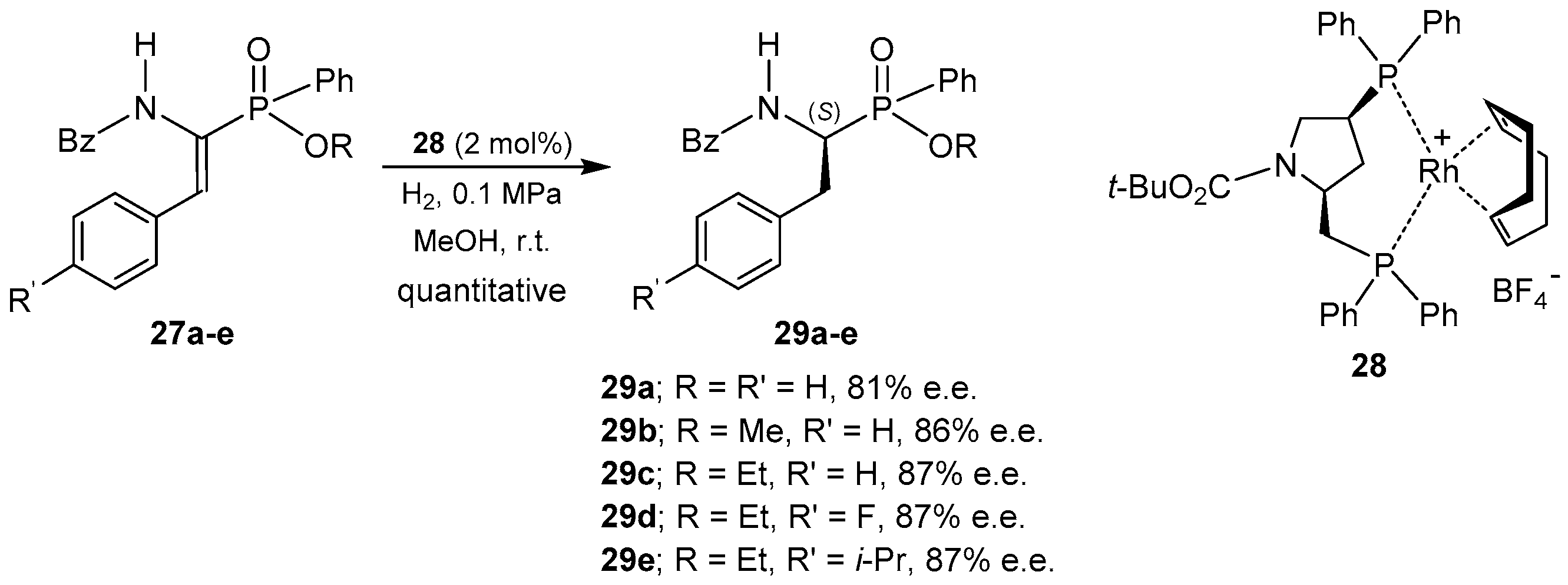
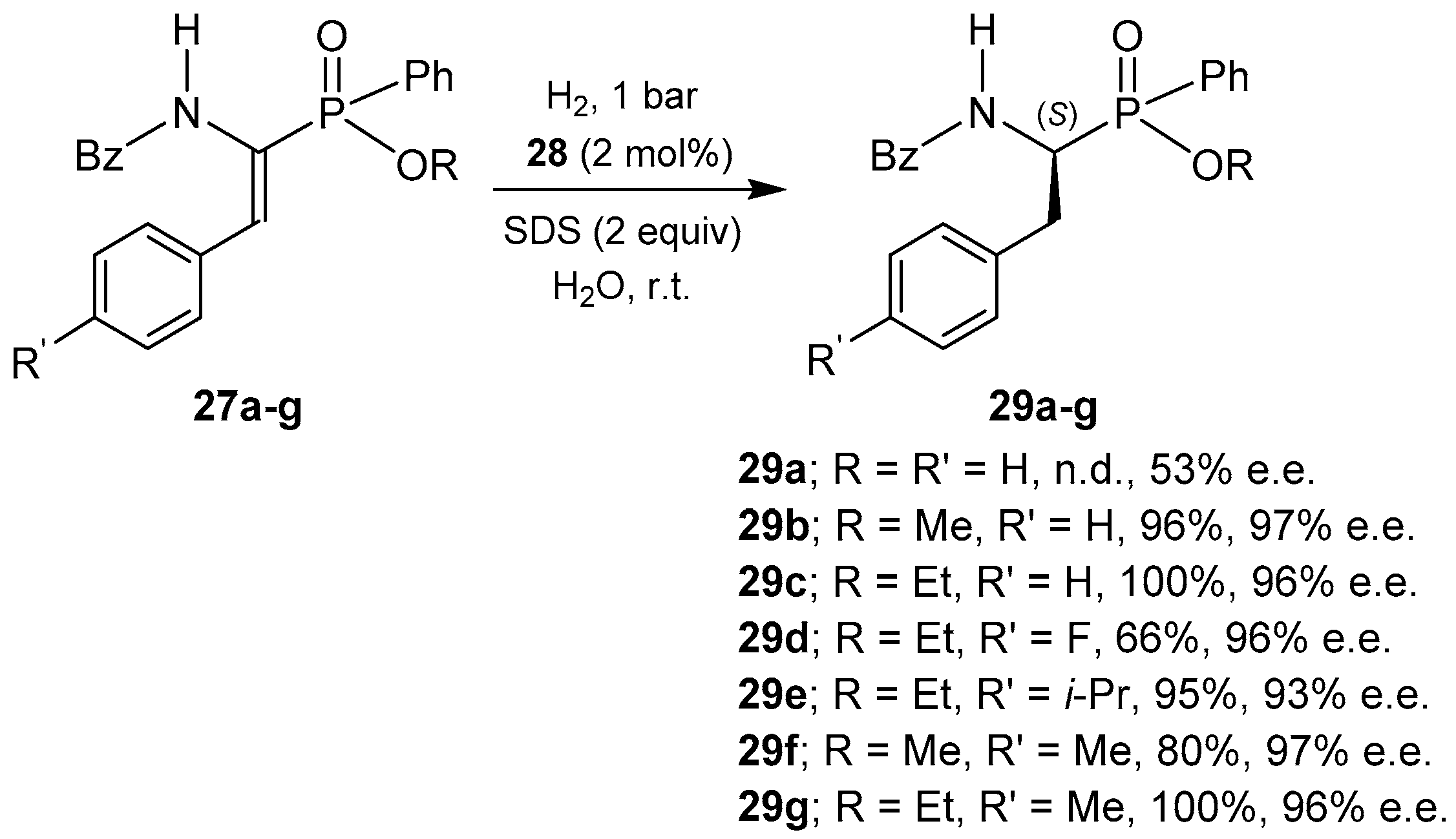



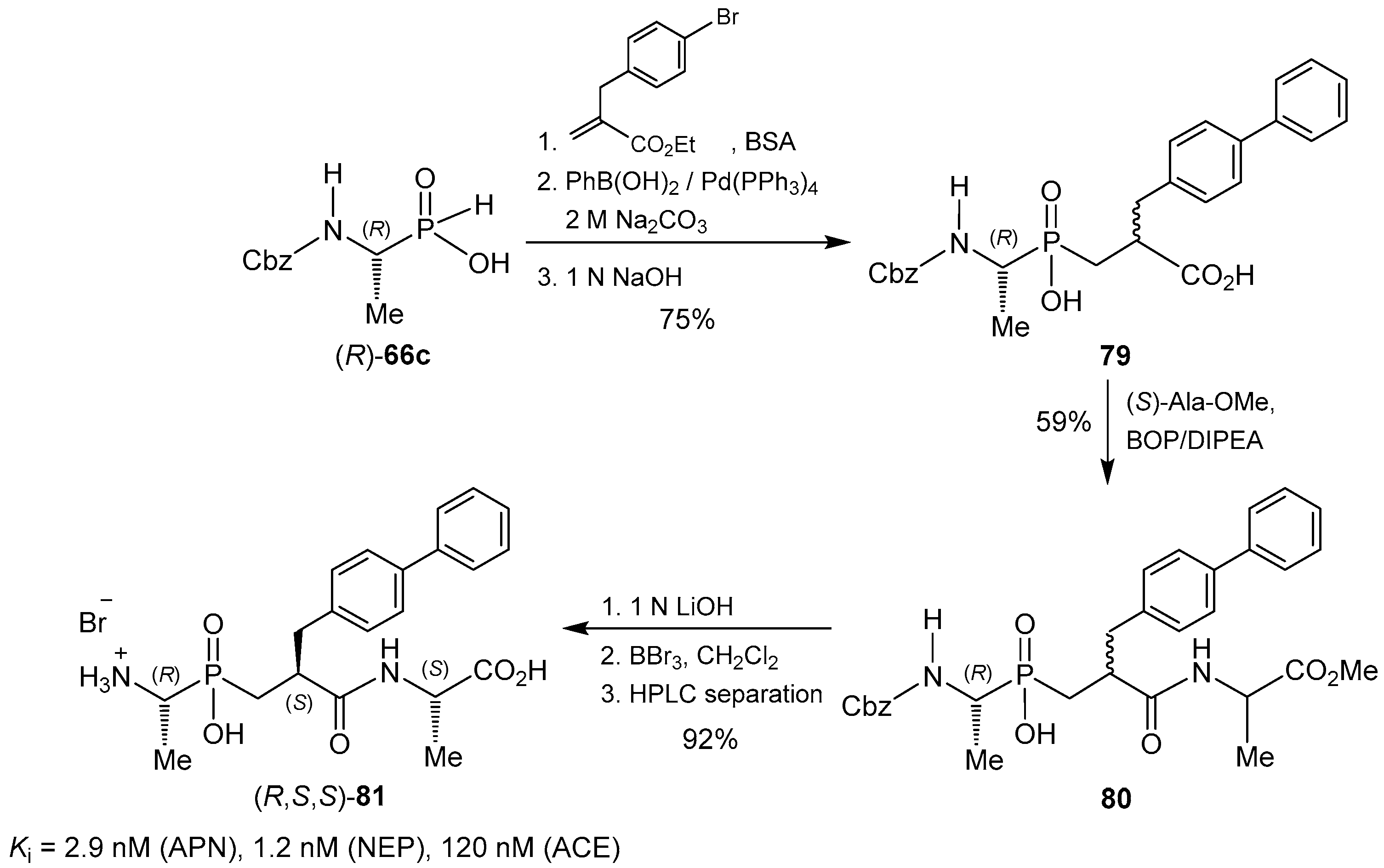
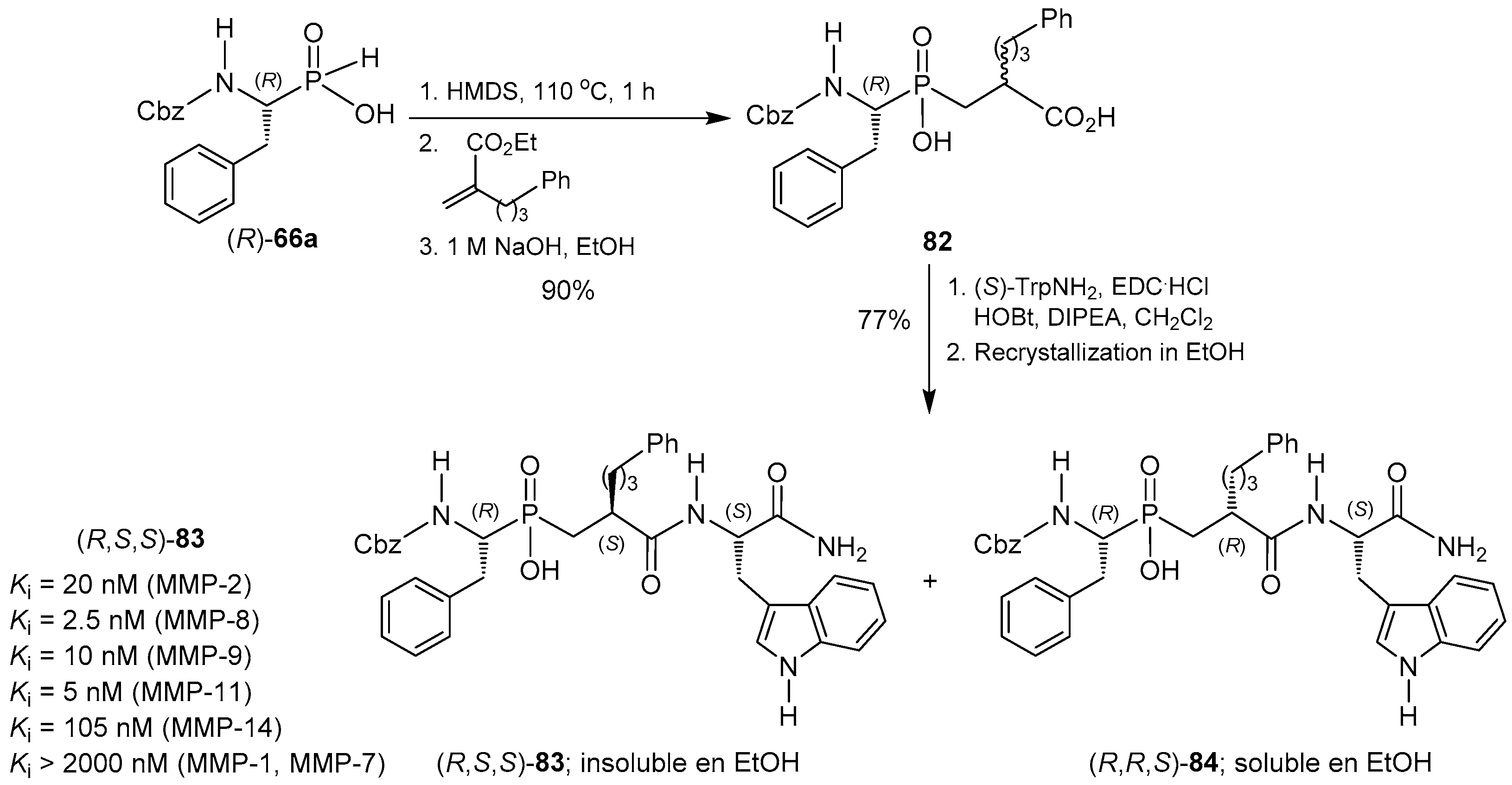
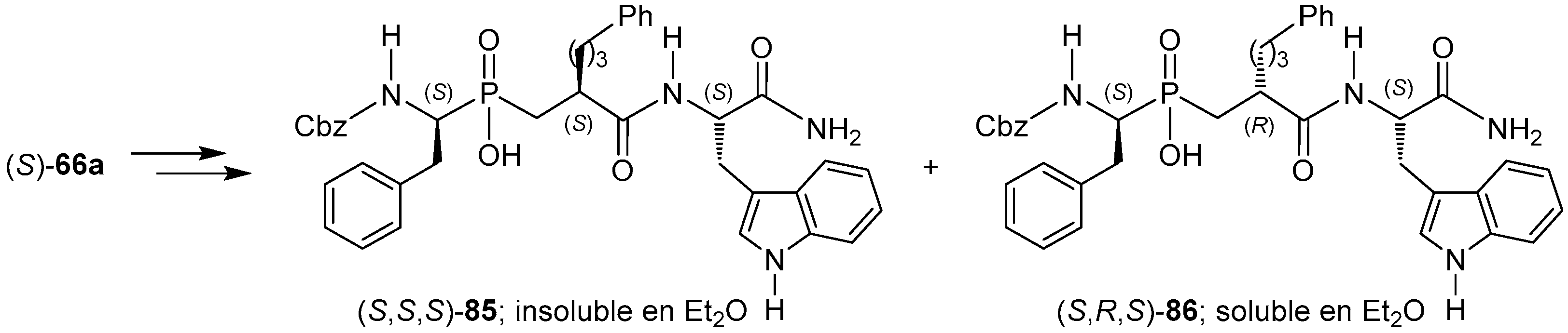

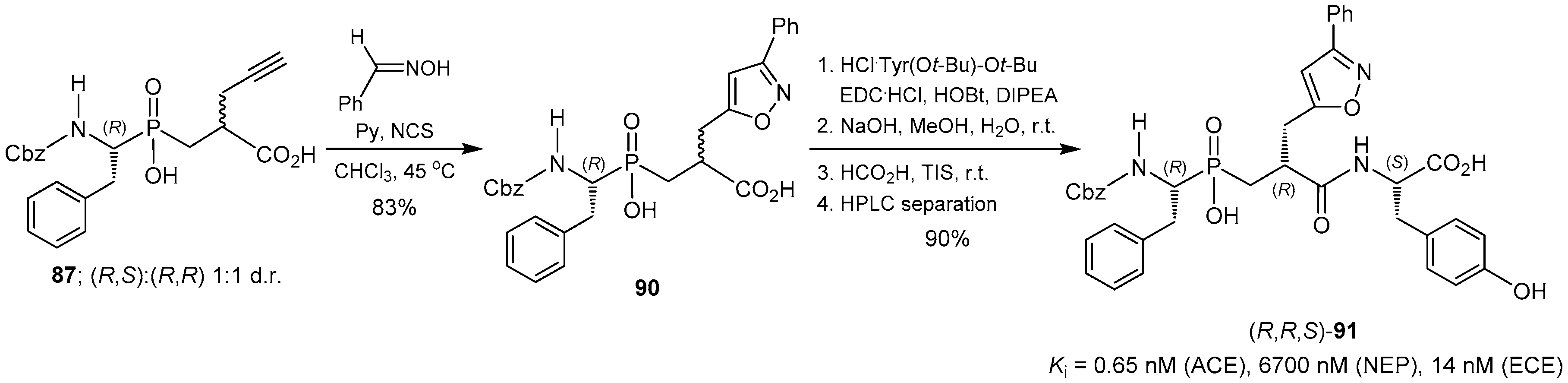






© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viveros-Ceballos, J.L.; Ordóñez, M.; Sayago, F.J.; Cativiela, C. Stereoselective Synthesis of α-Amino-C-phosphinic Acids and Derivatives. Molecules 2016, 21, 1141. https://doi.org/10.3390/molecules21091141
Viveros-Ceballos JL, Ordóñez M, Sayago FJ, Cativiela C. Stereoselective Synthesis of α-Amino-C-phosphinic Acids and Derivatives. Molecules. 2016; 21(9):1141. https://doi.org/10.3390/molecules21091141
Chicago/Turabian StyleViveros-Ceballos, José Luis, Mario Ordóñez, Francisco J. Sayago, and Carlos Cativiela. 2016. "Stereoselective Synthesis of α-Amino-C-phosphinic Acids and Derivatives" Molecules 21, no. 9: 1141. https://doi.org/10.3390/molecules21091141