In Vivo Anti-Cancer Mechanism of Low-Molecular-Weight Fucosylated Chondroitin Sulfate (LFCS) from Sea Cucumber Cucumaria frondosa
Abstract
:1. Introduction
2. Results and Discussion
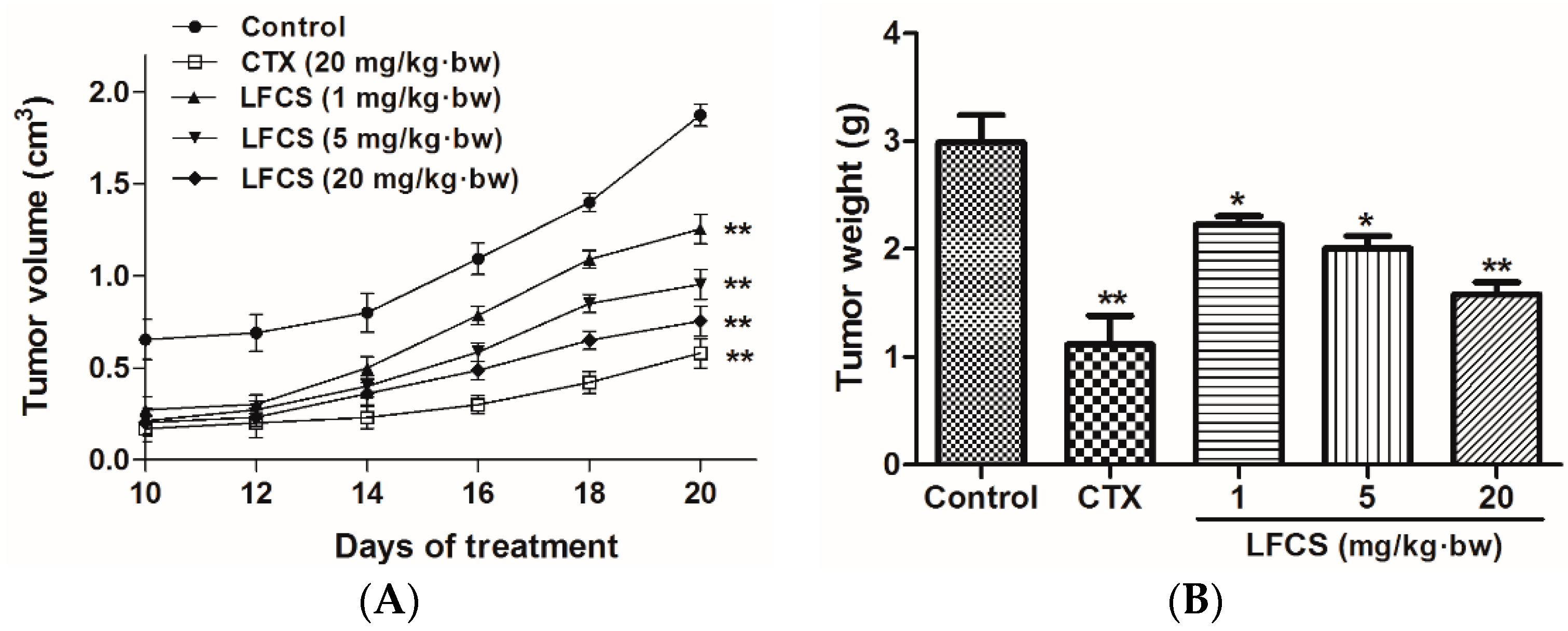
2.1. LFCS Inhibits LLC Tumor Growth in Vivo
2.2. LFCS Suppressed Lung Metastasis of LLC Cells In Vivo
2.3. LFCS Inhibited LLC Cell Proliferation and Cell Migration
2.4. LFCS Suppressed Proliferation and Migration of HUVEC
2.5. LFCS Induced Cell Cycle Arrest and Apoptosis by Uprgulating Protein Expression of p53, p21, and Caspase-3
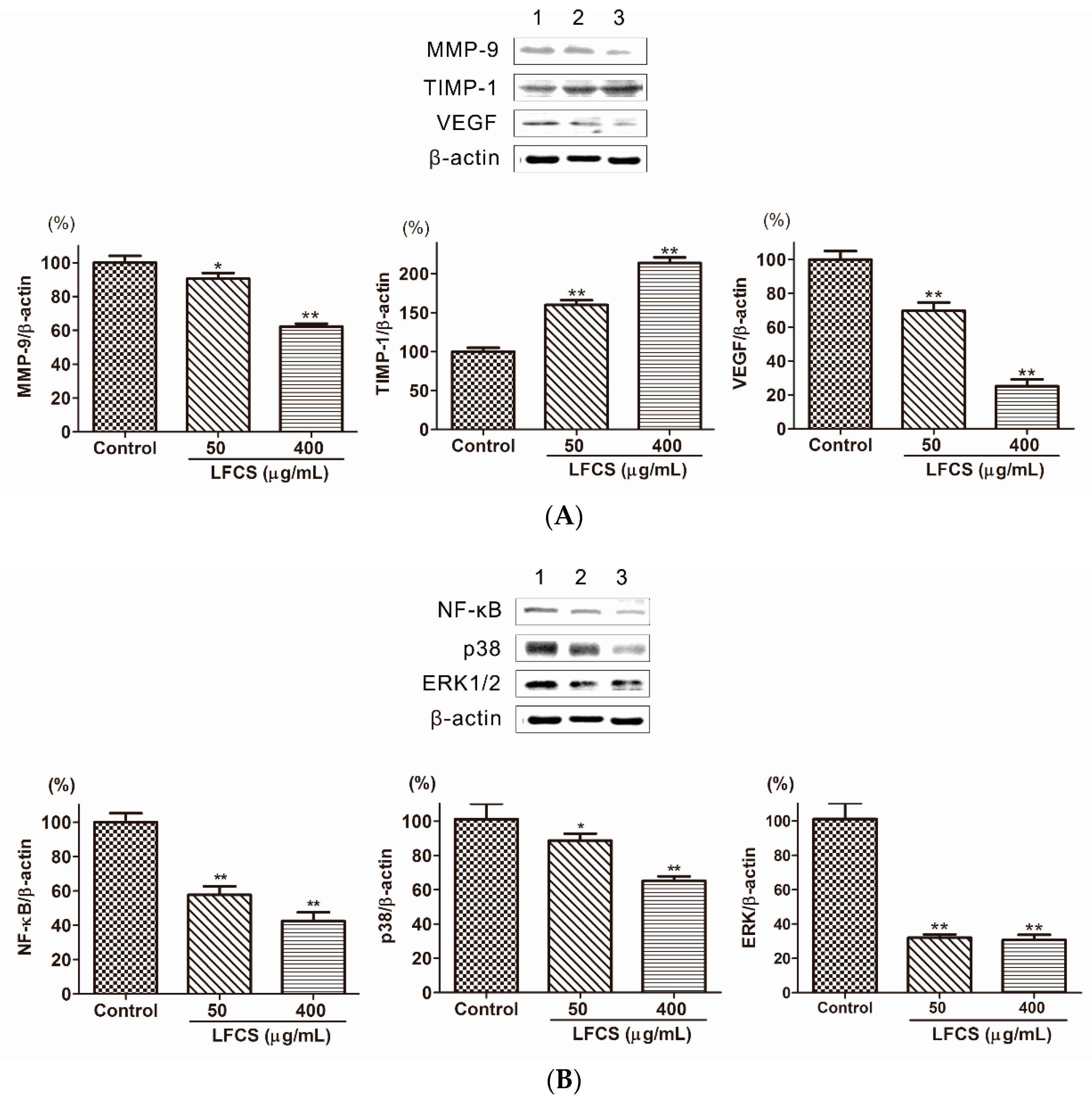
2.6. LFCS Suppressed Metastasis and Angiogenesis by Regulating Protein Expression of MMP-9, TIMP-1, VEGF, p38, ERK1/2, and NF-κB
3. Materials and Methods
3.1. Materials
3.2. Cells and Cell Culture
3.3. Animals and Ethical Approval
3.4. In Vivo LLC Cells Metastasis Model
3.5. Cell Proliferation Assays
3.6. Cell Migration Assays
3.7. Western Blot Analysis
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stewart, W.B.; Wild, P.C. World Cancer Report 2014; International Agency for Research on Cancer, WHO Press: Lyon, France, 2014; ISBN 9789283204329. [Google Scholar]
- Travis, W.D.; Elisabeth, B.; Nicholson, A.G.; Yasushi, Y.; Austin, J.H.M.; Mary Beth, B.; Chirieac, L.R.; Sanja, D.; Edwina, D.; Flieder, D.B. The 2015 world health organization classification of lung tumors: Impact of genetic, clinical and radiologic advances since the 2004 classification. J. Thorac. Oncol. 2015, 10, 1243–1260. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- López-Gómez, M.; Malmierca, E.; de Górgolas, M.; Casado, E. Cancer in developing countries: The next most preventable pandemic. The global problem of cancer. Crit. Rev. Oncol. Hematol. 2013, 88, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.E.; Cascone, T.; Gerber, D.E.; Heymach, J.V.; Minna, J.D. Targeted therapies for lung cancer. Cancer J. 2011, 17, 512–527. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, I.; Planchard, D. ALK inhibitors in non-small cell lung cancer: The latest evidence and developments. Ther. Adv. Med. Oncol. 2016, 8, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Meoni, G.; Cecere, F.L.; Lucherini, E.; di Costanzo, F. Medical treatment of advanced non-small cell lung cancer in elderly patients: A review of the role of chemotherapy and targeted agents. J. Geriatr. Oncol. 2013, 4, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Chaffer, C.L.; Weinberg, R.A. A perspective on cancer cell metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef] [PubMed]
- Millau, J.F.; Bastien, N.; Drouin, R. P53 transcriptional activities: A general overview and some thoughts. Mutat. Res. 2009, 681, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Woo, R.A.; Mclure, K.G.; Lees-Miller, S.P.; Rancourt, D.E.; Lee, P.W. DNA-dependent protein kinase acts upstream of p53 in response to DNA damage. Nature 1998, 394, 700–704. [Google Scholar] [PubMed]
- Xiong, Y.; Hannon, G.J.; Zhang, H.; Casso, D.; Kobayashi, R.; Beach, D. p21 is a universal inhibitor of cyclin kinases. Nature 1993, 366, 701–704. [Google Scholar] [CrossRef] [PubMed]
- Zhiyong, R.E.H.; Wei, W.; Stephen, D.; Jamesw, D.; Paul, C.; John, S.; Erica, H.; Kannanv, B.; Panayotis, P.; Jamesh, W. Role of p21 in apoptosis and senescence of human colon cancer cells treated with camptothecin. J. Biol. Chem. 2002, 277, 17154–17160. [Google Scholar]
- Stennicke, H.R.; Salvesen, G.S. Properties of the caspases. Cell Death Differ. 1999, 6, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G. Caspases: The executioners of apoptosis. Biochem. J. 1997, 326, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Li, T.M.; Chen, G.C.; Lin, J.G.; Yeh, C.C.; Cheng, K.C.; Chung, J.G. Ellagic acid induced p53/p21 expression, G1 arrest and apoptosis in human bladder cancer T24 cells. Anticancer Res. 2005, 25, 971–979. [Google Scholar] [PubMed]
- Stamenkovic, I. Matrix metalloproteinases in tumor invasion and metastasis. Semin. Cancer Biol. 2001, 10, 415–433. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wu, Q.; Yang, J.; Wang, H.; Ding, X.; Yang, F.; Xu, X. Prognostic significance of MMP-9 and TIMP-1 serum and tissue expression in breast cancer. Int. J. Cancer 2008, 122, 2050–2056. [Google Scholar] [CrossRef] [PubMed]
- D Amore, P.A. Vascular endothelial cell growth factor-A. Am. J. Pathol. 2007, 171, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, M.; Daniele, A.; Coviello, M.; Venneri, M.T.; Abbate, I.; Caringella, M.E.; Di, T.S.; Divella, R.; Trerotoli, P.; Di, G.M. MMP-2, MMP-9, VEGF and CA 15.3 in breast cancer. Anticancer Res. 2007, 27, 3593–3600. [Google Scholar] [PubMed]
- Huang, T.H.; Chiu, Y.H.; Chan, Y.L.; Chiu, Y.H.; Wang, H.; Huang, K.C.; Li, T.L.; Hsu, K.H.; Wu, C.J. Prophylactic administration of fucoidan represses cancer metastasis by inhibiting vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) in Lewis tumor-bearing mice. Mar. Drugs 2015, 13, 1882–1900. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.S.; Lee, H.; Kim, S.; Lee, E. Piceatannol suppresses breast cancer cell invasion through the inhibition of MMP-9: Involvement of PI3K/AKT and NF-κB pathways. J. Agric. Food Chem. 2012, 60, 4083–4089. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Pina, V.; Martinez, E.; Fernandez-Ruiz, I.; Del, F.C.; Soares-Schanoski, A.; Jurado, T.; Siliceo, M.; Toledano, V.; Fernandez-Palomares, R.; Garcia-Rio, F.; et al. Role of MMPs in orchestrating inflammatory response in human monocytes via a TREM-1-PI3K-NF-κB pathway. J. Leukoc. Biol. 2012, 91, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Tsai, C. CCL2 increases MMP-9 expression and cell motility in human chondrosarcoma cells via the Ras/Raf/MEK/ERK/NF-κB signaling pathway. Biochem. Pharmcol. 2012, 83, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; Huang, S.S.; Deng, J.S. Anti-inflammatory activities of inotilone from Phellinus linteus through the inhibition of MMP-9, NF-κB, and MAPK activation in vitro and in vivo. PLoS ONE 2012, 7, e35922. [Google Scholar] [CrossRef] [PubMed]
- Babykutty, S.; Priya, P.S.; Nandini, R.J.; Kumar, M.A.S.; Nair, M.S.; Srinivas, P.; Gopala, S. Nimbolide retards tumor cell migration, invasion, and angiogenesis by downregulating MMP-2/9 expression via inhibiting ERK1/2 and reducing DNA-binding activity of NF-κB in colon cancer cells. Mol. Carcinog. 2012, 51, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Al, S.S.; Sharaf, L.H.; Luqmani, Y.A. Signalling pathways involved in endocrine resistance in breast cancer and associations with epithelial to mesenchymal transition (Review). Int. J. Oncol. 2011, 38, 1197–1217. [Google Scholar]
- Reddy, K.B.; Nabha, S.M.; Atanaskova, N. Role of MAP kinase in tumor progression and invasion. Cancer Metastasis Rev. 2003, 22, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, Y.; Jiang, T.; Lv, Z. Novel branch patterns and anticoagulant activity of glycosaminoglycan from sea cucumber Apostichopus japonicus. Int. J. Biol. Macromol. 2015, 72, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Wen, D.; Gao, N.; Xiao, C.; Yang, L.; Xu, L.; Lian, W.; Peng, W.; Jiang, J.; Zhao, J. Anticoagulant and antithrombotic evaluation of native fucosylated chondroitin sulfates and their derivatives as selective inhibitors of intrinsic factor Xase. Eur. J. Med. Chem. 2015, 92, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Lian, W.; Wu, M.; Huang, N.; Gao, N.; Xiao, C.; Li, Z.; Zhang, Z.; Zheng, Y.; Peng, W.; Zhao, J. Anti-HIV-1 activity and structure-activity-relationship study of a fucosylated glycosaminoglycan from an echinoderm by targeting the conserved CD4 induced epitope. Biochim. Biophys. Acta 2013, 1830, 4681–4691. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, D.; Wang, S.; Tao, L.; Wang, A.; Chen, W.; Zhu, Z.; Zheng, S.; Gao, X.; Lu, Y. Holothurian glycosaminoglycan inhibits metastasis and thrombosis via targeting of nuclear factor-κB/tissue factor/Factor Xa pathway in melanoma B16F10 cells. PLoS ONE 2013, 8, e56557. [Google Scholar] [CrossRef] [PubMed]
- Borsig, L.; Wang, L.; Cavalcante, M.C.; Cardilo-Reis, L.; Ferreira, P.L.; Mourao, P.A.; Esko, J.D.; Pavao, M.S. Selectin blocking activity of a fucosylated chondroitin sulfate glycosaminoglycan from sea cucumber. Effect on tumor metastasis and neutrophil recruitment. J. Biol. Chem. 2007, 282, 14984–14991. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Z.; Lian, E.C. Aggregation of human platelets by acidic mucopolysaccharide extracted from Stichopus japonicus Selenka. Thromb. Haemost. 1988, 59, 435–439. [Google Scholar] [PubMed]
- Kitazato, K.; Kitazato, K.T.; Nagase, H.; Minamiguchi, K. DHG, a new depolymerized holothurian glycosaminoglycan, exerts an antithrombotic effect with less bleeding than unfractionated or low molecular weight heparin, in rats. Thromb. Res. 1996, 84, 111–120. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; Burman, R.; Mansour, A.; Turki, Z.; Boulos, L.; Gullbo, J.; Göransson, U. The traditional medical uses and cytotoxic activities of sixty-one Egyptian plants: Discovery of an active cardiac glycoside from Urginea maritima. J. Ethnopharmacol. 2013, 145, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Makrilia, N.; Lappa, T.; Xyla, V.; Nikolaidis, I.; Syrigos, K. The role of angiogenesis in solid tumours: An overview. Eur. J. Intern. Med. 2009, 20, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Xue, M.; Ge, Y.; Zhang, J.; Wang, Q.; Hou, L.; Liu, Y.; Sun, L.; Li, Q. Anticancer properties and mechanisms of fucoidan on mouse breast cancer in vitro and in vivo. PLoS ONE 2012, 7, e43483. [Google Scholar] [CrossRef] [PubMed]
- Wimberger, P.; Chebouti, I.; Kasimir-Bauer, S.; Lachmann, R.; Kuhlisch, E.; Kimmig, R.; Süleyman, E.; Kuhlmann, J.D. Explorative investigation of vascular endothelial growth factor receptor expression in primary ovarian cancer and its clinical relevance. Gynecol. Oncol. 2014, 133, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Adeel, Z.; Marianne, F. Role of nuclear factor-κB in breast and colorectal cancer. Curr. Allergy Asthma Rep. 2013, 13, 44–49. [Google Scholar]
- Sample Availability: Not available.




© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Liu, Y.; Hao, J.; Zhao, X.; Lang, Y.; Fan, F.; Cai, C.; Li, G.; Zhang, L.; Yu, G. In Vivo Anti-Cancer Mechanism of Low-Molecular-Weight Fucosylated Chondroitin Sulfate (LFCS) from Sea Cucumber Cucumaria frondosa. Molecules 2016, 21, 625. https://doi.org/10.3390/molecules21050625
Liu X, Liu Y, Hao J, Zhao X, Lang Y, Fan F, Cai C, Li G, Zhang L, Yu G. In Vivo Anti-Cancer Mechanism of Low-Molecular-Weight Fucosylated Chondroitin Sulfate (LFCS) from Sea Cucumber Cucumaria frondosa. Molecules. 2016; 21(5):625. https://doi.org/10.3390/molecules21050625
Chicago/Turabian StyleLiu, Xiaoxiao, Yong Liu, Jiejie Hao, Xiaoliang Zhao, Yinzhi Lang, Fei Fan, Chao Cai, Guoyun Li, Lijuan Zhang, and Guangli Yu. 2016. "In Vivo Anti-Cancer Mechanism of Low-Molecular-Weight Fucosylated Chondroitin Sulfate (LFCS) from Sea Cucumber Cucumaria frondosa" Molecules 21, no. 5: 625. https://doi.org/10.3390/molecules21050625
APA StyleLiu, X., Liu, Y., Hao, J., Zhao, X., Lang, Y., Fan, F., Cai, C., Li, G., Zhang, L., & Yu, G. (2016). In Vivo Anti-Cancer Mechanism of Low-Molecular-Weight Fucosylated Chondroitin Sulfate (LFCS) from Sea Cucumber Cucumaria frondosa. Molecules, 21(5), 625. https://doi.org/10.3390/molecules21050625







