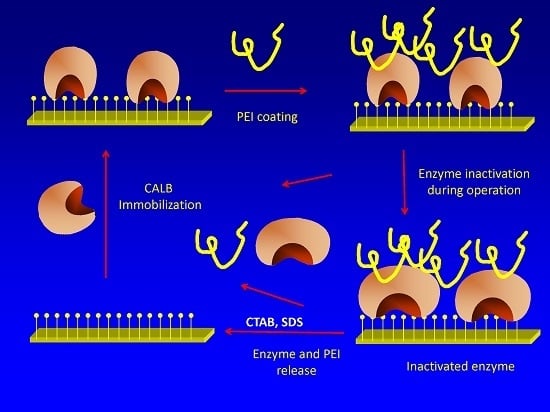
Stabilization of Candida antarctica Lipase B (CALB) Immobilized on Octyl Agarose by Treatment with Polyethyleneimine (PEI)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Immobilization of CALB on Octyl-Agarose
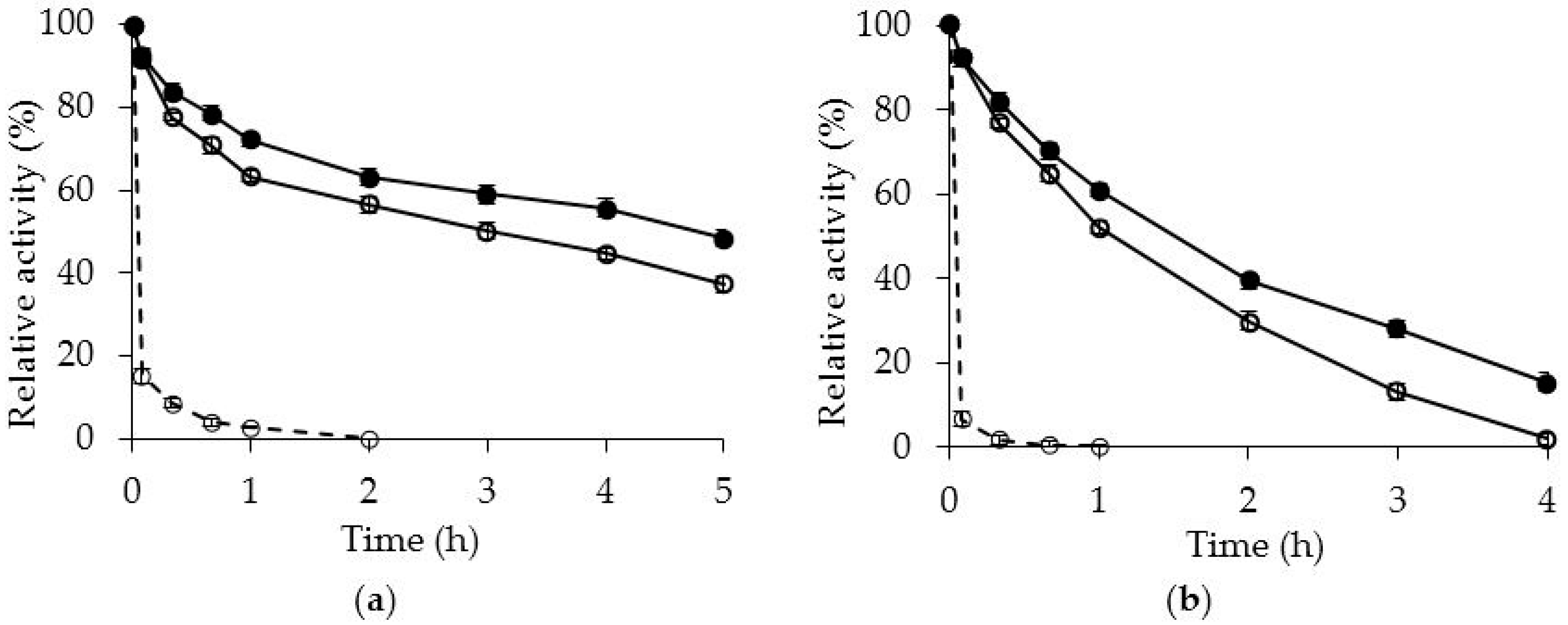
2.2. Effects of PEI Modification on Enzyme Activity/Stability
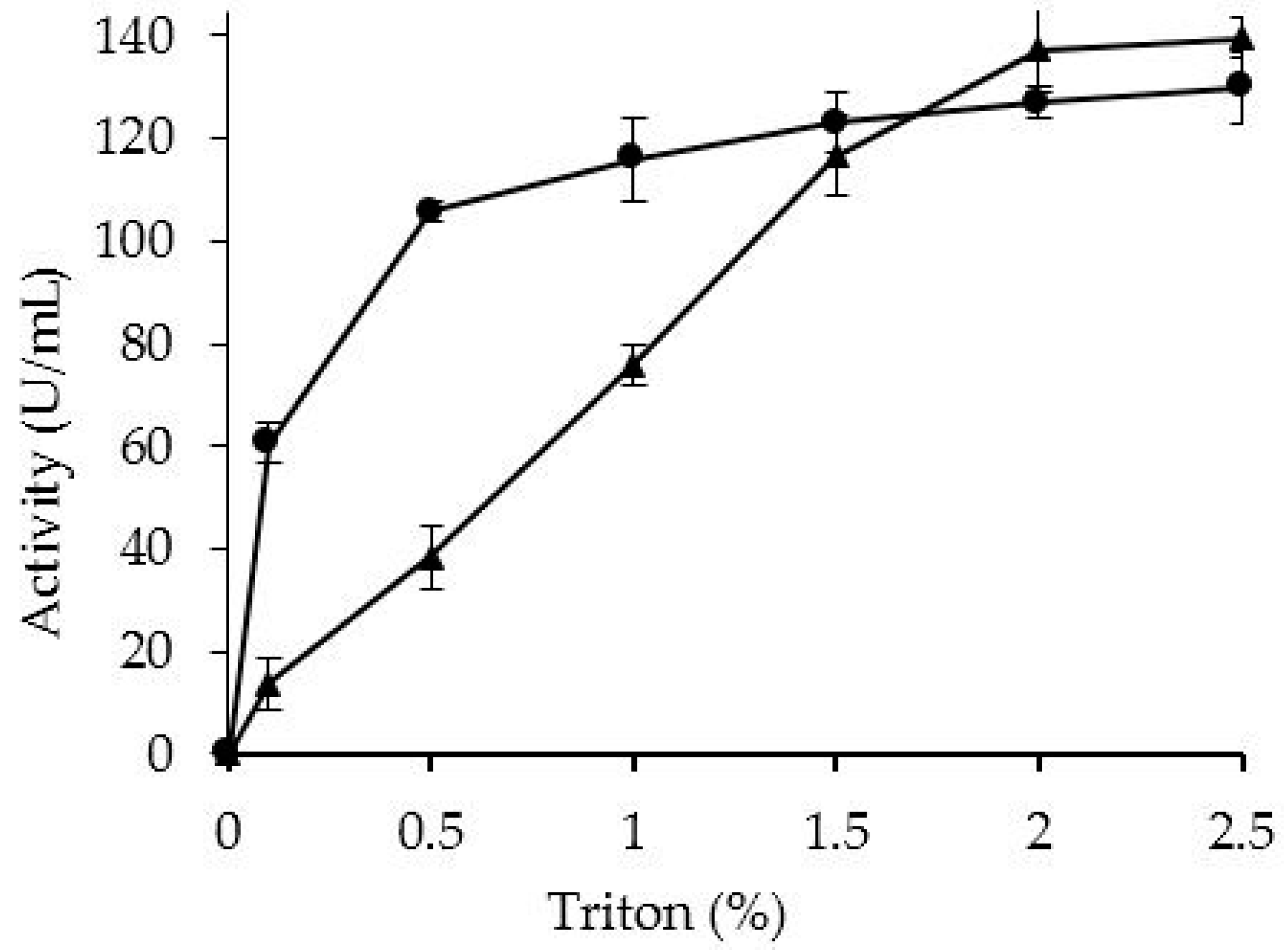
2.3. Effect of PEI Treatment in the Desorption of OCCALB by Incubation in non Ionic Detergents
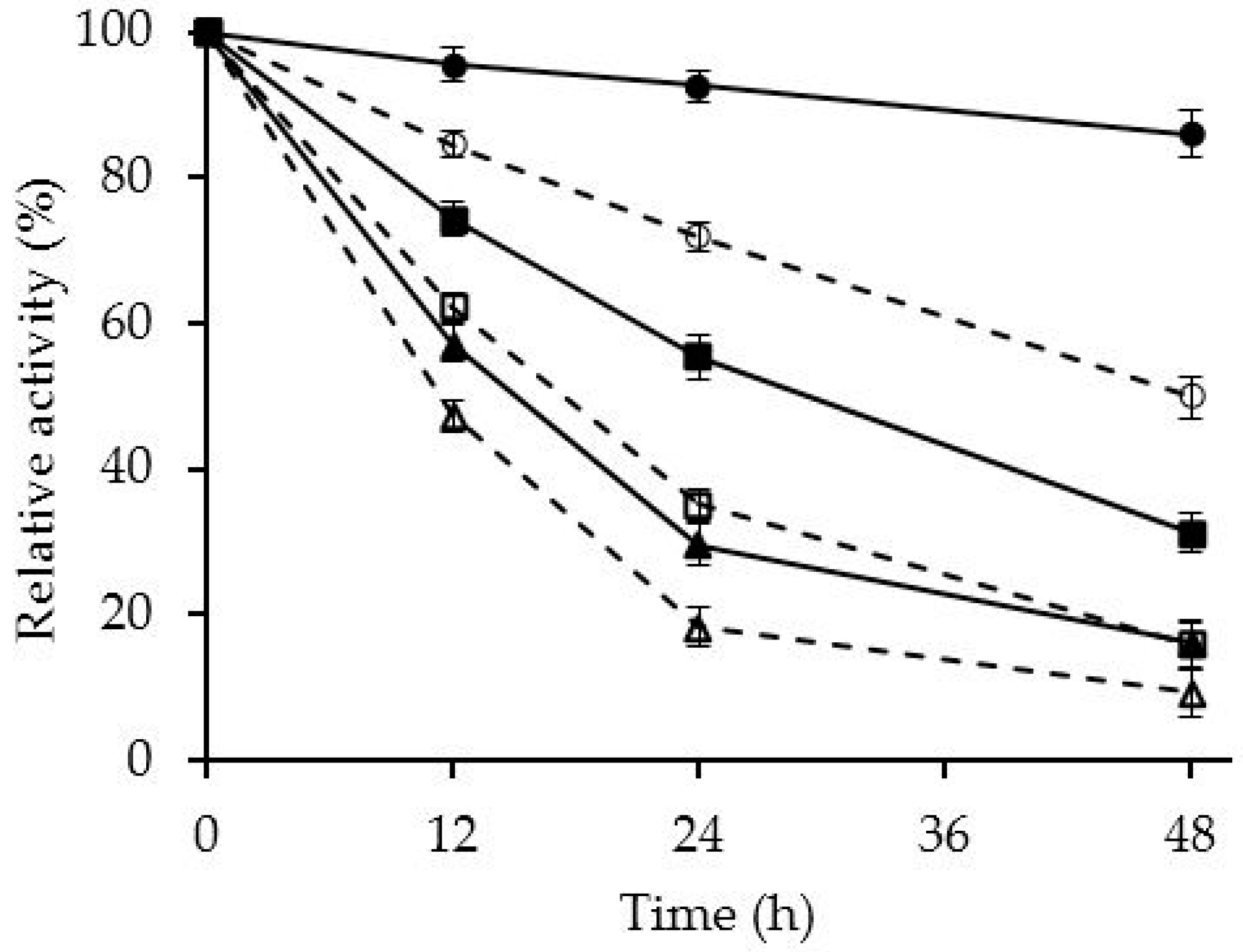
2.4. Effect of PEI Modification in the Release of CALB Molecules to the Medium during Inactivation
2.5. Reuse of the Support
3. Materials and Methods
3.1. Materials
3.2. Standard Measure of Enzyme Activity
3.3. Immobilization of CALB on Octyl (OC) Supports
3.4. Modification of OCCALB with PEI
3.5. Thermal Inactivation of OCCALB and OCCALB-PEI Preparations
3.6. Inactivation of OCCALB and OCCALB-PEI in the Presence of Organic Co-Solvents
3.7. Desorption of the CALB from the Supports
3.8. SDS-PAGE Experiments
3.9. Reuse of the Support
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CALB | Candida antarctica lipase B |
| OC | octyl-Sepharose |
| OCCALB | CALB immobilized on OC |
| PEI | polyethyleneimine |
| pnpb | p-nitrophenyl butyrate |
References
- Jaeger, K.-E.; Eggert, T. Lipases for biotechnology. Curr. Opin. Biotechnol. 2002, 13, 390–397. [Google Scholar] [CrossRef]
- Jaeger, K.-E.; Reetz, M.T. Microbial lipases form versatile tools for biotechnology. Trends Biotechnol. 1998, 16, 396–403. [Google Scholar] [CrossRef]
- Pandey, A.; Benjamin, S.; Soccol, C.R.; Nigam, P.; Krieger, N.; Soccol, V.T. The realm of microbial lipases in biotechnology. Biotechnol. Appl. Biochem. 1999, 29, 119–131. [Google Scholar] [PubMed]
- Schmid, R.D.; Verger, R. Lipases: Interfacial enzymes with attractive applications. Angew. Chem. Int. Ed. 1998, 37, 1608–1633. [Google Scholar] [CrossRef]
- Verger, R. “Interfacial activation” of lipases: Facts and artifacts. Trends Biotechnol. 1997, 15, 32–38. [Google Scholar] [CrossRef]
- Bastida, A.; Sabuquillo, P.; Armisen, P.; Fernández-Lafuente, R.; Huguet, J.; Guisán, J.M. A single step purification, immobilization, and hyperactivation of lipases via interfacial adsorption on strongly hydrophobic supports. Biotechnol. Bioeng. 1998, 58, 486–493. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R.; Armisén, P.; Sabuquillo, P.; Fernández-Lorente, G.; Guisán, J.M. Immobilization of lipases by selective adsorption on hydrophobic supports. Chem. Phys. Lipids 1998, 93, 185–197. [Google Scholar] [CrossRef]
- Fernández-Lorente, G.; Palomo, J.M.; Fuentes, M.; Mateo, C.; Guisán, J.M.; Fernández-Lafuente, R. Self-assembly of pseudomonas fluorescens lipase into bimolecular aggregates dramatically affects functional properties. Biotechnol. Bioeng. 2003, 82, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Palomo, J.M.; Peñas, M.M.; Fernández-Lorente, G.; Mateo, C.; Pisabarro, A.G.; Fernández-Lafuente, R.; Ramírez, L.; Guisán, J.M. Solid-phase handling of hydrophobins: Immobilized hydrophobins as a new tool to study lipases. Biomacromolecules 2003, 4, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, P. Immobilisation and application of lipases in organic media. Chem. Soc. Rev. 2013, 42, 6406–6436. [Google Scholar] [CrossRef] [PubMed]
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef] [PubMed]
- Liese, A.; Hilterhaus, L. Evaluation of immobilized enzymes for industrial applications. Chem. Soc. Rev. 2013, 42, 6236–6249. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, A.; Torres, R.; Fernandez-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lafuente, R. Stabilization of multimeric enzymes: Strategies to prevent subunit dissociation. Enzyme Microb. Technol. 2009, 45, 405–418. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Strategies for the one-step immobilization–purification of enzymes as industrial biocatalysts. Biotechnol. Adv. 2015, 33, 435–456. [Google Scholar] [CrossRef] [PubMed]
- Palomo, J.M.; Muñoz, G.; Fernández-Lorente, G.; Mateo, C.; Fernández-Lafuente, R.; Guisán, J.M. Interfacial adsorption of lipases on very hydrophobic support (octadecyl-sepabeads): Immobilization, hyperactivation and stabilization of the open form of lipases. J. Mol. Catal. B Enzym. 2002, 19–20, 279–286. [Google Scholar] [CrossRef]
- Manoel, E.A.; dos Santos, J.C.S.; Freire, D.M.G.; Rueda, N.; Fernandez-Lafuente, R. Immobilization of lipases on hydrophobic supports involves the open form of the enzyme. Enzyme Microb. Technol. 2015, 71, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lorente, G.; Cabrera, Z.; Godoy, C.; Fernandez-Lafuente, R.; Palomo, J.M.; Guisan, J.M. Interfacially activated lipases against hydrophobic supports: Effect of the support nature on the biocatalytic properties. Process Biochem. 2008, 43, 1061–1067. [Google Scholar] [CrossRef]
- Rueda, N.; dos Santos, J.C.S.; Torres, R.; Ortiz, C.; Barbosa, O.; Fernandez-Lafuente, R. Improved performance of lipases immobilized on heterofunctional octyl-glyoxyl agarose beads. RSC Adv. 2015, 5, 11212–11222. [Google Scholar] [CrossRef]
- Pizarro, C.; Brañes, M.C.; Markovits, A.; Fernández-Lorente, G.; Guisán, J.M.; Chamy, R.; Wilson, L. Influence of different immobilization techniques for Candida cylindracea lipase on its stability and fish oil hydrolysis. J. Mol. Catal. B Enzym. 2012, 78, 111–118. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G.; Filice, M.; Lopez-Vela, D.; Pizarro, C.; Wilson, L.; Betancor, L.; Avila, Y.; Guisan, J.M. Cross-linking of lipases adsorbed on hydrophobic supports: Highly selective hydrolysis of fish oil catalyzed by RML. J. Am. Oil Chem. Soc. 2010, 88, 801–807. [Google Scholar] [CrossRef]
- Barbosa, O.; Torres, R.; Ortiz, C.; Fernandez-Lafuente, R. The slow-down of the CALB immobilization rate permits to control the inter and intra molecular modification produced by glutaraldehyde. Process Biochem. 2012, 47, 766–774. [Google Scholar] [CrossRef]
- Guajardo, N.; Bernal, C.; Wilson, L.; Cabrera, Z. Selectivity of R-α-monobenzoate glycerol synthesis catalyzed by Candida antarctica lipase B immobilized on heterofunctional supports. Process Biochem. 2015, 50, 1870–1877. [Google Scholar] [CrossRef]
- Guajardo, N.; Bernal, C.; Wilson, L.; Cabrera, Z. Asymmetric hydrolysis of dimethyl-3-phenylglutarate in sequential batch reactor operation catalyzed by immobilized Geobacillus thermocatenulatus lipase. Catal. Today 2015, 255, 21–26. [Google Scholar] [CrossRef]
- Bernal, C.; Illanes, A.; Wilson, L. Heterofunctional hydrophilic-hydrophobic porous silica as support for multipoint covalent immobilization of lipases: Application to lactulose palmitate synthesis. Langmuir 2014, 30, 3557–3566. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lopez, L.; Rueda, N.; Bartolome-Cabrero, R.; Rodriguez, M.D.; Albuquerque, T.L.; dos Santos, J.C.S.; Barbosa, O.; Fernandez-Lafuente, R. Improved immobilization and stabilization of lipase from Rhizomucor miehei on octyl-glyoxyl agarose beads by using CaCl2. Process Biochem. 2016, 51, 48–52. [Google Scholar] [CrossRef]
- Suescun, A.; Rueda, N.; dos Santos, J.C.S.; Castillo, J.J.; Ortiz, C.; Torres, R.; Barbosa, O.; Fernandez-Lafuente, R. Immobilization of lipases on glyoxyl-octyl supports: Improved stability and reactivation strategies. Process Biochem. 2015, 50, 1211–1217. [Google Scholar] [CrossRef]
- Rueda, N.; Santos, J.C.S.D.; Ortiz, C.; Barbosa, O.; Fernandez-Lafuente, R.; Torres, R. Chemical amination of lipases improves their immobilization on octyl-glyoxyl agarose beads. Catal. Today 2016, 259, 107–118. [Google Scholar] [CrossRef]
- Albuquerque, T.L.D.; Rueda, N.; dos Santos, J.C.S.; Barbosa, O.; Ortiz, C.; Binay, B.; Özdemir, E.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Easy stabilization of interfacially activated lipases using heterofunctional divinyl sulfone activated-octyl agarose beads. Modulation of the immobilized enzymes by altering their nanoenvironment. Process Biochem. 2016, 51, 865–874. [Google Scholar] [CrossRef]
- Rueda, N.; dos Santos, J.C.S.; Torres, R.; Barbosa, O.; Ortiz, C.; Fernandez-Lafuente, R. Reactivation of lipases by the unfolding and refolding of covalently immobilized biocatalysts. RSC Adv. 2015, 5, 55588–55594. [Google Scholar] [CrossRef]
- Rueda, N.; dos Santos, C.S.; Rodriguez, M.D.; Albuquerque, T.L.; Barbosa, O.; Torres, R.; Ortiz, C.; Fernandez-Lafuente, R. Reversible immobilization of lipases on octyl-glutamic agarose beads: A mixed adsorption that reinforces enzyme immobilization. J. Mol. Catal. B-Enzym. 2016, 128, 10–18. [Google Scholar] [CrossRef]
- Rueda, N.; Albuquerque, T.L.; Bartolome-Cabrero, R.; Fernandez-Lopez, L.; Torres, R.; Ortiz, C.; dos Santos, J.C.S.; Barbosa, O.; Fernandez-Lafuente, R. Reversible immobilization of lipases on heterofunctional octyl-amino agarose beads prevents enzyme desopriton. Molecules 2016, 21, 646. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Wei, Y.; Luo, H.; Li, X.; Wang, X.; Liang, C.; Chang, Y.; Yu, H.; Shen, Z. Immobilization and stabilization of cephalosporin C acylase on aminated support by crosslinking with glutaraldehyde and further modifying with aminated macromolecules. Biotechnol. Prog. 2015, 31, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Galan, C.; Barbosa, O.; Fernandez-Lafuente, R. Stabilization of the hexameric glutamate dehydrogenase from Escherichia coli by cations and polyethyleneimine. Enzyme Microb. Technol. 2013, 52, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rochefort, D. Activity, conformation and thermal stability of laccase and glucose oxidase in poly(ethyleneimine) microcapsules for immobilization in paper. Process Biochem. 2011, 46, 993–1000. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Rocha-Martin, J.; Mateo, C.; Cava, F.; Berenguer, J.; Fernandez-Lafuente, R.; Guisan, J.M. Coating of soluble and immobilized enzymes with ionic polymers: Full stabilization of the quaternary structure of multimeric enzymes. Biomacromolecules 2009, 10, 742–747. [Google Scholar] [CrossRef] [PubMed]
- Breccia, J.D.; Andersson, M.M.; Hatti-Kaul, R. The role of poly(ethyleneimine) in stabilization against metal-catalyzed oxidation of proteins: A case study with lactate dehydrogenase. Biochim. Biophys. Acta Gen. Subj. 2002, 1570, 165–173. [Google Scholar] [CrossRef]
- Andersson, M.M.; Breccia, J.D.; Hatti-Kaul, R. Stabilizing effect of chemical additives against oxidation of lactate dehydrogenase. Biotechnol. Appl. Biochem. 2000, 32, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.M.; Hatti-Kaul, R. Protein stabilising effect of polyethyleneimine. J. Biotechnol. 1999, 72, 21–31. [Google Scholar] [CrossRef]
- Bryjak, J. Storage stabilization of enzyme activity by poly(ethyleneimine). Bioprocess Eng. 1995, 13, 177–181. [Google Scholar]
- Mateo, C.; Fernandes, B.; van Rantwijk, F.; Stolz, A.; Sheldon, R.A. Stabilisation of oxygen-labile nitrilases via co-aggregation with poly(ethyleneimine). J. Mol. Catal. B Enzym. 2006, 38, 154–157. [Google Scholar] [CrossRef]
- Guisan, J.M.; Sabuquillo, P.; Fernandez-Lafuente, R.; Fernandez-Lorente, G.; Mateo, C.; Halling, P.J.; Kennedy, D.; Miyata, E.; Re, D. Preparation of new lipases derivatives with high activity-stability in anhydrous media: Adsorption on hydrophobic supports plus hydrophilization with polyethylenimine. J. Mol. Catal. B Enzym. 2001, 11, 817–824. [Google Scholar] [CrossRef]
- Dos Santos, J.C.S.; Garcia-Galan, C.; Rodrigues, R.C.; de Sant’Ana, H.B.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Stabilizing hyperactivated lecitase structures through physical treatment with ionic polymers. Process Biochem. 2014, 49, 1511–1515. [Google Scholar] [CrossRef]
- Dos Santos, J.C.S.; Garcia-Galan, C.; Rodrigues, R.C.; de Sant’ Ana, H.B.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. Improving the catalytic properties of immobilized lecitase via physical coating with ionic polymers. Enzyme Microb. Technol. 2014, 60, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.M.; Larsson, K.M.; Kirk, O. One biocatalyst—many applications: The use of Candida antarctica B-lipase in organic synthesis. Biocatal. Biotransform. 1998, 16, 181–204. [Google Scholar] [CrossRef]
- Gotor-Fernández, V.; Busto, E.; Gotor, V. Candida antarctica lipase B: An ideal biocatalyst for the preparation of nitrogenated organic compounds. Adv. Synth. Catal. 2006, 348, 797–812. [Google Scholar] [CrossRef]
- Peirce, S.; Virgen-Ortíz, J.J.; Tacias-Pascacio, V.G.; Rueda, N.; Bartolome-Cabrero, R.; Fernandez-Lopez, L.; Russo, M.E.; Marzocchella, A.; Fernandez-Lafuente, R. Development of simple protocols to solve the problems of enzyme coimmobilization. Application to coimmobilize a lipase and a β-galactosidase. RSC Adv. 2016. submitted. [Google Scholar]
- Uppenberg, J.; Hansen, M.T.; Patkar, S.; Jones, T.A. The sequence, crystal structure determination and refinement of two crystal forms of lipase B from Candida antarctica. Structure 1994, 2, 293–308. [Google Scholar] [CrossRef]
- Virgen-Ortíz, J.J.; Peirce, S.; Tacias-Pascacio, V.G.; Marzocchella, A.; Russo, M.E.; Fernandez-Lafuente, R. Reuse of anion exchangers as supports for enzyme immobilization: Reinforcement of the enzyme-support multiinteraction after enzyme inactivation. Process Biochem. 2016. Accepted for publication. [Google Scholar]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Coupling chemical modification and immobilization to improve the catalytic performance of enzymes. Adv. Synth. Catal. 2011, 353, 2216–2238. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernandez-Lafuente, R. Amination of enzymes to improve biocatalyst performance: Coupling genetic modification and physicochemical tools. RSC Adv. 2014, 4, 38350–38374. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the biocatalyst are available from the authors for colaboraiton.




© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peirce, S.; Tacias-Pascacio, V.G.; Russo, M.E.; Marzocchella, A.; Virgen-Ortíz, J.J.; Fernandez-Lafuente, R. Stabilization of Candida antarctica Lipase B (CALB) Immobilized on Octyl Agarose by Treatment with Polyethyleneimine (PEI). Molecules 2016, 21, 751. https://doi.org/10.3390/molecules21060751
Peirce S, Tacias-Pascacio VG, Russo ME, Marzocchella A, Virgen-Ortíz JJ, Fernandez-Lafuente R. Stabilization of Candida antarctica Lipase B (CALB) Immobilized on Octyl Agarose by Treatment with Polyethyleneimine (PEI). Molecules. 2016; 21(6):751. https://doi.org/10.3390/molecules21060751
Chicago/Turabian StylePeirce, Sara, Veymar G. Tacias-Pascacio, Maria Elena Russo, Antonio Marzocchella, José J. Virgen-Ortíz, and Roberto Fernandez-Lafuente. 2016. "Stabilization of Candida antarctica Lipase B (CALB) Immobilized on Octyl Agarose by Treatment with Polyethyleneimine (PEI)" Molecules 21, no. 6: 751. https://doi.org/10.3390/molecules21060751