1. Introduction
Lipases are ubiquitous enzymes which can be found in animals, plants, fungi and bacteria [
1,
2]. Naturally, lipases catalyze the hydrolysis of esters formed of glycerol and long-chain fatty acids [
3,
4]. However, under particular conditions, they can catalyze esterification and transesterification [
5] with high activity, specificity and selectivity. Therefore, lipases represent a significant class of industrially relevant enzymes [
6].
Lipases represent a peculiar mechanism of action, the so called interfacial activation [
7,
8]. Due to a loop containing α-helicoidal elements that covers the active site, a large proportion of lipases have two different conformations (the closed and the open forms) [
4,
9]. In an aqueous environment, the equilibrium is shifted towards the water soluble closed form and the active site is secluded from reaction medium [
4,
10,
11]. Catalysis takes place in the open-lid form which can be stabilized by interfacial activation at the interface of the drops of the oily phase containing hydrophobic substrates [
12]. These structural changes related to the interfacial activation are crucial to provide the substrates access to the active site. The performance of enzymes in organic solvents facilitated their application as biocatalysts [
13], even in supercritical medium [
14].
The lipase B from
Candida antarctica (
CaLB) is one of the most frequently used lipase in biotechnology due to its excellent biochemical properties such as resistance to organic solvents, high stereo- and enantioselectivity [
2,
9,
15]. The three-dimensional structure of
CaLB has been resolved by Uppenberg [
16,
17].
CaLB—having a molecular weight of 33 kDa and isoelectric point of 6.8—contains only a small lid covering the active site [
18]. Therefore, it was thought that the typical interfacial activation of lipases [
7,
8] is not a characteristic feature of this enzyme [
19]. However, recent structural investigations revealed the open and closed states of
CaLB and the mechanism of interfacial activation [
20].
Replacing soluble enzymes with immobilized preparations in synthetic processes can significantly reduce the cost of enzyme production and complex downstream processing [
21,
22]. Immobilization of enzymes is a powerful biochemical tool with a potential for improving enzyme activity, temperature stability, and selectivity [
23]. Retaining the active conformation during immobilization is very important for enzyme activity [
24]; therefore, finding an immobilization protocol that increases enzyme activity and stability should be a target of real importance [
25]. Several immobilization techniques are available for protein immobilization: adsorption, covalent attachment onto solid supports, cross-linking and entrapment of protein in matrices. Generally, a support for enzyme immobilization must meet two requirements: it should contain an adequate amount of functional groups on the surface and it should be mechanically stable for repeated use or in application in continuous processes [
21]. Adsorption of lipases on hydrophobic surfaces is easy to perform because they resemble the surface of their natural substrates [
4], but enzyme leaching could be a serious disadvantage [
22,
26]. After adsorption on the hydrophobic surface, post-immobilization with glutaraldehyde (GA) is frequently used [
2]. GA modifies primary amino groups of proteins and it is also an effective cross linker used to produce CLEAs (Cross-linked enzyme aggregate) [
27]. GA can be used for activating the primary amino groups of the support followed by covalent immobilization [
3,
25]. Entrapment could be a proper immobilization method for enzymes which can be easily deactivated as in this way the three dimensional structure of enzyme could be stabilized via rigidification by the entrapping matrix [
28].
In the past few decades, intensive studies were performed on development of supports for lipase immobilization. The most straightforward strategy was to utilize hydrophobic supports for lipase immobilization in their active conformation by simple adsorption advancing interfacial activation. Hydrophobic organic materials such as octyl-sepharose or octadecyl-polyacrylate [
4] and polyethyleneimine-agarose [
29] were successfully applied in adsorptive immobilization where lipase stayed fully active even after a very long incubation in organic solvents. Polymer supports with core-shell morphology could tune lipase properties during immobilization [
12]. Similarly, surface-grafted mesoporous silica gel as inorganic material proved to beneficial for adsorptive immobilization of lipases [
2,
30,
31,
32].
Although lipases after adsorptive immobilization can retain their activity in organic media but under aqueous conditions they can release the enzyme causing decrease of activity and product contamination. To circumvent the problem of enzyme leaching, covalent immobilization offered a solution. For example, activated natural polymers (epichlorohydrin- or glutaraldehyde-activated chitosan or agarose gels) proved to be suitable carriers for multipoint covalent immobilization of proteins [
3,
33] or silica gel grafted with glyoxyl groups enabled covalent enzyme immobilization [
34].
Epoxy or multifunctional epoxy-supports were suitable for covalent immobilization of enzymes [
32,
35,
36]. Epoxy supports—while being stable at neutral pH values for a long time in wide temperature range—could react with different nucleophilic groups (such as amine, thiol or carboxylate) on the protein surface under mild conditions by forming stable covalent bonds which prevents enzyme leakage [
21,
37,
38]. The usefulness of epoxy-functionalized carriers [
33,
34,
35,
36,
37,
38,
39] led to the idea of using bisepoxides for enzyme immobilization. Thus, poly(ethylene glycol) diglycidyl ether was applied for binding various oxidases onto biosensor microelectrode [
40]. Later, glycerol diglycidyl ether was used as a cross-linking agent for immobilization of lipases and phenylalanine ammonia-lyase (PAL) as cross-linked enzyme aggregates (CLEA) [
41] or binding enzymes such as PAL [
42] or
CaLB [
43] onto carbon nanotubes.
Besides enzyme immobilization, reactor technology can also affect the efficiency of biotransformations [
44]. Nowadays, continuous-flow systems are of ever-increasing importance since they offer facile automation, reproducibility and safety [
45,
46]. In addition to the ease of studies on substrate concentration, temperature and flow rate and how these factors effect enzyme-catalyzed reactions in such systems, the catalytic efficiency of the immobilized enzymes proved to be higher in packed-bed reactors operated in continuous-flow mode than in batch mode [
47,
48,
49]. Noteworthy, the mechanical damages reducing the operational stability of the immobilized biocatalysts in stirred or shaken batch reactors is much less pronounced in packed bed reactors. The influence of various immobilization conditions [
48,
50] and of ionic liquids [
51] on biocatalytic properties of
CaLB was already evaluated in continuous-flow processes.
The aim of this study was to investigate the activation of mesoporous aminoalkyl polymer resins using low-cost bisepoxides in covalent immobilization of lipase B from Candida antarctica (CaLB) offering a less-toxic, inexpensive and easy-to-handle alternative over glutaraldehyde-activation of such carriers. A major concern was to fine-tune the length, flexibility and hydrophobicity of the spacer arm by varying aminoalkyl functions and bisepoxides, thereby resulting in significant variation in the properties of the CaLB biocatalysts by altering the microenvironment surrounding the enzyme molecule during and after immobilization. Variation of the spacer arm offers tuning and optimization possibilities in biocatalyst design and development and can lead to improved properties useful for extended use in recycled batch reactions or in continuous-flow systems even at elevated temperatures.
2. Results and Discussion
Selection of the supports was a crucial point for this study on surface fine-tuning exploring variations created by different bisepoxide spacer arms. Besides the functions on the surface, pore size and particle size were important parameters influencing the final properties of resins in various applications.
2.1. Selection and Bisepoxide Activation of Carriers Used for Covalent Immobilization of CaLB
Because a final goal was to apply the immobilized
CaLB derivatives in continuous-flow reactors, particle size influencing pressure drop and pore diameter affecting specific area for immobilization and mass transfer properties were important parameters. Thus, two commercially available macroporous polymethyl methacrylate enzyme carriers functionalized with ethylamine (EA) and hexylamine (HA) at their surface were selected as starting supports for this study. The properties of the resins and approximate density of ethylamine and hexylamine functional groups on resin surfaces are presented in
Table 1. Worth mentioning is that the different lengths of ethylamine and hexylamine functional groups on the two starting carriers can contribute to the different properties of the final spacer arms between the surface of carriers and the target protein.
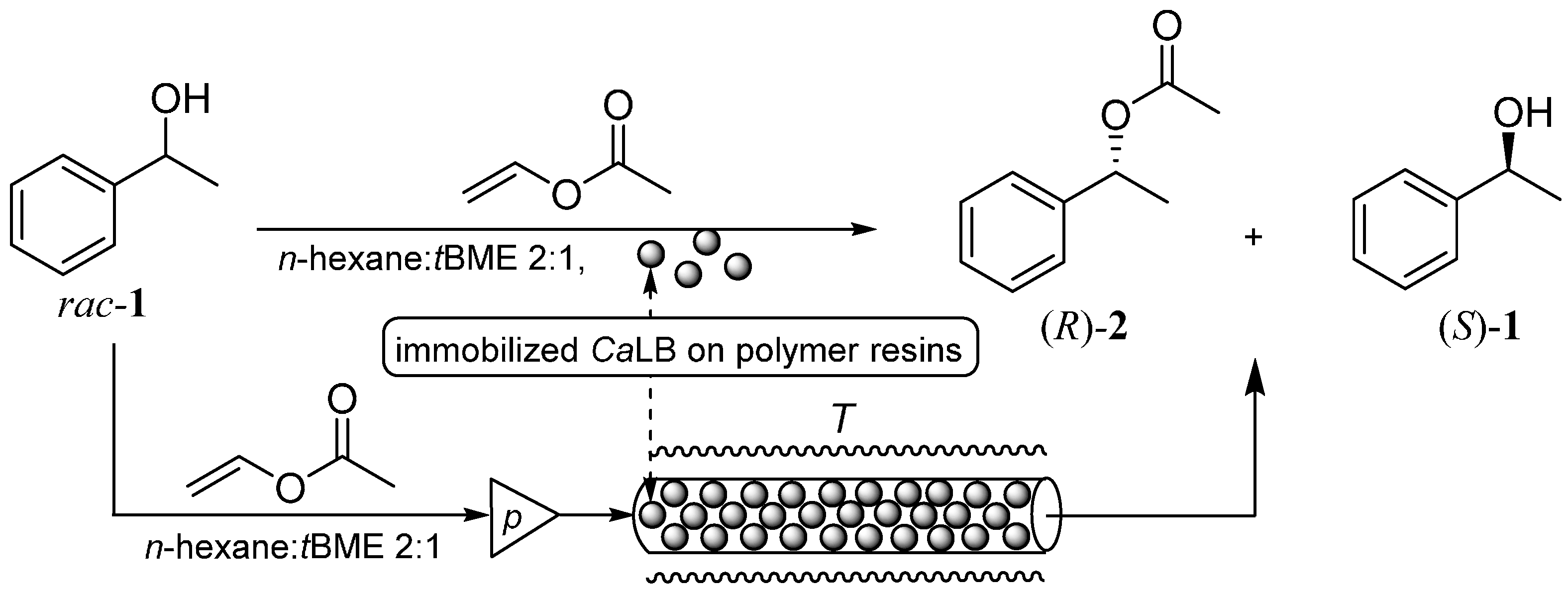
2.2. Covalent Immobilization of CaLB on Bisepoxide-Activated Supports and Its Properties in Kinetic Resolution of 1-Phenylethanol rac-1
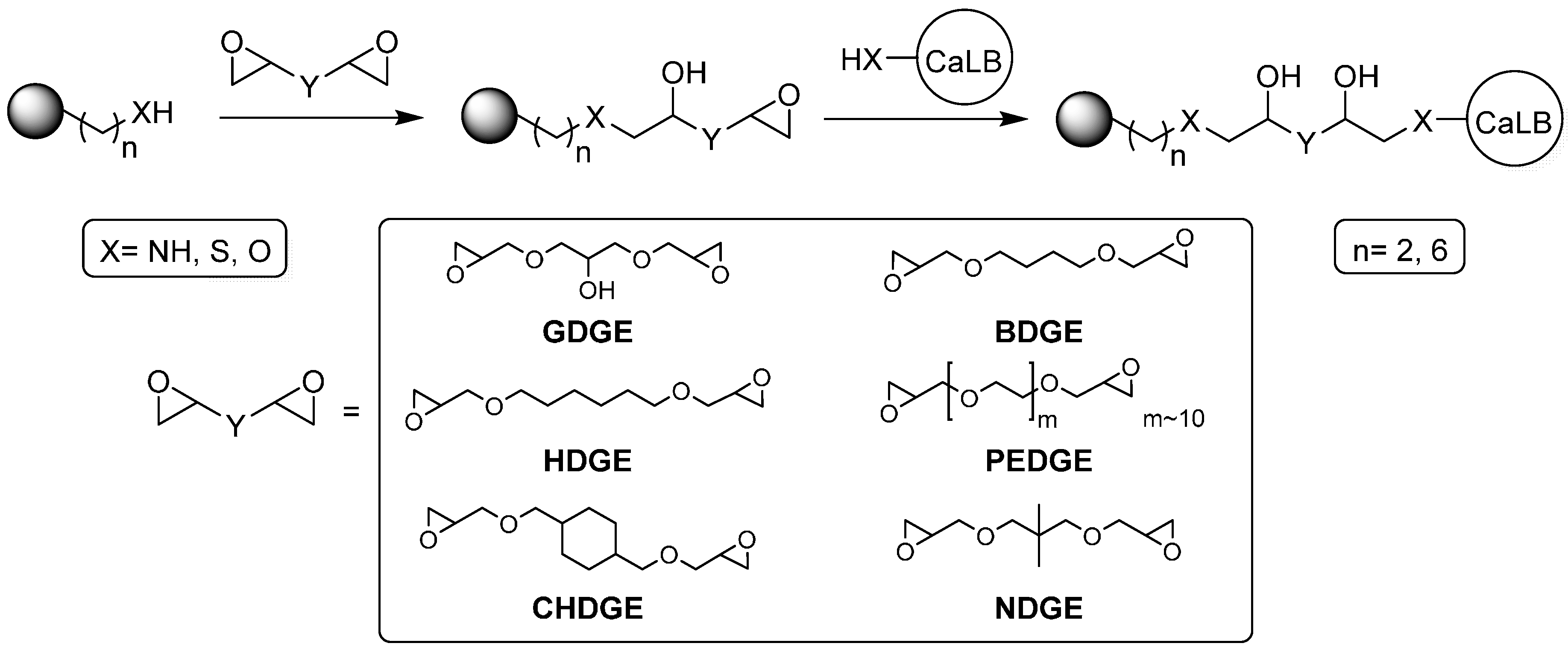
Due to their quite high content of water (~70%), drying of the resins was required to determine their real mass before surface modification (see
Section 3.3.). In the first set of experiments, six different types of bisepoxides with different lengths and hydrophobicities were selected as activating agents for the two aminoalkyl carriers (
Figure 1). As reference, glutaraldehyde activation of the two carriers was also performed. After pre-activation, immobilization of
CaLB was carried out in phosphate buffer (pH 7.5) at room temperature (24 h).
Because the epoxide functions may form covalent bonds not only via the surface exposed amine functions of Lys residues but also via the S- and O-atoms of Cys, Tyr, Asp, Glu, covalent bond formation probability may be higher with the bisepoxide-activated supports than with the GA-activated ones, especially in the case of proteins having only a few surface exposed lysine residues. These differences may explain the higher activity of the immobilized CaLB achieved with several bisepoxide-activated carriers compared to the GA-activated ones. Moreover, the C−X bonds (X = NH, S, O) forming by immobilization with the bisepoxide-activated carriers, unlike the C=N bonds forming in GA-activated ones, are not susceptible to hydrolysis.
Worth mentioning is that GA-activated resins are not suitable for long-term storage and they should be used for immobilization shortly after activation. On the other hand, the bisepoxide-activated supports—similarly to the usual epoxy carriers—can be stored for a long time enabling real separation of the carrier activation and enzyme immobilization steps in time.
To characterize the catalytic performance of the biocatalysts, kinetic resolution of 1-phenylethanol (
rac-
1) was selected as the test reaction (
Figure 2). This test enabled gaining information on the activity and enantiomer selectivity of the
CaLB variants. To distinguish between the fraction of
CaLB retained by only physical adsorption from the one retained by stronger covalent bonds, the activity of the preparations was tested before and after washing with Triton X-100 non-ionic detergent solution which could remove enzyme molecules attached only by hydrophobic interactions onto the surface.
Table 2 shows the wash resistance representing the covalent ratio of binding onto activated EA- and HA-resins and the biocatalytic performance of the covalently bound
CaLB preparations.
For comparison, a commercial preparation (EP
CaLB) was selected as a standard in which
CaLB was covalently attached to macroporous acrylic beads of 150–300 µm particle size by the aid of epoxy functions (EP). In the case of EP
CaLB, washing the preparation with Triton X-100 solution resulted in 125% activation possibly due to a slight bioimprinting effect [
53]. In case of EA
CaLB, the 7% wash resistance was consistent with the fact that the non-activated resin did not contain groups for covalent attachment and only ionic interactions could retain the enzyme molecules causing residual activity. In case of HA
CaLB, the alkyl chain of hexylamine function rendered the surface more hydrophobic compared to EA-resin, thus a combination of the increased hydrophobicity and ionic interactions could rationalize the slightly higher wash resistance (11%).
When polyethylene diglycidyl ether (PEDGE) was used as activating bisepoxide, the wash resistance remained quite similar as observed for the unmodified resins (7% for EA-PEDGE CaLB and 17% for EA-PEDGE CaLB). This result indicating good adsorption ability but only occasional covalent bond formation could be explained by assuming that during the activation both epoxy functions of the bisepoxide with a long spacer arm of high flexibility could react with the surface amine functions. Thus, only a few epoxy functions of PEDGE remained intact after the activation step explaining the low frequency of covalent bond formation of the PEDGE-activated resin with the enzyme.
In contrast, high wash resistance was obtained with
CaLB immobilized on 1,4-cyclohexanedimethanol diglycidyl ether (CHDGE)-, 1,6-hexanediol diglycidyl ether (HDGE)- and glycerol diglycidyl ether (GDGE)-activated resins (81%, 58% and 53%, respectively). This result can be attributed to the hydrophobic character of CHDGE, since cyclohexyl rings increased the hydrophobicity of support. Barbosa
et al. proved that proteins become immobilized on epoxy supports via a two-step mechanism: physical adsorption occurs in the first place then covalent linkages are formed between the nucleophilic residues on the enzyme molecule and epoxy functions of the support [
15]. Thus, enhanced hydrophobicity of the spacer arm with cyclohexyl ring could result in stronger hydrophobic activation and consequently lead to
CaLB forms of higher activity. A comparison of the activation of the alkylamino resins with bisepoxides to the widely applied glutaraldehyde activation indicated the value of the activation by a properly selected bisepoxide. The usefulness of a bisepoxide-activation-based approach was indicated clearly by the approximately four-fold activity of the
CaLB attached to CHDGE-activated supports (
Ub = 39.6 µmol·min
−1·g
−1 for EA-CHDGE
CaLB and
Ub = 47.6 µmol·min
−1·g
−1 for HA-CHDGE
CaLB) compared to the activity of the corresponding
CaLB form immobilized by the aid of the widely used glutaraldehyde-activation (
Ub = 10.2 µmol·min
−1·g
−1 for EA-GA
CaLB and
Ub = 11.9 µmol·min
−1·g
−1 for HA-GA
CaLB). The importance of the length of spacer arms became apparent by analysis of the differences between bisepoxide-activation by various bisepoxides. Within both series (EA-resin-based or HA-resin-based), the highest degree of covalent immobilization (wash resistance) was achieved after CHDGE activation (92% with HA-resin and 81% with EA-resin). HDGE and GDGE activation resulted in a somewhat lower degree of covalent binding (82% and 64% with HA-resin and 58% and 53% with EA-resin, respectively). However, only a modest degree of covalent binding was observed after BDGE-activation (51% with HA-resin and 22% with EA-resin).
Analysis of the activities of the various forms of CaLB covalently immobilized on pre-activated resins indicated a role of the alkylamino function of the resins influencing the properties of immobilized CaLB. Residual activity of CaLB attached to the modified HA-resins after activation with any bisepoxide or glutaraldehyde was higher than that attached to the corresponding pre-activated EA-resin. Because the trends within the two series were the same, optimization of the bisepoxide-activation conditions for CaLB immobilization on either alkylamino resins was performed only with the three best bisepoxides (CHDGE, HDGE and GDGE).
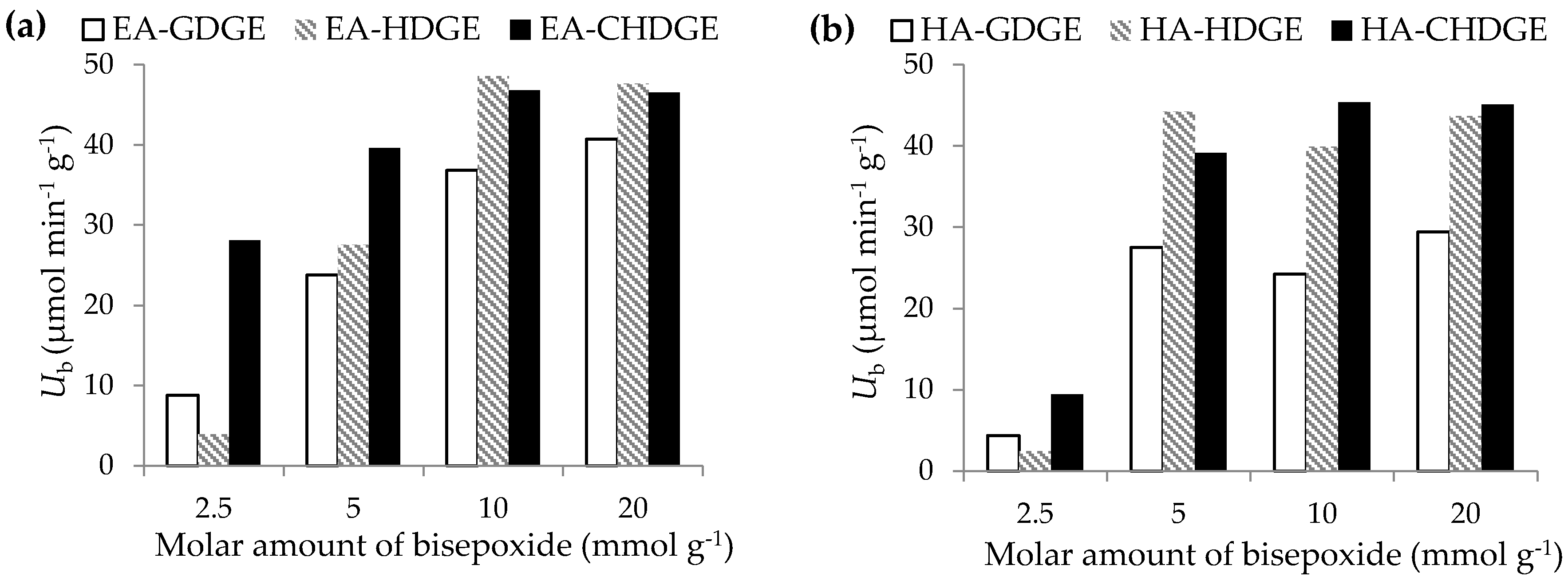
2.3. Optimization of the Bisepoxide Activation for Covalent Immobilization of CaLB
The microenvironment of enzymes after immobilization can be tuned by proper optimization of the process parameters. Our preliminary results applying bisepoxides in 5 mmol·g
−1 of carrier amounts showed (
Table 2) that aminoalkyl (EA or HA)-resin-activation by three bisepoxides were efficient for immobilization of
CaLB. In this series of experiments, the amount of bisepoxides applied for surface modification of the two aminoalkyl resins was varied in order to investigate the effect of final epoxy group density on enzyme activity and immobilization yield (
Figure 3). Reduction of the amount of bisepoxides to 2.5 mmol·g
−1 carrier caused dramatic decrease in specific activity of EA-HDGE
CaLB and EA-GDGE
CaLB. Increase of the molar amount of bisepoxides from 5 mmol·g
−1 carrier to 10 mmol·g
−1 carrier increased the specific activity of the immobilized
CaLB preparations. A further increase of the amount of bisepoxides to 20 mmol·g
−1 carrier resulted in negligible increase of activity of the immobilized
CaLB compared to that of achievable by
CaLB on the carriers treated by 10 mmol bisepoxide g
−1 carrier. Thus, for both aminoalkyl resins, the optimal amount of bisepoxide for surface modification was 10 mmol·g
−1 carrier.
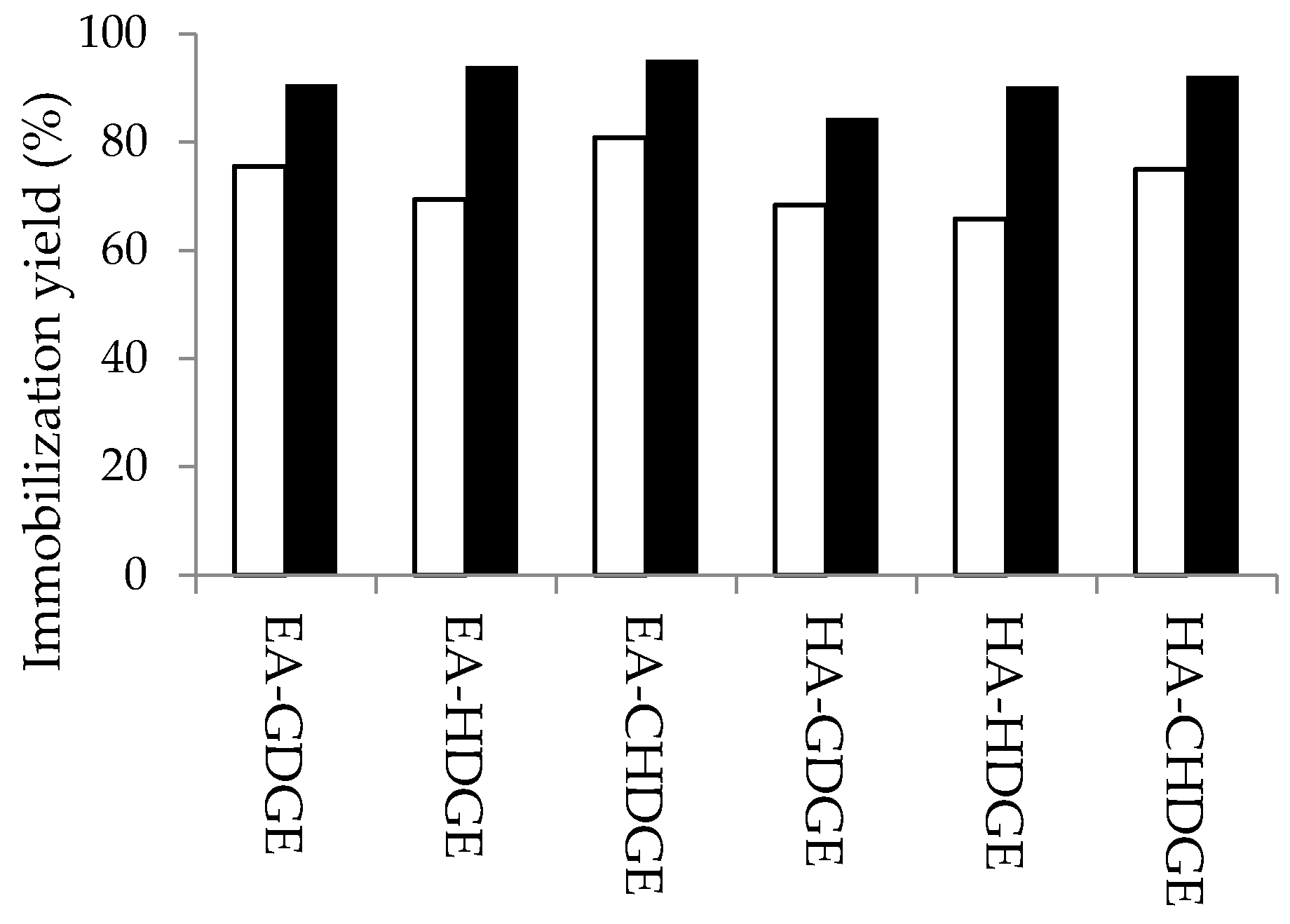
This result was confirmed for the other types of bisepoxides by protein concentration assays as well. Immobilization yields for 12 variants of bisepoxide-activated supports were determined by protein concentration measurements in the supernatant before and after immobilization of
CaLB according to Bradford’s method. The results for EA- and HA-resins modified with 2.5 and 10 mmol·g
−1 bisepoxides (CHDGE, HDGE and GDGE) are shown in
Figure 4.
Our results indicated that immobilization yields with the other two bisepoxides (CHDGE, HDGE) were also significantly higher after bisepoxide-activation with the optimal amount of bisepoxides (10 mmol·g
−1 carrier) as compared to the resins modified with only 2.5 mmol bisepoxide g
−1 carrier. Supports modified with CHDGE showed highest immobilization yields (EA-CHDGE
CaLB: 95% and HA-CHDGE
CaLB: 92%) which also confirmed the role of proper hydrophobicity of the linker which is beneficial for the first lipase adsorption step during the covalent immobilization on epoxy-activated supports [
15].
2.4. Operational Stability of the CaLB Preparations
One of the great advantages of immobilized enzyme preparations is the possibility of re-use in repeated batch reactions, thus making the process more cost-effective [
54]. Five
CaLB preparations (EA-GDGE
CaLB, EA-CHDGE
CaLB, HA-GDGE
CaLB, HA-CHDGE
CaLB, HA-HDGE
CaLB) were selected for studying their stability during recycling. To test their operational stability, the
CaLB biocatalysts were tested by repetitive cycles of KRs of
rac-1 30 °C for 1 h in a shaken test tube (1000 rpm, in hexane/
t-butyl methyl ether/vinyl acetate 6/3/1). Between each cycle, the
CaLB biocatalysts were washed with
n-hexane. The relative residual activities of repeated batch experiments compared to the first experiment as 100% are presented in
Figure 5. Although every biocatalyst used in this study lost its initial activity cycle-by-cycle, HA-HDGE
CaLB retained 90% of its initial activity after 13 cycles. This demonstrated that spacer arms with proper length and hydrophobicity between enzyme molecule and support could contribute not only to the initial activity but also to the long-term stability of the immobilized enzyme. The EA-GDGE
CaLB in which the enzyme was attached by the shortest carbon chain retained only 70% of its initial activity after 13 cycles. Furthermore,
CaLB immobilized on EA- or HA-resin activated with the cyclohexyl ring containing CHDGE preserved 82% of their initial activity regardless of which resin was used.
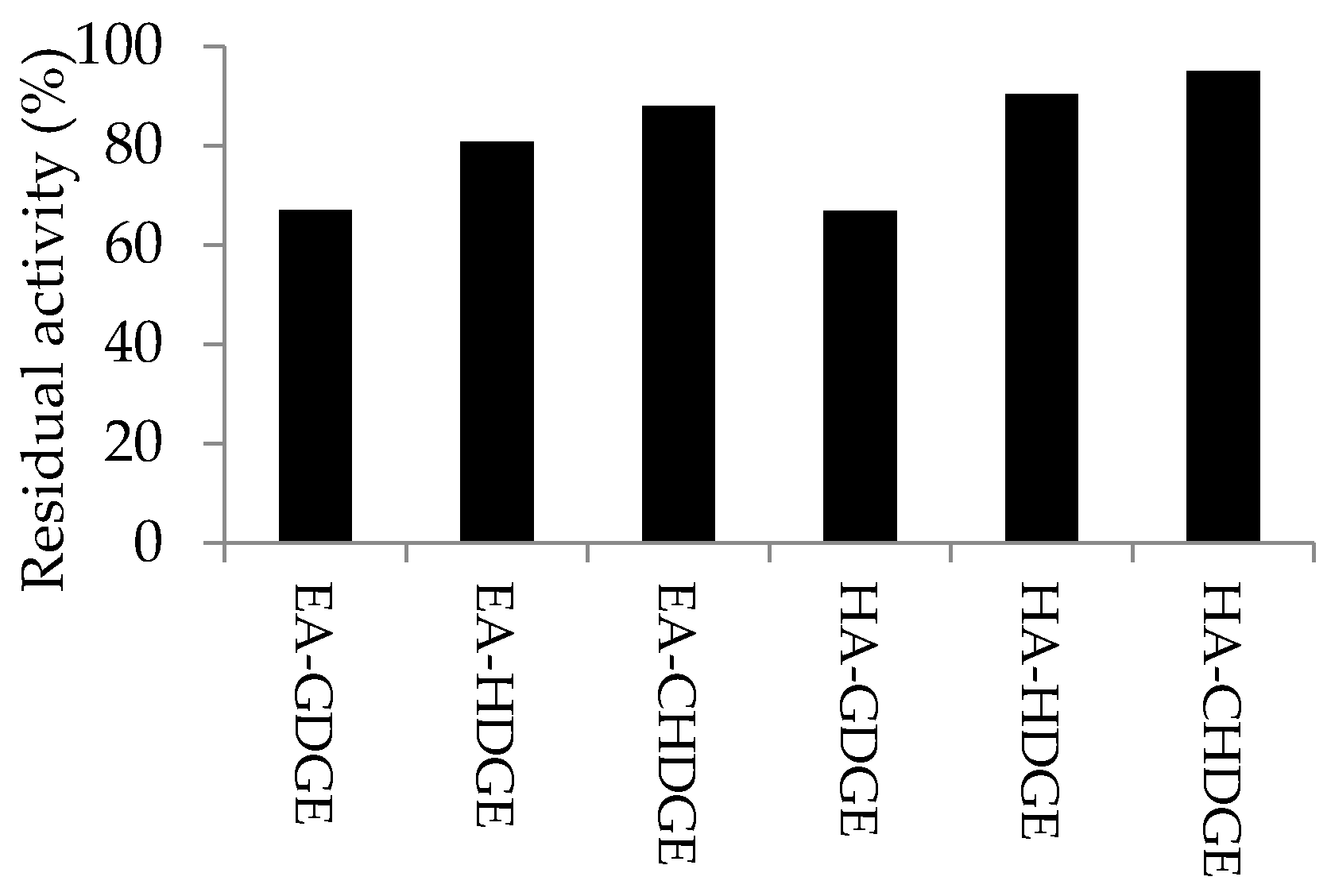
2.5. Long-Term Storage Stability of the CaLB Preparations
The long-term storage stability of
CaLB preparations were studied by storing the dry biocatalysts in screw capped vials at 4 °C for 12 months. After 12 months, the residual activity of the stored
CaLB preparations was compared to the activity of the freshly prepared biocatalysts (results are shown in
Figure 6). After 12-month storage, HA-CHDGE
CaLB and HA-HDGE
CaLB preserved most of their initial activity (95% and 90%, respectively). Presence of the cyclohexyl ring in the linker was beneficial to preserve enzyme activity in case of
CaLB attached to modified EA-resin (88% residual activity with EA-CHDGE
CaLB), while EA-GDGE
CaLB preserved only 67% of its initial activity.
2.6. Continuous-Flow Kinetic Resolution of Racemic 1-Phenylethanol (rac-1) Catalyzed by CaLB Preparations on Bisepoxide-Activated Resins—Substrate Concentration and Temperature Effects
Immobilization of enzymes and retention of biocatalysts played a major role in development of continuous-flow processes [
42,
45,
46,
47,
55,
56]. It was previously shown that the mode of immobilization [
46] and operation temperature [
45,
50] could significantly influence KR processes in continuous-flow bioreactors. Therefore, six different
CaLB preparations from this study were selected to investigate the effect of substrate concentration and temperature on KR of
rac-
1 catalyzed by the covalently immobilized enzyme. As a reference, this study was extended with the commercially available EP
CaLB which contained
CaLB covalently immobilized onto epoxy-functionalized acrylic resin.
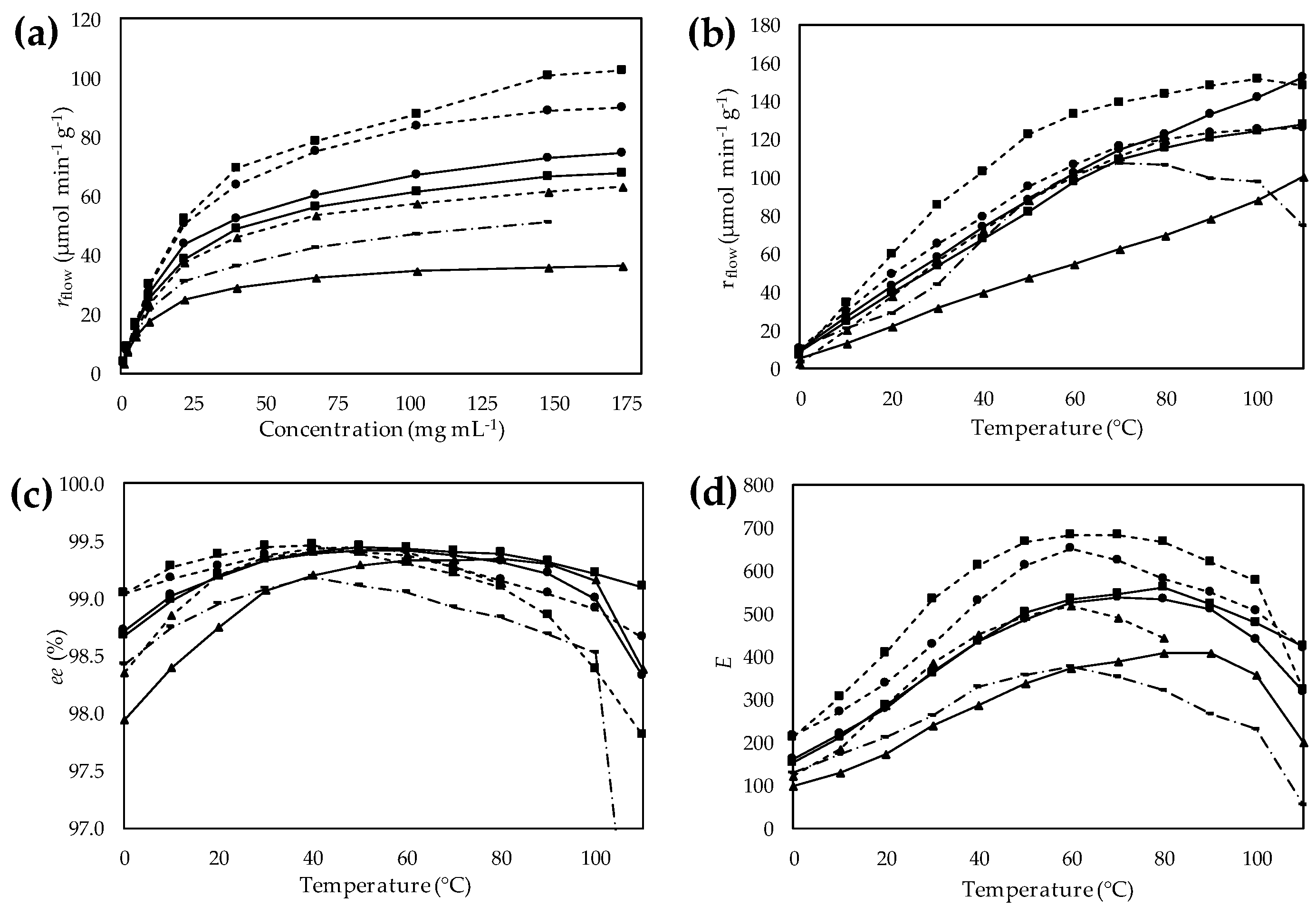
During the substrate concentration dependency investigations, stainless-steel columns filled with the actual immobilized
CaLB preparation were thermostated at 30 °C and a solution containing vinyl acetate and the substrate at various concentrations was pumped through the column at a constant flow rate. The productivity of the various
CaLB preparations (
rflow, µmol·g
−1·min
−1) was investigated as a function of substrate concentration (
Figure 7a). The 2.5-fold higher activity of the most active HA-HDGE
CaLB than that of the commercially available EP
CaLB indicated the significance of fine-tuning the bisepoxide activation for covalent immobilization. Similar to KRs of
rac-
1 in batch,
CaLB on EA- and HA-resins activated with GDGE showed the lowest activity (EA-GDGE
CaLB 29.2 µmol·g
−1·min
−1; HA-GDGE
CaLB 46.1 µmol·g
−1·min
−1) among tested derivatives. The behavior of
CaLB on resin-surfaces grafted with epoxy function via a linker containing cyclohexyl ring (EA-CHDGE
CaLB and HA-CHDGE
CaLB derivatives) demonstrated that this lipase interacting with hydrophobic groups became more active in flow systems as well. After an initial quasi-linear range, the productivity–substrate concentration curve showed the typical saturation with all seven
CaLB preparations (
Figure 7a). The rapidly increasing range of
rflow–[(
R)-
1] curves ended at around 40 mg·mL
−1; therefore, the temperature effect studies were performed at this substrate concentration.
A major goal of this study was to gain information on the thermal stability of
CaLB linked to polymer resins by spacer arms with different length and hydrophobicity. Thus, KR of
rac-
1 was carried out in bioreactors filled with the selected
CaLB preparations similarly as in the substrate-concentration tests but varying the temperature in the range of 0–110 °C in 10 °C steps (
Figure 7b–d).
Productivity (
rflow) of the six selected
CaLB derivatives on bisepoxide-activated resins as a function of temperature was compared to EP
CaLB containing the lipase immobilized on epoxy-functionalized acrylic resin (
Figure 7b). The thermal behavior of
CaLB attached to bisepoxide activated resins differed significantly from EP
CaLB (linking the enzyme to the resin by a quite short linker). The
rflow of EP
CaLB increased only up to 70 °C, then a significant drop of enzyme activity could be observed. In contrast, productivity of
CaLB immobilized on EA-resin activated by CHDGE and GDGE bisepoxide increased almost linearly with increasing temperature up to 110 °C indicating the importance of properly selected linkers to achieve significant improvement of thermal stability. The productivity of
CaLB immobilized via longer and more flexible linkers (the HDGE-activated EA-resin and the CHDHE- or GDGE-activated HA-resins) increased also uninterruptedly up to 110 °C, although with a slowdown in the increase starting at around 70 °C. HA-HDGE
CaLB involving the longest and most flexible linker proved to be the least thermostable of all the six
CaLB forms attached to bisepoxide-activated resins with a drop of enzyme activity at 100 °C.
Besides the productivity of the immobilized enzyme, dependence of enantiomer selectivity of the KR (
R)-
1 on temperature is also an important issue. Thus, enantiomer selectivity of the process was characterized as a function of temperature by the enantiomeric excess of product (
R)-
1 (
ee) and by the enantiomeric ratio (
E) (
Figure 7c,d, respectively). The
ee–temperature (
Figure 7c) and
E–temperature (
Figure 7d) curves exhibited maxima at various temperatures between 50 and 80 °C. This behavior was in agreement with previously found maxima of
E–temperature curves in kinetic resolutions of secondary alcohols [
45,
46,
50], although different resins revealed these maxima at different temperatures (
Figure 7d). Characteristic differences of enantiomer selectivity optima were found between
CaLB on bisepoxide activated EA-resins (~80 °C) compared to
CaLB on bisepoxide activated HA-resins (~60 °C) indicating that increased flexibility of the linker allows a lower degree of selectivity. At 60 °C,
CaLB immobilized on HA-resin activated by HDGE showed the highest enantiomer selectivity among all tested preparations (
E ~ 700 with
ee(R)-2 = 99.3%), while the HA-GDGE
CaLB with shorter and less hydrophobic spacer arm showed a lower degree of enantiomer selectivity (
E ~ 490 with
ee(R)-2 = 99.2%). On the other hand,
CaLB immobilized on EA-resins activated by the three bisepoxides (HDGE, CHDGE and GDGE) displayed maxima in enantioselectivity at around 80 °C (
E ~ 550, 530 and 380, respectively). This is a remarkable shift compared to the enantiomer selectivity maxima of
CaLB immobilized on HA-resins at a 20 °C lower temperature. Worth mentioning is that enantiomer selectivity maxima of both bisepoxide-activated series exceeded the 50 °C optimum found for EP
CaLB without a long spacer between the resin surface and the enzyme.
2.7. Characterization of Support Morphology after Recycling and Application in Continuous-Flow Reactors
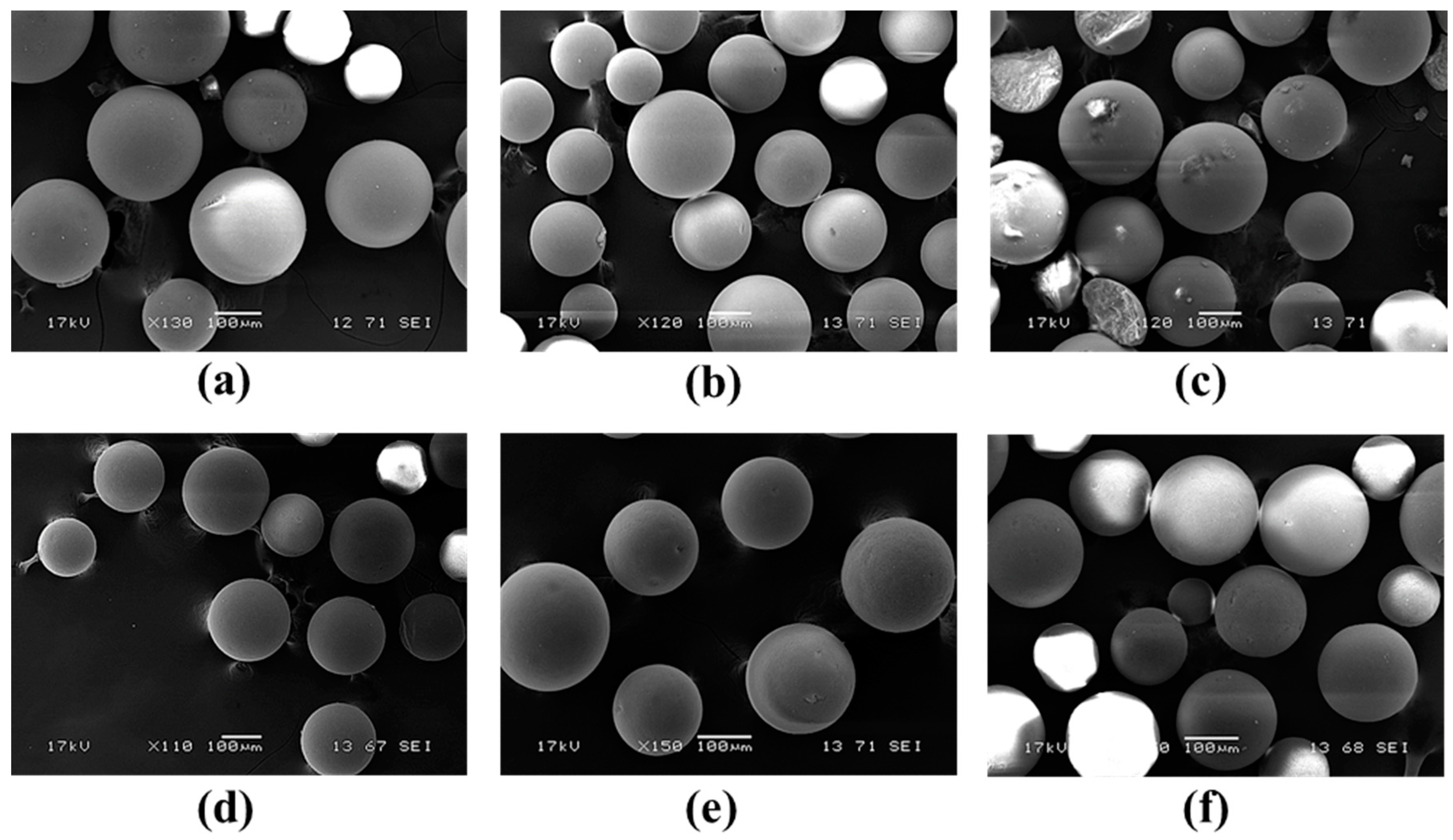
In addition to their biocatalytic properties, mechanical stability of the immobilized biocatalysts is also an important issue. Thus, morphology of two selected
CaLB forms (on the GDGE-activated EA-resin and on the HDGE-activated HA-resin) representing both aminoalkyl-resin based series was investigated by scanning electron microscopy (SEM) before their use, after the recycling study and after the temperature dependence study (
Figure 8).
SEM investigations of EA-GDGE
CaLB and HA-HDGE
CaLB before their use in biotransformations revealed intact morphology of spherical beads with diameters 150–300 µm (
Figure 8a,d, respectively), indicating no mechanical damage during bisepoxide activation and enzyme immobilization steps. Remarkably, both
CaLB preparations proved to be mechanically quite durable and remained intact even after 13 cycles of KR experiments in batch mode (
Figure 8b,e). However, the SEM pictures after the temperature dependency study of KRs, performed in packed-bed reactors using continuous-flow mode in the 0–110 °C temperature range, indicated limitations in the temperature stability of these
CaLB forms on polymer beads (
Figure 8c,f). Although data sheets of the basic EA- or HA-resins declared them to be stable up to 60 °C, we tested
CaLB on their bisepoxide-activated derivatives up to 110 °C in continuous-flow reactors. Thus, the result indicating fracture of EA-GDGE polymer beads at this elevated temperature was not surprising (
Figure 8c). Remarkably, HA-HDGE beads remained apparently unaltered even after the temperature-dependency study ended at 110 °C.
3. Materials and Methods
3.1. Materials
Bisepoxides [1,6-hexanediol diglycidyl ether (HDGE), neopentylglycol diglycidyl ether (NDGE), 1,4-cyclohexanedimethanol diglycidyl ether (CHDGE), 1,4-butanediol diglycidyl ether (BDGE), poly(ethylene glycol) diglycidyl ether (PEDGE)] were products of Ipox Chemicals Ltd. (Budapest, Hungary). Racemic 1-phenylethanol (rac-1), vinyl acetate, glutaraldehyde (GA) solution (25% w/v in H2O); hydrochloric acid, glycerol diglycidyl ether (GDGE) and Triton™ X-100 [4-(1,1,3,3-tetramethylbutyl)phenyl-polyethylene glycol] were purchased from Sigma-Aldrich (Milwaukee, WI, USA). NaH2PO4, Na2HPO4 and sodium hydroxide were purchased from Merck KGaA (Darmstadt, Germany).
Solvents (n-hexane, t-butyl methyl ether, ethanol, Patosolv® (mixture of ethanol/isopropanol, t-butanol), propan-1-ol and isopropanol from Molar Chemicals Kft. (Budapest, Hungary) were dried and/or freshly distilled prior to use.
Ethylamine (EA)- and hexylamine (HA)-functionalized methacrylic polymer resins (ReliZyme™ EA 403 and ReliZyme™ HA 403; polymethyl methacrylate supports, particle size 150–300 µm, pore size 400–600 Å) were purchased from Resindion S.r.l. (Rome, Italy).
Lipase B from Candida antarctica (CaLB, lyophilized) was purchased from c-LEcta GmbH (Leipzig, Germany). EP CaLB (Candida antarctica lipase B, T2-150, covalently attached to dry acrylic beads with a 150–300 µm particle size) was the product of ChiralVision BV (Leiden, The Netherlands).
3.2. Analytical Methods
GC analyses were carried out on Agilent 4890 instrument equipped with FID detector using H2 carrier gas (injector: 250 °C, FID: 250 °C, head pressure: 12 psi, split ratio: 50:1) on a Hydrodex β-6TBDM column (25 m × 0.25 mm × 0.25 μm film with heptakis-(2,3-di-O-methyl-6-O-t-butyldimethylsilyl)-β-cyclodextrine; Macherey & Nagel GmbH). tr (min): for 1 and 2 (oven: 120 °C, 8 min), 3.62 [(S)-2], 3.97 [(R)-2], 5.09 [(R)-1], 5.35 [(S)-1]. Conversion (c), enantiomeric excess (ee) and enantiomeric ratio (E) were determined by GC measurements with base-line separations of the enantiomers of 1 and 2 using precise integration methods.
Enantiomeric ratio (
E) was calculated from the conversion (
c) and enantiomeric excess of the product (
ee(R)-2) using the equation
E = ln[1 −
c(1 +
ee(R)-2)]/ln[1 −
c(1 −
ee(R)-2)] [
52]. Due to sensitivity to experimental errors,
E values calculated in the 100–200 range are given as >100, in the 200–500 range as >200 and above 500 as >>200.
To characterize the productivity of the biocatalysts, specific reaction rate (or specific biocatalyst activity) in batch reactions before (
Ub0) and after (
Ub) washing with Triton X-100 solution (for details of washing see
Section 3.6) were calculated using the equation
Ub =
n(R)-2/(
t ×
mB) (where
n(R)-2 [μmol] is the amount of the product,
t [min] is the reaction time and
mB [g] is the mass of the applied biocatalyst).
Specific reaction rates in continuous-flow systems (
rflow) were calculated using the equation
rflow = [(
R)-
2] ×
v/
mB (where [(
R)-
2] [μmol mL
−1] is the molar concentration of the product (
R)-
2,
v [mL·min
−1] is the flow rate and
mB [g] is the mass of the applied biocatalyst) [
45].
The surface morphology of the samples was investigated with a JEOL JSM-5500LV scanning electron microscope (SEM). The samples of immobilized CaLB were coated with gold prior to analysis. Electron beam energy of 25 kV was used for the investigations.
3.3. Drying of the Aminoalkyl Polymer Resins
The original water content (~60%–70%) of the ethylamine- (EA) and hexylamine-functionalized (HA) beads was removed prior to further use according to the following procedure: In a proper-sized flask, 50.0 g of resins were swirled with ethanol (2 × 100 mL; 2 × 5 min) and hexane (1 × 100 mL; 2 × 5 min), including filtration between the washing steps. After the final filtration, resins were dried at room temperature for 2 h followed by further drying in a VDL 23 vacuum drying chamber, Binder GmbH (Tuttlingen, Germany) at room temperature until the vacuum level dropped below 2 mbar.
3.4. Surface Activation of Aminoalkyl Polymer Resins with Bisepoxides or Glutaraldehyde
Dry polymer support (EA- or HA-resin, 500.0 mg) was added to a solution of bisepoxide (2.5 mmol, GDGE, HDGE, NDGE, CHDGE, BDGE or PEDGE) or glutaraldehyde (2.5 mmol) in propan-1-ol (15 mL). The suspension of polymer support in activating agent solution was shaken at 400 rpm for 24 h at 25 °C. The activated support was filtered off on a glass filter (G3), washed with Patosolv® (3 × 10 mL), dried at room temperature (4 h) and stored at 4 °C (in case of the GA-activated support, under argon).
3.5. Immobilization of CaLB on Bisepoxide-Activated Polymer Resins
CaLB (40.0 mg, lyophilized powder) was dissolved in phosphate buffer (10.0 mL, 100 mM, pH 7.5), and then the support (200.0 mg) was added to the solution. The enzyme-support suspension was shaken at 400 rpm for 24 h at 25 °C. The immobilized
CaLB preparation was filtered off on a glass filter (G3), washed with isopropanol (2 × 15 mL) and hexane (15 mL), dried for 4 h at room temperature and stored at 4 °C. Protein concentration of the
CaLB solution before immobilization and in supernatant after immobilization were determined according to Bradford’s method [
57]. As a standard, BSA was used.
Immobilization yield (IY) was calculated according to equation IY(%) = P/P0 × 100 (where P0 [mg·mL−1] is the initial protein concentration before immobilization, and P [mg·mL−1] is the protein concentration in supernatant after immobilization).
3.6. Lipase Desorption Tests
Samples of the immobilized CaLB preparations (80.0 mg of each) were washed at room temperature for 1.5 h with a 1% (v/v) solution of Triton X-100 in phosphate buffer (7.5 mL; 100 mM; pH 7.5) in a shaker at 400 rpm. The samples were then filtered off on a glass filter (G3) and resuspended in distilled water (15 mL) and incubated for 30 minutes at 400 rpm, filtered off and washed several times with distilled water, isopropanol (2 × 10 mL) and hexane (5 mL).
The “Wash resistance” was calculated using the equation Wash resistance (%) =
Ub/
Ub0 × 100 (where
Ub0 [μmol min
−1 g
−1] is the specific reaction rate achieved with the immobilized
CaLB right after immobilization and
Ub [μmol min
−1 g
−1] is the specific reaction rate after washing with Triton X-100 solution. For a definition of
Ub, see
Section 3.2).
3.7. Kinetic Resolution of 1-Phenylethanol (rac-1) Catalyzed by the CaLB Preparations in Batch Mode
In a screw capped glass vial (4 mL), the immobilized
CaLB biocatalyst (25.0 mg) was added to a solution of 1-phenylethanol (
rac-
1: 50.0 mg, 0.409 mmol) in a mixture of hexane/t-butyl methyl ether/vinyl acetate 6/3/1 (2.0 mL) and the mixture was shaken (1000 rpm) at 30 °C for 4 h. The reactions were analyzed by GC after 1, 2, and 4 h as described in
Section 2.2. All test reactions were performed in triplicates. Standard deviations of conversion were below 5%, standard deviations of enantiomeric excess were below 0.4%.
3.8. Optimization of the Bisepoxide Activation of Alkylamino Polymer Resins
Bisepoxide (GDGE, HDGE or CHDGE) in various molar amounts (1.25 mmol, 2.5 mmol, 5 mmol or 10 mmol) was dissolved in propan-1-ol (15 mL) and then polymer support (EA or HA, 500.0 mg) was added to the solution. The mixture was shaken (400 rpm) at 25 °C for 24 h. The bisepoxide-activated support was filtered off on a glass filter (G3), washed with Patosolv
® (3 × 10 mL), dried at room temperature (4 h) and stored at 4 °C. Catalytic properties of the
CaLB on the bisepoxide-activated support was tested (for details of immobilization, washing and testing see
Section 3.5,
Section 3.6 and
Section 3.7, respectively).
3.9. Recycling of the Immobilized CaLB Biocatalysts
Immobilized CaLB biocatalyst (25.0 mg) was added to a solution of 1-phenylethanol (rac-1, 50.0 mg, 0.409 mmol) in hexane/t-butyl methyl ether/vinyl acetate 6/3/1 (1.0 mL) in an Eppendorf tube and the mixture was shaken (1000 rpm) for 1 h at 30 °C. After 1 h, the reaction mixture was centrifuged, the immobilized CaLB biocatalyst was washed with hexane (2 × 1.0 mL), then fresh solution of rac-1 (50.0 mg, 0.409 mmol) in hexane/t-butyl methyl ether/vinyl acetate 6/3/1 (1.0 mL) was added to biocatalysts and samples were shaken again (1000 rpm) at 30 °C for 1 h. In this way, immobilized CaLB biocatalysts were tested in 13 consecutive cycles.
3.10. Long-Term Stability of CaLB Derivatives (Storage at 4 °C)
Residual activities of enzyme preparations (EA-GDGE
CaLB, EA-HDGE
CaLB, EA-CHDGE
CaLB, HA-GDGE
CaLB, HA-HDGE
CaLB, HA-CHDGE
CaLB) stored at 4 °C in glass vials closed with screw caps under argon for 12 months in kinetic resolution of
rac-
1 were determined as described in
Section 3.7.
3.11. Packed-Bed Columns Filled with Immobilized CaLB
Immobilized CaLB preparations [EP CaLB, EA-GDGE CaLB, EA-HDGE CaLB, EA-CHDGE CaLB, HA-GDGE CaLB, HA-HDGE CaLB, HA-CHDGE CaLB) were packed into stainless steel CatCart™ columns (stainless steel, inner diameter: 4 mm; total length: 70 mm; packed length: 65 mm; inner volume: 0.816 mL) according to the filling process of ThalesNano Inc (Budapest, Hungary). The columns were settled by silver metal [Sterlitech Silver Membrane from Sigma-Aldrich (Milwaukee, WI, USA), Z623237, pore size 0.45 µm; purity: 99.97%] and PTFE [Whatman® Sigma-Aldrich, Milwaukee, WI, USA, WHA10411311, pore size 0.45 µm] filter membranes. Two CatCart™ columns per enzyme preparation (separate columns for temperature and substrate concentration test) were packed (filling weights: EP CaLB, 250 ± 10 mg; HA-GDGE CaLB, 230 ± 13 mg; HA-HDGE CaLB, 220 ± 2 mg; HA-CHDGE CaLB, 230 ± 15 mg; EA-GDGE CaLB, 229 ± 5 mg; EA-HDGE CaLB, 212 ± 9 mg; EA-CHDGE CaLB, 214 ± 14 mg).
3.12. Kinetic Resolution of 1-Phenylethanol (rac-1) Catalyzed by the CaLB Preparations in Continuous-Flow Mode
Continuous-flow kinetic resolutions were performed in a laboratory flow reactor built from a Knauer K-120 isocratic HPLC pump attached to CatCart™columns filled with the immobilized CaLB biocatalysts in an in–house made aluminum metal block column holder with precise temperature control. The CaLB-filled columns were washed with a dry hexane/t-butyl methyl ether 2/1 mixture (1 mL·min−1, 20 min) before reactions with rac-1.
To study the effect of substrate concentration on the biocatalytic properties of immobilized
CaLB biocatalysts, the solution of 1-phenylethanol (
rac-
1) at different concentrations (1, 2.5, 5, 9.5, 22, 40, 68, 103, 148, 174 mg·mL
−1) and vinyl acetate (5 equiv.) in dry hexane/
t-butyl methyl ether 2/1 was pumped through the enzyme-filled columns set to 30 °C at a flow rate of 0.2 mL·min
−1. Samples were analyzed by GC every 10 min up to 40 min to allow the establishment of a stationary state. Samples were collected during stationary operation (40 min after changing the parameters) and analyzed as described in
Section 3.2.
3.13. Effect of Temperature on Kinetic Resolution of 1-Phenylethanol (rac-1) Catalyzed by the CaLB Preparations in Continuous-Flow Mode
The solution of 1-phenylethanol rac-1 (40.0 mg·mL−1) and vinyl acetate (5 equiv.) in hexane/t-butyl methyl ether 2/1 was pumped through the biocatalyst-filled columns thermostated to various temperatures (0–110 °C) at a flow rate of 0.2 mL·min−1. Samples were collected during stationary operation (40 min after changing the parameters) and analyzed as described above. The experiments were performed with 10 °C steps in a temperature range between 0 and 110 °C.
After completing experiments at different substrate concentrations and temperatures, the columns were routinely washed with hexane/
t-butyl methyl ether 2/1 mixture (0.5 mL·min
−1, 20 min) and then analyzed by scanning electron microscope (for details on SEM, see
Section 3.2).
 ) or hexylamine (
) or hexylamine (  ) resin activated by CHDGE (●), GDGE (▲) or HDGE (■). Relative activity (%) = activity in given cycle/activity in first cycle × 100 was determined in kinetic resolution of rac-1. Experiments were performed as described in Section 3.9.
) resin activated by CHDGE (●), GDGE (▲) or HDGE (■). Relative activity (%) = activity in given cycle/activity in first cycle × 100 was determined in kinetic resolution of rac-1. Experiments were performed as described in Section 3.9.
 ) or hexylamine (
) or hexylamine (  ) resin activated by CHDGE (●), GDGE (▲) or HDGE (■). Relative activity (%) = activity in given cycle/activity in first cycle × 100 was determined in kinetic resolution of rac-1. Experiments were performed as described in Section 3.9.
) resin activated by CHDGE (●), GDGE (▲) or HDGE (■). Relative activity (%) = activity in given cycle/activity in first cycle × 100 was determined in kinetic resolution of rac-1. Experiments were performed as described in Section 3.9.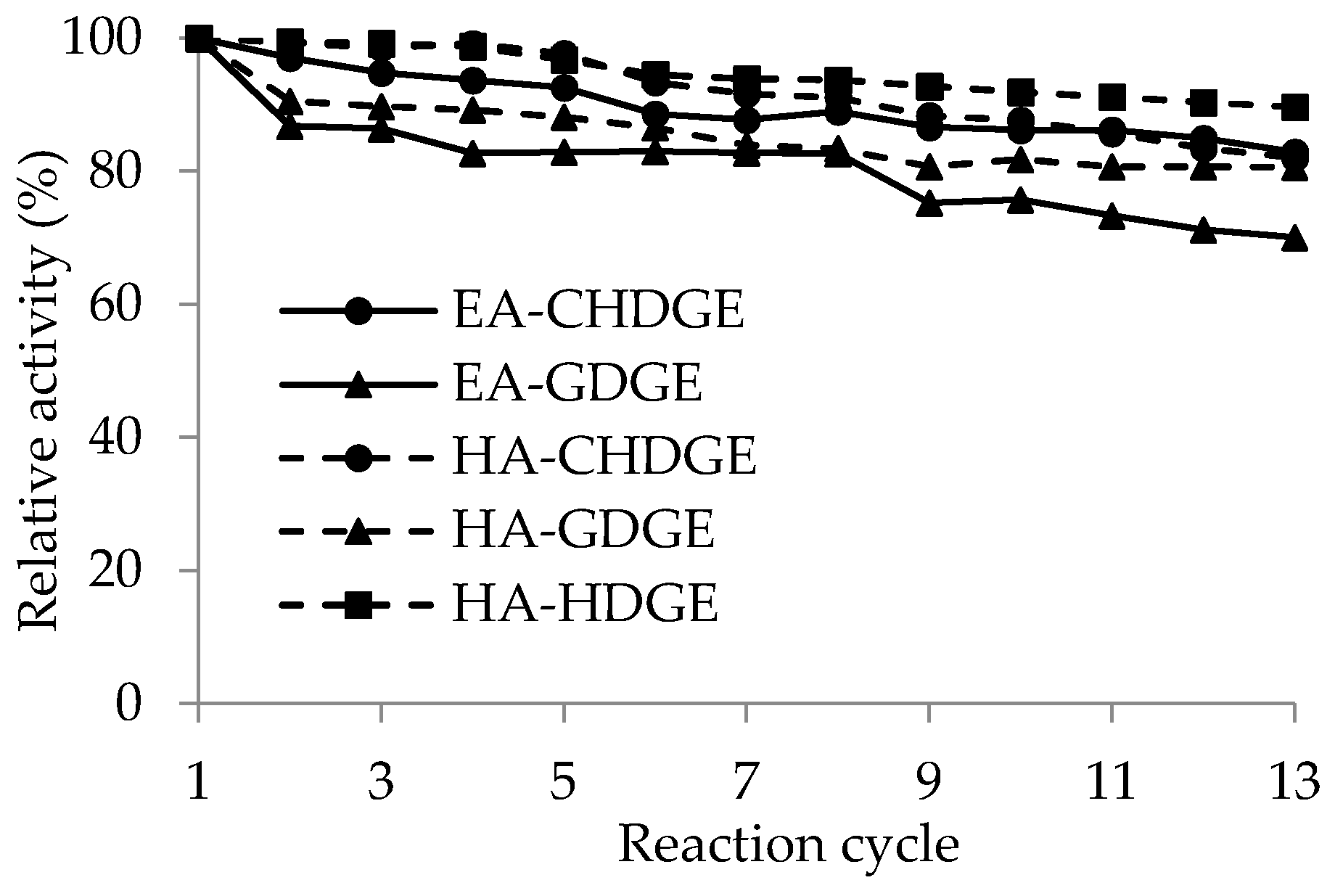
 ) or hexylamine (
) or hexylamine (  ) resin activated by CHDGE (●), GDGE (▲) or HDGE (■), compared to EP CaLB (
) resin activated by CHDGE (●), GDGE (▲) or HDGE (■), compared to EP CaLB (  in
in  ). (a) Effect of the substrate concentration on specific reaction rate (rflow); (b) Effect of temperature on specific reaction rate (rflow); (c) Effect of temperature on enantiomeric excess (ee); (d) Effect of temperature on enantiomeric ratio (E).
). (a) Effect of the substrate concentration on specific reaction rate (rflow); (b) Effect of temperature on specific reaction rate (rflow); (c) Effect of temperature on enantiomeric excess (ee); (d) Effect of temperature on enantiomeric ratio (E).
 ) or hexylamine (
) or hexylamine (  ) resin activated by CHDGE (●), GDGE (▲) or HDGE (■), compared to EP CaLB (
) resin activated by CHDGE (●), GDGE (▲) or HDGE (■), compared to EP CaLB (  in
in  ). (a) Effect of the substrate concentration on specific reaction rate (rflow); (b) Effect of temperature on specific reaction rate (rflow); (c) Effect of temperature on enantiomeric excess (ee); (d) Effect of temperature on enantiomeric ratio (E).
). (a) Effect of the substrate concentration on specific reaction rate (rflow); (b) Effect of temperature on specific reaction rate (rflow); (c) Effect of temperature on enantiomeric excess (ee); (d) Effect of temperature on enantiomeric ratio (E).