Synthesis and in Vitro Antiproliferative Evaluation of C-13 Epimers of Triazolyl-d-Secoestrone Alcohols: The First Potent 13α-d-Secoestrone Derivative
Abstract
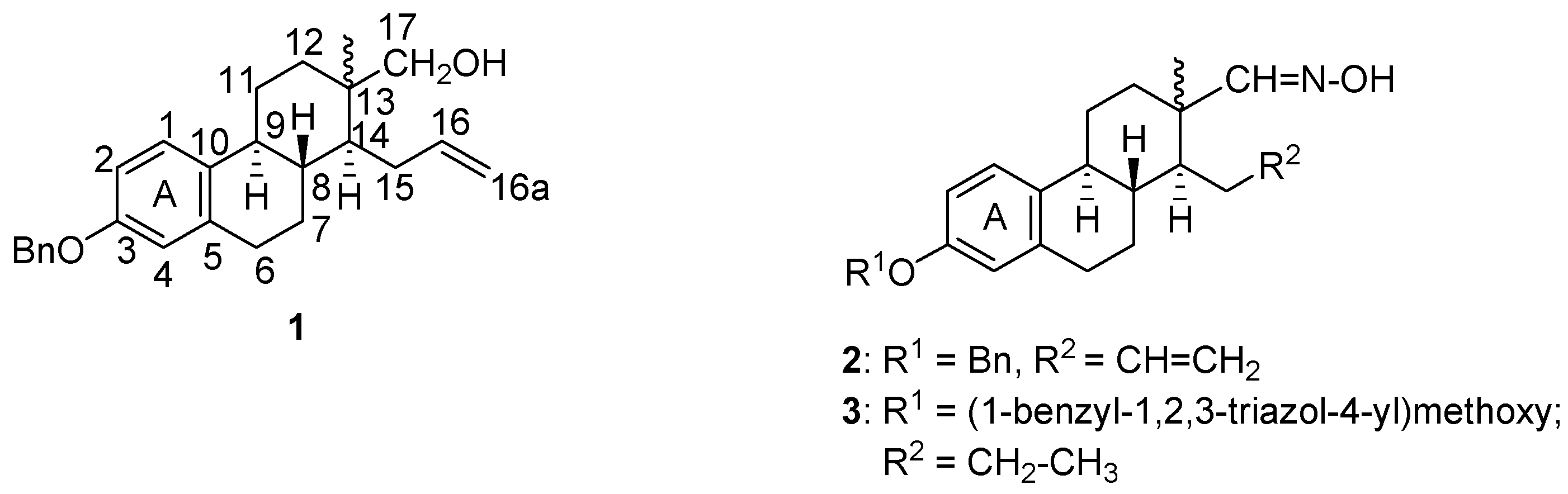
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. Chemistry
4.2.1. General Procedure for the Synthesis of 3-Benzyloxy-d-secoalcohols 1, 6
4.2.2. General Procedure for the Synthesis of 3-Hydroxy-d-secoestrones 7, 8
4.2.3. General Procedure for the Synthesis of 3-(Prop-2-inyloxy)-d-secoestrones 9, 10
4.2.4. General Procedure for the “Click” Reaction
4.3. Determination of Antiproliferative Activities
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products, derivatives and mimics as antitumor agents. J. Nat. Prod. 2011, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Khuda-Bukhsh, A.R. Molecular approaches towards development of purified natural products and their structurally known derivatives as efficient anti-cancer drugs: Current trends. Eur. J. Pharm. 2013, 714, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Kumar, S.B.; Negi, A.S. Current status on development of steroids as anticancer agents. J. Steroid Biochem. Mol. Biol. 2013, 137, 242–270. [Google Scholar] [CrossRef] [PubMed]
- Numazawa, M.; Ando, M.; Watari, Y.; Tominaga, T.; Hayata, Y.; Yoshimura, A. Structure-activity relationships of 2-, 4-, or 6-substituted estrogens as aromatase inhibitors. J. Steroid Biochem. Mol. Biol. 2005, 96, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Schönecker, B.; Lange, C.; Kötteritzsch, M.; Günther, W.; Weston, J.; Anders, E.; Görls, H. Conformational design for 13α-steroids. J. Org. Chem. 2000, 65, 5487–5497. [Google Scholar] [CrossRef] [PubMed]
- Ayan, D.; Roy, J.; Maltais, R.; Poirier, D. Impact of estradiol structural modifications (18-methyl and/or 17-hydroxy inversion of configuration) on the in vitro and in vivo estrogenic activity. J. Steroid Biochem. Mol. Biol. 2011, 127, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Penov Gasi, K.M.; Miljkovic, D.A.; Medic Mijacevic, L.D.; Djurendic, E.A.; Stojanovic, S.Z.; Sakac, M.N.; Djurendic, M.D.; Stankovic, S.M.; Lazar, D.; Andric, S.; et al. Synthesis, X-ray crystal structure and biological activity of 16-amino-17-substituted-d-homo steroid derivatives. Steroids 2003, 68, 667–676. [Google Scholar] [CrossRef]
- Jovanovic-Santa, S.; Petrovic, J.; Andric, S.; Kovacevic, R.; Durendic, E.; Sakac, M.; Lazar, D.; Stankovic, S. Synthesis, structure, and screening of estrogenic and antiestrogenic activity of new 3,17-substituted-16,17-seco-estratriene derivatives. Bioorg. Chem. 2003, 1, 475–484. [Google Scholar] [CrossRef]
- Nikolic, A.R.; Petri, E.T.; Klisuric, O.R.; Celic, A.S.; Jakimov, D.S.; Djurendic, E.A.; Penov Gasi, K.M.; Sakac, M.N. Synthesis and anticancer cell potential of steroidal 16,17-seco-16,17a-dinitriles: Identification of a selective inhibitor of hormone-independent breast cancer cells. Bioorg. Med. Chem. 2015, 23, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Mernyak, E.; Szabo, J.; Huber, J.; Schneider, G.; Minorics, R.; Bozsity, N.; Zupko, I.; Varga, M.; Bikadi, Z.; Hazai, E.; et al. Synthesis and antiproliferative effects of d-homo- and d-secoestrones. Steroids 2014, 87, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Mernyak, E.; Fiser, G.; Szabo, J.; Bodnar, B.; Schneider, G.; Kovacs, I.; Ocsovszki, I.; Zupko, I.; Wölfling, J. Synthesis and in vitro antiproliferative evaluation of d-secooxime derivatives of 13β- and 13α-estrone. Steroids 2014, 89, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Astruc, D. The copper(I)-catalyzed alkyne-azide cycloaddition (CuAAC) “click” reaction and its applications. An overview. Coord. Chem. Rev. 2011, 255, 2933–2945. [Google Scholar] [CrossRef]
- Pedersen, D.S.; Abell, A. 1,2,3-Triazoles in peptidomimetic chemistry. Eur. J. Org. Chem. 2011, 2011, 2399–2411. [Google Scholar] [CrossRef]
- Deobald, A.M.; Camargo, L.R.S.; Alves, D.; Zukerman-Schpector, J.; Corrêa, A.G.; Paixão, M.W. Click chemistry: an efficient synthesis of heterocycles substituted with steroids, saponins, and digitalis analogues. Synthesis 2011, 24, 4003–4010. [Google Scholar]
- Szabo, J.; Bacsa, I.; Wölfling, J.; Schneider, G.; Zupko, I.; Varga, M.; Herman, B.E.; Kalmar, L.; Szecsi, M.; Mernyak, E. Synthesis and in vitro pharmacological evaluation of N-[(1-benzyl-1,2,3-triazol-4-yl)methyl]-carboxamides on d-secoestrone scaffolds. J. Enzyme Inhib. Med. Chem. 2015. [Google Scholar] [CrossRef]
- Kadar, Z.; Baji, A.; Zupko, I.; Bartok, T.; Wölfling, J.; Frank, E. Efficient approach to novel 1α-triazolyl-5α-androstane derivatives as potent antiproliferative agents. Org. Biomol. Chem. 2011, 9, 8051–8057. [Google Scholar] [CrossRef] [PubMed]
- Kadar, Z.; Kovacs, D.; Frank, E.; Schneider, G.; Huber, J.; Zupko, I.; Bartok, T.; Wölfling, J. Synthesis and in vitro antiproliferative activity of novel androst-5-ene triazolyl and tetrazolyl derivatives. Molecules 2011, 16, 4786–4806. [Google Scholar] [CrossRef] [PubMed]
- Frank, E.; Molnar, J.; Zupko, I.; Kadar, Z.; Wölfling, J. Synthesis of novel steroidal 17α-triazolyl-derivatives via Cu(I)-catalyzed azide-alkyne cycloaddition, and an evaluation of their cytotoxic activity in vitro. J. Steroids 2011, 76, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Kadar, Z.; Frank, E.; Schneider, G.; Molnar, J.; Zupko, I.; Koti, J.; Schönecker, B.; Wölfling, J. Efficient synthesis of novel A-ring-substituted 1,2,3-triazolylcholestane derivatives via catalytic azide-alkyne cycloaddition. Arkivoc 2012, 3, 279–296. [Google Scholar]
- Kadar, Z.; Molnar, J.; Schneider, G.; Zupko, I.; Frank, E. A facile click approach to novel 15β-triazolyl-5α-androstane derivatives, and an evaluation of their antiproliferative activities in vitro. Bioorg. Med. Chem. 2012, 20, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Mernyak, E.; Kovacs, I.; Minorics, R.; Sere, P.; Czegany, D.; Sinka, I.; Wölfling, J.; Schneider, G.; Ujfaludi, Z.; Boros, I.; et al. Synthesis of trans-16-triazolyl-13α-methyl-17-estradiol diastereomers and the effects of structural modifications on their in vitro antiproliferative activities. J. Steroid Biochem. Mol. Biol. 2015, 150, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Jurasek, M.; Dzubak, P.; Sedlak, D.; Dvorzakova, H.; Hajduch, M.; Bartunek, P.; Drašar, P. Preparation, preliminary screening of new types of steroid conjugates and their activities on steroid receptors. Steroids 2013, 78, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Bózsity, N.; Minorics, R.; Szabó, J.; Mernyák, E.; Schneider, G.; Wölfling, J.; Wang, H.C.; Wu, C.C.; Ocsovszki, I.; Zupkó, I. Mechanism of antiproliferative action of a new d-secoestrone-triazole derivative in cervical cancer cells and its effect on cancer cell motility. J. Steroid Biochem. Mol. Biol. under review.
- Doorbar, J.; Quint, W.; Banks, L.; Bravo, I.G.; Stoler, M.; Broker, T.R.; Stanley, M.A. The biology and life-cycle of human papillomaviruses. Vaccine 2012, 30S, F55–F70. [Google Scholar] [CrossRef] [PubMed]
- Pater, M.M.; Pater, A. Human papillomavirus types 16 and 18 sequences in carcinoma cell lines of the cervix. Virology 1985, 145, 313–318. [Google Scholar] [CrossRef]
- Neve, R.M.; Chin, K.; Fridlyand, J.; Yeh, J.; Baehner, F.L.; Fevr, T.; Clark, L.; Bayani, N.; Coppe, J.P.; Tong, F.; et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 2006, 10, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Huber, J.; Wölfling, J.; Schneider, G.; Ocsovszki, I.; Varga, M.; Zupkó, I.; Mernyák, E. Synthesis of antiproliferative 13α-d-homoestrones via Lewis acid-promoted one-pot Prins-Ritter reactions of d-secosteroidal δ-alkenyl-aldehydes. Steroids 2015, 102, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Pardin, C.; Roy, I.; Lubell, W.D.; Keillor, J.W. Reversible and competitive cinnamoyl triazole inhibitors of tissue transglutaminase. Chem. Biol. Drug Des. 2008, 72, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Maycock, C.D.; Santos, J.P.; Duarte, M.F.; Frenandez, M.T.; Costa, M.L. Study of selected benzyl azides by UV photoelectron spectroscopy and mass spectrometry. J. Mol. Struct. 2010, 980, 163–171. [Google Scholar]
- Pötzsch, R.; Voit, B. Thermal and photochemical crosslinking of hyperbranched polyphenylene with organic azides. Macromol. Rapid Commun. 2012, 33, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Barr, L.; Lincoln, S.F.; Easton, C.J. A cyclodextrin molecular reactor for the regioselective synthesis of 1,5-disubstituted-1,2,3-triazoles. Supramol. Chem. 2005, 17, 547–555. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available.

| Comp. | Conc. (µM) | Inhibition (%) ± SEM (Calculated IC50) 1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| A2780 | Hela | SiHa | C33A | MCF-7 | T47D | MDA-MB-231 | MDA-MB-361 | ||
| 1 | 10 | 42.3 ± 0.9 | 31.4 ± 1.6 | - 2 | 39.2 ± 0.6 | 81.3 ± 0.7 | 26.1 ± 2.0 | - | 28.2 ± 0.4 |
| 30 | 97.5 ± 0.1 | 97.9 ± 0.3 | 84.3 ± 0.9 | 86.9 ± 0.7 | 97.4 ± 0.3 | 87.2 ± 0.9 | 84.5 ± 0.9 | 87.6 ± 0.4 | |
| (6.4) | |||||||||
| 6 | 10 | 43.6 ± 2.4 | 20.1 ± 1.8 | - | 40.4 ± 2.1 | - | - | - | - |
| 30 | 55.4 ± 2.5 | 60.3 ± 1.4 | 49.7 ± 1.4 | 53.8 ± 1.1 | 36.4 ± 1.2 | 59.6 ± 0.8 | 49.3 ± 1.6 | 77.2 ± 1.3 | |
| 7 | 10 | - | - | - | 40.5 ± 0.8 | 24.3 ± 2.6 | - | - | - |
| 30 | 35.1 ± 0.8 | 40.7 ± 1.7 | - | 42.3 ± 1.8 | 51.6 ± 2.9 | - | - | - | |
| 8 | 10 | - | 23.7 ± 0.9 | - | - | - | - | - | - |
| 30 | - | 64.5 ± 1.1 | - | - | - | 23.0 ± 1.7 | - | - | |
| 9 | 10 | 22.4 ± 1.0 | 21.5 ± 0.8 | - | 30.0 ± 1.1 | - | - | 36.8 ± 2.7 | - |
| 30 | 70.7 ± 0.4 | 89.3 ± 1.9 | 84.5 ± 0.5 | 70.1 ± 0.7 | 52.5 ± 1.0 | 37.7 ± 1.3 | 81.0 ± 1.1 | 59.0 ± 2.9 | |
| 10 | 10 | - | - | - | - | - | - | - | - |
| 30 | 29.7 ± 1.9 | 36.9 ± 1.8 | - | 39.9 ± 1.1 | 23.3 ± 0.6 | 45.5 ± 0.6 | 28.2 ± 2.2 | - | |
| 12a | 10 | 81.5 ± 1.1 | 85.4 ± 0.3 | 21.2 ± 1.1 | 90.0 ± 0.3 | 66.3 ± 0.3 | 51.0 ± 1.1 | 53.5 ± 1.2 | 59.3 ± 1.4 |
| 30 | 88.0 ± 0.1 | 91.7 ± 0.3 | 34.5 ± 1.2 | 95.1 ± 0.2 | 74.5 ± 1.7 | 54.4 ± 1.8 | 59.6 ± 1.8 | 45.2 ± 1.1 | |
| (0.9) | (1.1) [23] | (1.8) [23] | (1.5) | ||||||
| 12b | 10 | 96.8 ± 0.2 | 52.6 ± 0.9 | 48.1 ± 0.8 | 86.8 ± 0.8 | 71.6 ± 1.0 | 65.7 ± 1.4 | 58.3 ± 0.7 | 87.2 ± 0.5 |
| 30 | 97.4 ± 0.1 | 65.4 ± 0.9 | 64.3 ± 1.0 | 93.9 ± 0.9 | 73.9 ± 1.0 | 66.4 ± 1.2 | 86.1 ± 0.3 | 89.3 ± 1.1 | |
| (3.8) | (5.0) | (5.0) | (8.3) | (4.4) | |||||
| 12c | 10 | 83.3 ± 0.5 | 27.1 ± 1.7 | - | 57.5 ± 1.8 | - | - | 33.6 ± 0.8 | 47.1 ± 2.9 |
| 30 | 93.4 ± 0.1 | 66.0 ± 2.4 | 35.6 ± 0.3 | 84.6 ± 0.9 | 66.1 ± 1.7 | 53.0 ± 1.6 | 48.9 ± 0.7 | 45.6 ± 0.6 | |
| (5.4) | (8.3) | ||||||||
| 12d | 10 | 20.5 ± 1.2 | 30.9 ± 3.0 | - | 25.3 ± 1.5 | - | - | - | - |
| 30 | 29.0 ± 2.0 | 45.9 ± 1.1 | - | 64.0 ± 1.8 | 29.5 ± 2.7 | 28.9 ± 0.9 | - | 45.5 ± 1.2 | |
| 12e | 10 | 86.4 ± 0.3 | 46.3 ± 2.5 | 25.5 ± 2.0 | 81.4 ± 1.9 | 61.1 ± 1.6 | 41.5 ± 2.0 | 49.8 ± 0.7 | 47.5 ± 0.7 |
| 30 | 89.6 ± 0.3 | 72.5 ± 1.1 | 23.2 ± 1.2 | 88.9 ± 0.6 | 63.4 ± 0.9 | 49.7 ± 2.5 | 48.8 ± 0.9 | 46.7 ± 1.0 | |
| (4.6) | (5.4) | (6.6) | |||||||
| 13a | 10 | 73.4 ± 0.9 | 70.0 ± 0.7 | 41.8 ± 1.7 | 80.0 ± 0.5 | 39.4 ± 1.2 | 36.6 ± 0.7 | 38.2 ± 2.2 | 65.8 ± 1.0 |
| 30 | 83.8 ± 0.8 | 90.9 ± 0.3 | 37.1 ± 1.0 | 93.9 ± 0.1 | 73.8 ± 1.1 | 69.1 ± 0.6 | 59.7 ± 1.6 | 48.6 ± 1.4 | |
| (3.0) | (5.3) | (4.4) | |||||||
| 13b | 10 | 54.3 ± 0.8 | 33.9 ± 1.1 | - | 26.6 ± 1.8 | - | 43.2 ± 0.3 | 36.7 ± 1.8 | - |
| 30 | 81.0 ± 0.1 | 74.4 ± 0.9 | 49.4 ± 0.8 | 86.1 ± 0.6 | 65.6 ± 1.9 | 89.3 ± 0.8 | 56.8 ± 1.2 | 48.7 ± 1.0 | |
| (9.8) | |||||||||
| 13c | 10 | - | 21.3 ± 2.3 | - | - | - | - | - | - |
| 30 | 52.7 ± 1.9 | 40.6 ± 0.9 | - | 41.7 ± 1.2 | 20.1 ± 0.8 | 39.4 ± 1.7 | 28.1 ± 0.7 | 25.7 ± 2.8 | |
| 13d | 10 | 20.0 ± 1.9 | 24.4 ± 1.3 | - | 26.1 ± 0.9 | - | - | - | - |
| 30 | 44.8 ± 1.1 | 36.9 ± 0.8 | 23.0 ± 1.2 | 66.3 ± 0.6 | 30.8 ± 1.7 | 44.4 ± 1.7 | 37.5 ± 1.6 | 40.3 ± 2.1 | |
| 13e | 10 | - | - | - | - | - | - | - | - |
| 30 | 29.1 ± 2.3 | 48.3 ± 2.0 | - | 34.2 ± 1.3 | - | 22.8 ± 1.9 | - | - | |
| Cisplatin | 10 | 83.6 ± 1.2 | 42.6 ± 2.3 | 88.6 ± 0.5 | 83.8 ± 0.8 | 66.9 ± 1.8 | 51.0 ± 2.0 | - | 67.5 ± 1.0 |
| 30 | 95.0 ± 0.3 | 99.9 ± 0.3 | 90.2 ± 1.8 | 94.0 ± 0.6 | 96.8 ± 0.4 | 57.9 ± 1.5 | 71.5 ± 1.2 | 87.8 ± 1.1 | |
| (1.3) | (12.4) | (7.8) | (3.7) | (5.8) | (9.8) | (19.1) | (3.7) | ||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szabó, J.; Jerkovics, N.; Schneider, G.; Wölfling, J.; Bózsity, N.; Minorics, R.; Zupkó, I.; Mernyák, E. Synthesis and in Vitro Antiproliferative Evaluation of C-13 Epimers of Triazolyl-d-Secoestrone Alcohols: The First Potent 13α-d-Secoestrone Derivative. Molecules 2016, 21, 611. https://doi.org/10.3390/molecules21050611
Szabó J, Jerkovics N, Schneider G, Wölfling J, Bózsity N, Minorics R, Zupkó I, Mernyák E. Synthesis and in Vitro Antiproliferative Evaluation of C-13 Epimers of Triazolyl-d-Secoestrone Alcohols: The First Potent 13α-d-Secoestrone Derivative. Molecules. 2016; 21(5):611. https://doi.org/10.3390/molecules21050611
Chicago/Turabian StyleSzabó, Johanna, Nóra Jerkovics, Gyula Schneider, János Wölfling, Noémi Bózsity, Renáta Minorics, István Zupkó, and Erzsébet Mernyák. 2016. "Synthesis and in Vitro Antiproliferative Evaluation of C-13 Epimers of Triazolyl-d-Secoestrone Alcohols: The First Potent 13α-d-Secoestrone Derivative" Molecules 21, no. 5: 611. https://doi.org/10.3390/molecules21050611






