Carnosol and Related Substances Modulate Chemokine and Cytokine Production in Macrophages and Chondrocytes
Abstract
:1. Introduction
2. Results
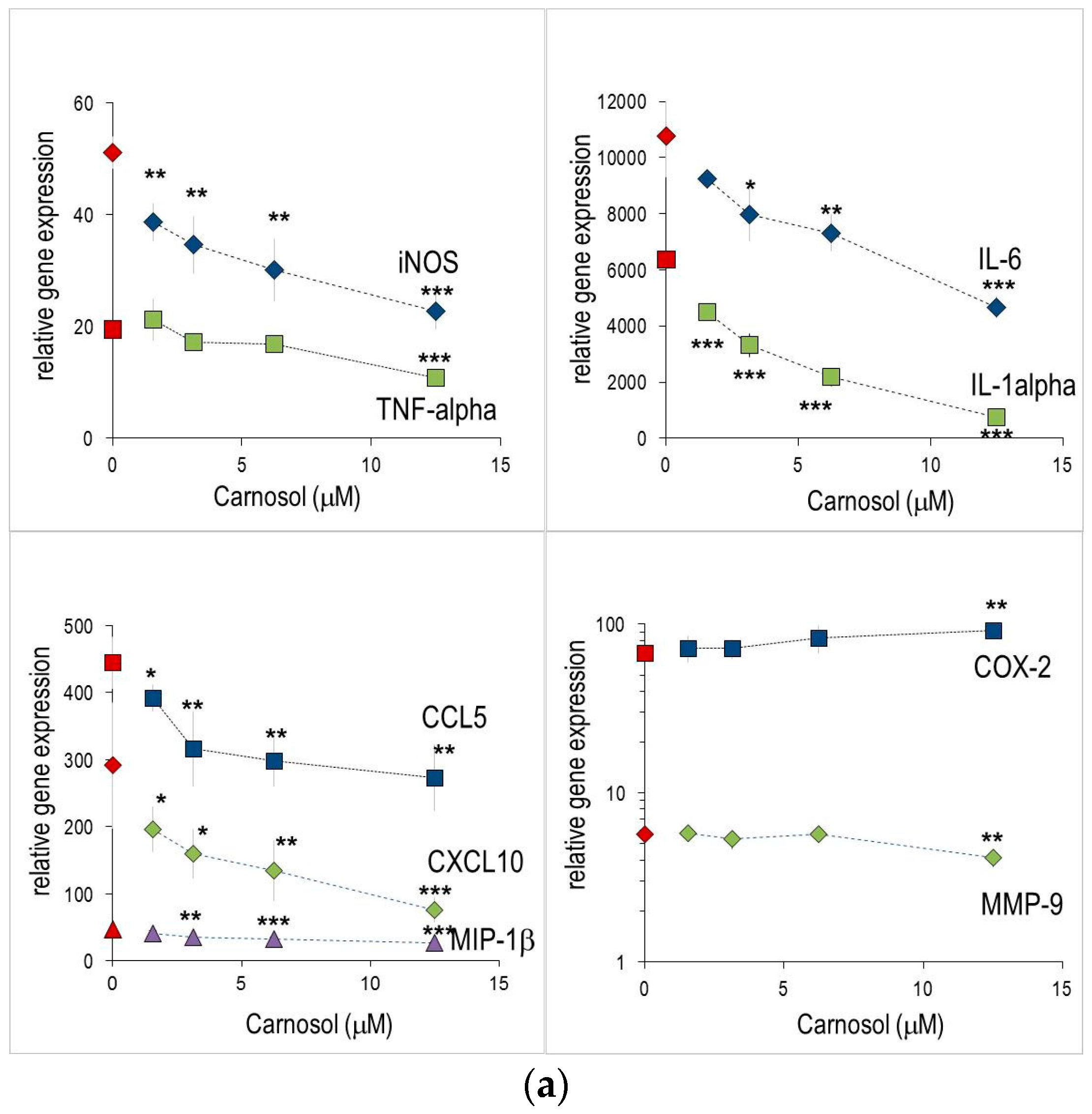
2.1. Anti-Inflammatory Effects Measured in Macrophages
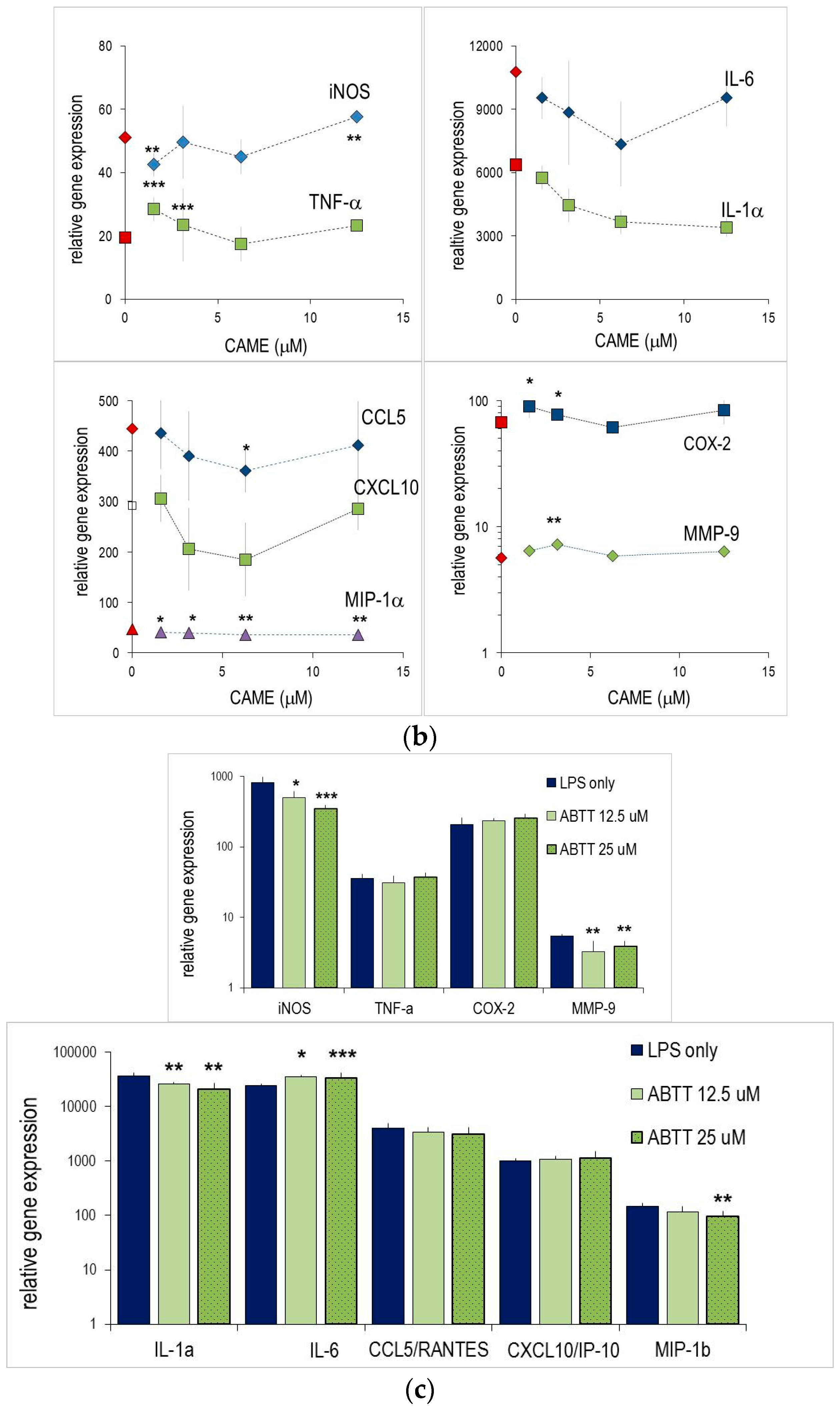
2.2. Abietane Diterpenes Modulate the Expression of Cytokine and Chemokine Genes in Macrophages
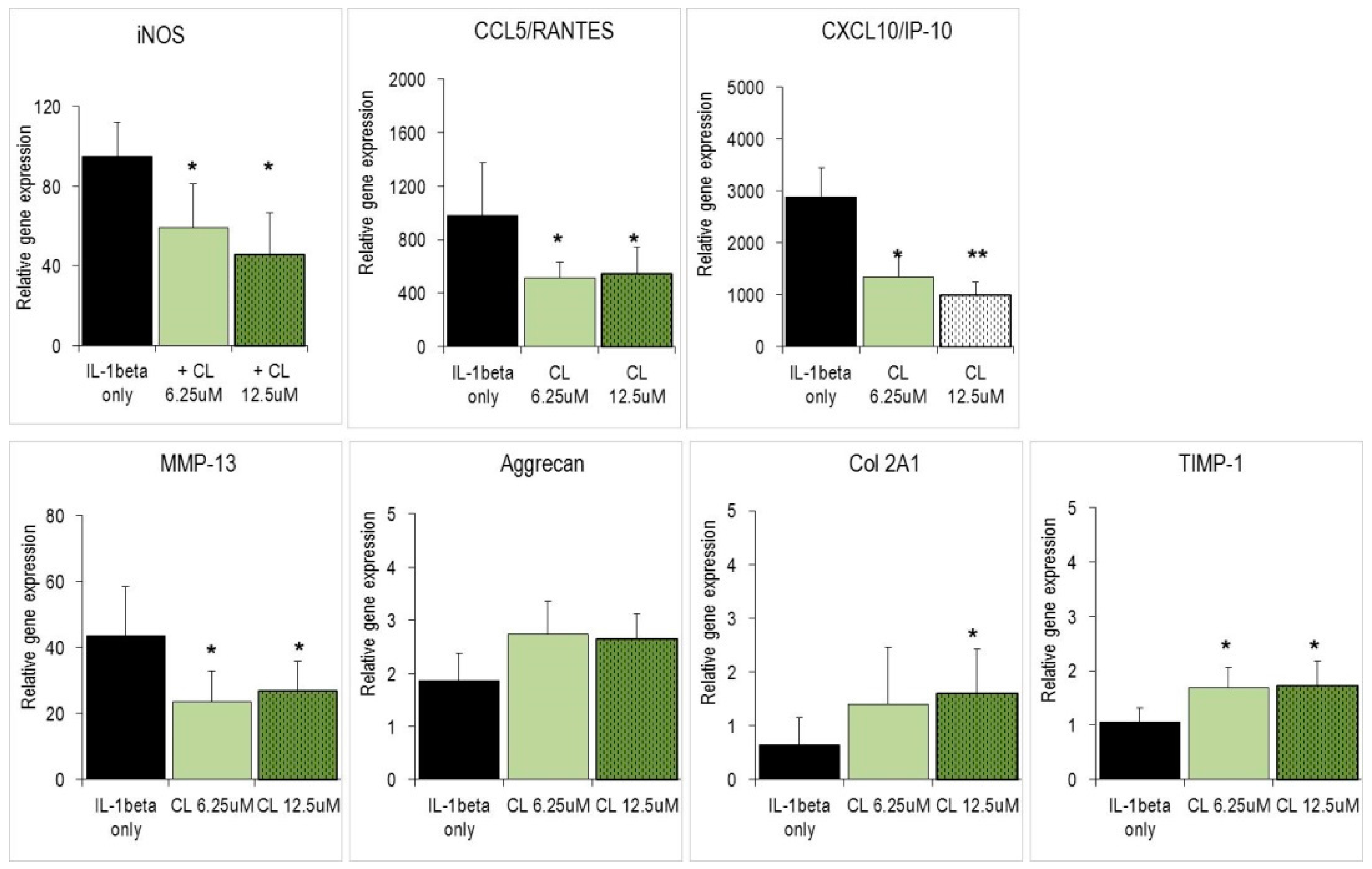
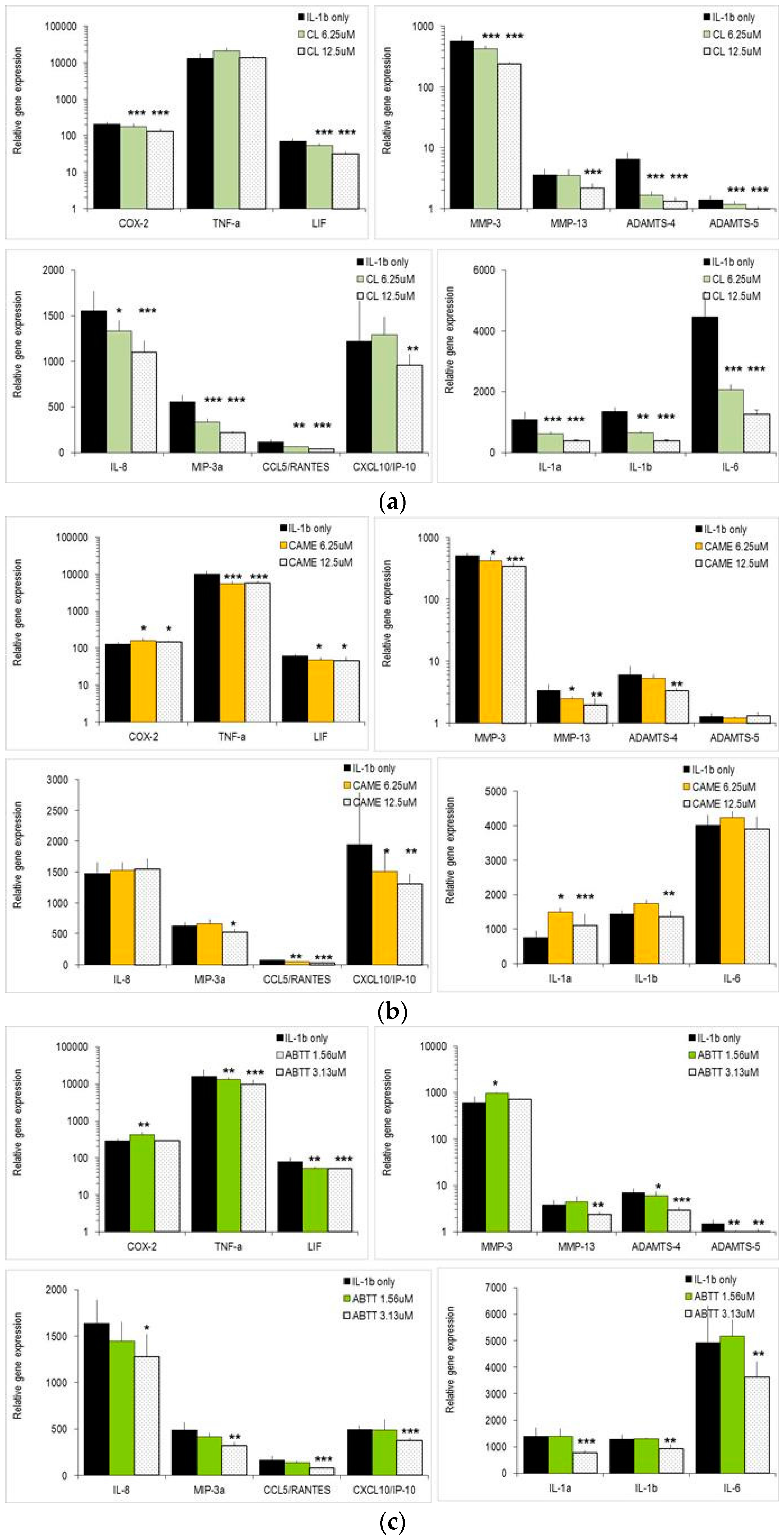
2.3. Carnosol and Related Diterpenes Impair Expression of Chemokine and Catabolic Genes in Chondrosarcoma Cells and Primary Chondrocytes
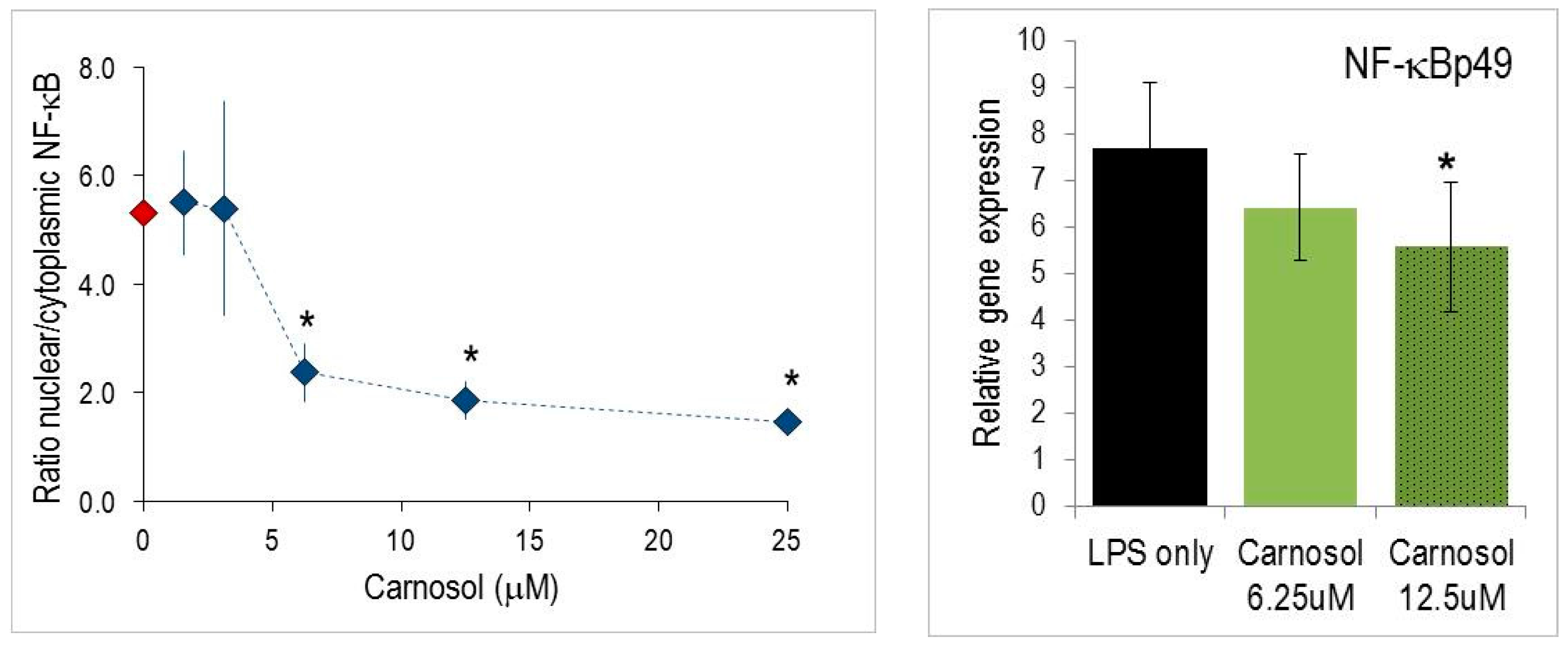
2.4. Nuclear Translocation of NF-κB
3. Discussion
4. Materials and Methods
4.1. Phytochemicals and Reagents
4.2. Cell Culture
4.3. RNA Isolation, cDNA Synthesis and RT-PCR
4.4. Measurement of Nitric Oxide and PGE2 Determination
4.5. NF-κB Translocation Experiments in Chondrocytes
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ABTT | 8,11,13-Abietatriene-11,12,20-triol |
| CA | Carnosic acid |
| CL | Carnosol |
| CAME | Carnosic acid 12-methyl-ether |
| NHAC-kn | Normal human articulocytes-knee |
| NO | Nitric oxide |
| PGE2 | Prostaglandin E2 |
References
- Aruoma, O.I.; Halliwell, B.; Aeschbach, R.; Loligers, J. Antioxidant and pro-oxidant properties of active rosemary constituents: Carnosol and carnosic acid. Xenobiotica 1992, 22, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Spencer, J.P.; Rossi, R.; Aeschbach, R.; Khan, A.; Mahmood, N.; Munoz, A.; Murcia, A.; Butler, J.; Halliwell, B. An evaluation of the antioxidant and antiviral action of extracts of rosemary and provencal herbs. Food Chem. Toxicol. 1996, 34, 449–456. [Google Scholar] [CrossRef]
- Chan, M.M.; Ho, C.T.; Huang, H.I. Effects of three dietary phytochemicals from tea, rosemary and turmeric on inflammation-induced nitrite production. Cancer Lett. 1995, 96, 23–29. [Google Scholar] [CrossRef]
- Lo, A.H.; Liang, Y.C.; Lin-Shiau, S.Y.; Ho, C.T.; Lin, J.K. Carnosol, an antioxidant in rosemary, suppresses inducible nitric oxide synthase through down-regulating nuclear factor-kappab in mouse macrophages. Carcinogenesis 2002, 23, 983–991. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Kikuzaki, H.; Nakatani, N. Antioxidant activity of chemical components from sage (Salvia officinalis L.) and thyme (Thymus vulgaris L.) measured by the oil stability index method. J. Agric. Food Chem. 2002, 50, 1845–1851. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.H.; Tu, P.F.; Zhou, K.; Wang, H.; Wang, B.H.; Lu, J.F. Antioxidant properties of phenolic diterpenes from rosmarinus officinalis. Acta Pharmacol. Sin. 2001, 22, 1094–1098. [Google Scholar] [PubMed]
- Dorrie, J.; Sapala, K.; Zunino, S.J. Carnosol-induced apoptosis and downregulation of BcL-2 in B-lineage leukemia cells. Cancer Lett. 2001, 170, 33–39. [Google Scholar] [CrossRef]
- Huang, M.T.; Ho, C.T.; Wang, Z.Y.; Ferraro, T.; Lou, Y.R.; Stauber, K.; Ma, W.; Georgiadis, C.; Laskin, J.D.; Conney, A.H. Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Res. 1994, 54, 701–708. [Google Scholar] [PubMed]
- Johnson, J.J.; Syed, D.N.; Heren, C.R.; Suh, Y.; Adhami, V.M.; Mukhtar, H. Carnosol, a dietary diterpene, displays growth inhibitory effects in human prostate cancer PC3 cells leading to G2-phase cell cycle arrest and targets the 5′-AMP-activated protein kinase (AMPK) pathway. Pharm. Res. 2008, 25, 2125–2134. [Google Scholar] [CrossRef] [PubMed]
- Mace, K.; Offord, E.A.; Harris, C.C.; Pfeifer, A.M. Development of in vitro models for cellular and molecular studies in toxicology and chemoprevention. Arch. Toxicol. Suppl. 1998, 20, 227–236. [Google Scholar] [PubMed]
- Moran, A.E.; Carothers, A.M.; Weyant, M.J.; Redston, M.; Bertagnolli, M.M. Carnosol inhibits beta-catenin tyrosine phosphorylation and prevents adenoma formation in the C57bl/6j/min/+ (min/+) mouse. Cancer Res. 2005, 65, 1097–1104. [Google Scholar] [PubMed]
- Visanji, J.M.; Thompson, D.G.; Padfield, P.J. Induction of G2/M phase cell cycle arrest by carnosol and carnosic acid is associated with alteration of cyclin a and cyclin b1 levels. Cancer Lett. 2006, 237, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.J. Carnosol: A promising anti-cancer and anti-inflammatory agent. Cancer Lett. 2011, 305, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, J.S.; Cho, H.S.; Lee, H.J.; Kim, S.Y.; Kim, S.; Lee, S.Y.; Chun, H.S. Carnosol, a component of rosemary (Rosmarinus officinalis L.) protects nigral dopaminergic neuronal cells. Neuroreport 2006, 17, 1729–1733. [Google Scholar] [CrossRef] [PubMed]
- Park, K.W.; Kundu, J.; Chae, I.G.; Kim, D.H.; Yu, M.H.; Kundu, J.K.; Chun, K.S. Carnosol induces apoptosis through generation of ROS and inactivation of STAT3 signaling in human colon cancer HCT116 cells. Int. J. Oncol. 2014, 44, 1309–1315. [Google Scholar] [PubMed]
- Peng, C.H.; Su, J.D.; Chyau, C.C.; Sung, T.Y.; Ho, S.S.; Peng, C.C.; Peng, R.Y. Supercritical fluid extracts of rosemary leaves exhibit potent anti-inflammation and anti-tumor effects. Biosci. Biotechnol. Biochem. 2007, 71, 2223–2232. [Google Scholar] [CrossRef] [PubMed]
- Poeckel, D.; Greiner, C.; Verhoff, M.; Rau, O.; Tausch, L.; Hornig, C.; Steinhilber, D.; Schubert-Zsilavecz, M.; Werz, O. Carnosic acid and carnosol potently inhibit human 5-lipoxygenase and suppress pro-inflammatory responses of stimulated human polymorphonuclear leukocytes. Biochem. Pharmacol. 2008, 76, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Wijeratne, S.S.; Cuppett, S.L. Potential of rosemary (Rosemarinus officinalis L.) diterpenes in preventing lipid hydroperoxide-mediated oxidative stress in Caco-2 cells. J. Agric. Food Chem. 2007, 55, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Chen, Y.; Zhang, L.; He, X.; He, X.; Lian, L.; Wu, X.; Lan, P. Carnosol inhibits cell adhesion molecules and chemokine expression by tumor necrosis factor-alpha in human umbilical vein endothelial cells through the nuclear factor-κb and mitogen-activated protein kinase pathways. Mol. Med. Rep. 2014, 9, 476–480. [Google Scholar] [PubMed]
- Martin, D.; Rojo, A.I.; Salinas, M.; Diaz, R.; Gallardo, G.; Alam, J.; De Galarreta, C.M.; Cuadrado, A. Regulation of heme oxygenase-1 expression through the phosphatidylinositol 3-kinase/AKT pathway and the Nrf2 transcription factor in response to the antioxidant phytochemical carnosol. J. Biol. Chem. 2004, 279, 8919–8929. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Tabuchi, T.; Tamaki, Y.; Kosaka, K.; Takikawa, Y.; Satoh, T. Carnosic acid and carnosol inhibit adipocyte differentiation in mouse 3T3-L1 cells through induction of phase2 enzymes and activation of glutathione metabolism. Biochem. Biophys. Res. Commun. 2009, 382, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.; Ho, C.T.; Lin-Shiau, S.Y.; Lin, J.K. Carnosol inhibits the invasion of B16/F10 mouse melanoma cells by suppressing metalloproteinase-9 through down-regulating nuclear factor-κb and c-jun. Biochem. Pharmacol. 2005, 69, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Subbaramaiah, K.; Cole, P.A.; Dannenberg, A.J. Retinoids and carnosol suppress cyclooxygenase-2 transcription by CREB-binding protein/p300-dependent and -independent mechanisms. Cancer Res. 2002, 62, 2522–2530. [Google Scholar] [PubMed]
- Bai, N.; He, K.; Roller, M.; Lai, C.S.; Shao, X.; Pan, M.H.; Ho, C.T. Flavonoids and phenolic compounds from Rosmarinus officinalis. J. Agric. Food Chem. 2010, 58, 5363–5367. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, M.; Saas, J.; Sohler, F.; Haag, J.; Soder, S.; Pieper, M.; Bartnik, E.; Beninga, J.; Zimmer, R.; Aigner, T. Comparison of the chondrosarcoma cell line SW1353 with primary human adult articular chondrocytes with regard to their gene expression profile and reactivity to IL-1beta. Osteoarthr. Cartil. 2005, 13, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Berenbaum, F. The regulation of chondrocyte function by proinflammatory mediators: Prostaglandins and nitric oxide. Clin. Orthop. Relat. Res. 2004, S37–S46. [Google Scholar] [CrossRef]
- Goldring, S.R.; Goldring, M.B. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin. Orthop. Relat. Res. 2004, S27–S36. [Google Scholar] [CrossRef]
- Sandell, L.J.; Xing, X.; Franz, C.; Davies, S.; Chang, L.W.; Patra, D. Exuberant expression of chemokine genes by adult human articular chondrocytes in response to IL-1β. Osteoarthr. Cartil. 2008, 16, 1560–1571. [Google Scholar] [CrossRef] [PubMed]
- Schwager, J.; Hoeller, U.; Wolfram, S.; Richard, N. Rose hip and its constituent galactolipids confer cartilage protection by modulating cytokine, and chemokine expression. BMC Complement. Altern. Med. 2011, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.; Horcajada, M.N.; Membrez Scalfo, F.; Ameye, L.; Offord, E.; Henrotin, Y. Carnosol inhibits pro-inflammatory and catabolic mediators of cartilage breakdown in human osteoarthritic chondrocytes and mediates cross-talk between subchondral bone osteoblasts and chondrocytes. PLoS ONE 2015, 10, e0136118. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.J.; Fischer, P.A.; Boltz, R.C.; Schmidt, J.A.; Colaianne, J.J.; Gough, A.; Rubin, R.A.; Miller, D.K. Characterization and quantitation of nf-kappab nuclear translocation induced by interleukin-1 and tumor necrosis factor-alpha. Development and use of a high capacity fluorescence cytometric system. J. Biol. Chem. 1998, 273, 28897–28905. [Google Scholar] [CrossRef] [PubMed]
- Del Bano, M.J.; Lorente, J.; Castillo, J.; Benavente-Garcia, O.; del Rio, J.A.; Ortuno, A.; Quirin, K.W.; Gerard, D. Phenolic diterpenes, flavones, and rosmarinic acid distribution during the development of leaves, flowers, stems, and roots of rosmarinus officinalis. Antioxidant activity. J. Agric. Food Chem. 2003, 51, 4247–4253. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, I.C.; Andres, L.S.; Leon, L.G.; Machin, R.P.; Padron, J.M.; Luis, J.G.; Delgadillo, J. Abietane diterpenoids from salvia pachyphylla and S. clevelandii with cytotoxic activity against human cancer cell lines. J. Nat. Prod. 2006, 69, 1803–1805. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Jin, Y.R.; Lim, Y.; Hong, J.T.; Kim, T.J.; Chung, J.H.; Yun, Y.P. Antiplatelet activity of carnosol is mediated by the inhibition of TXA2 receptor and cytosolic calcium mobilization. Vasc. Pharmacol. 2006, 45, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Jin, Y.R.; Lee, J.H.; Yu, J.Y.; Han, X.H.; Oh, K.W.; Hong, J.T.; Kim, T.J.; Yun, Y.P. Antiplatelet activity of carnosic acid, a phenolic diterpene from Rosmarinus officinalis. Planta Med. 2007, 73, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, D.W.; Lawrence, T.; Perretti, M.; Rossi, A.G. Inflammatory resolution: New opportunities for drug discovery. Nat. Rev. Drug Discov. 2004, 3, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Singletary, K.W. Rosemary extract and carnosol stimulate rat liver glutathione-s-transferase and quinone reductase activities. Cancer Lett. 1996, 100, 139–144. [Google Scholar] [CrossRef]
- Amin, A.R.; Attur, M.; Patel, R.N.; Thakker, G.D.; Marshall, P.J.; Rediske, J.; Stuchin, S.A.; Patel, I.R.; Abramson, S.B. Superinduction of cyclooxygenase-2 activity in human osteoarthritis-affected cartilage. Influence of nitric oxide. J. Clin. Investig. 1997, 99, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Murrell, G.A.; Jang, D.; Williams, R.J. Nitric oxide activates metalloprotease enzymes in articular cartilage. Biochem. Biophys. Res. Commun. 1995, 206, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Vuolteenaho, K.; Moilanen, T.; Knowles, R.G.; Moilanen, E. The role of nitric oxide in osteoarthritis. Scand. J. Rheumatol. 2007, 36, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Melchiorri, C.; Meliconi, R.; Frizziero, L.; Silvestri, T.; Pulsatelli, L.; Mazzetti, I.; Borzi, R.M.; Uguccioni, M.; Facchini, A. Enhanced and coordinated in vivo expression of inflammatory cytokines and nitric oxide synthase by chondrocytes from patients with osteoarthritis. Arthritis Rheum. 1998, 41, 2165–2174. [Google Scholar] [CrossRef]
- Richard, N.; Porath, D.; Radspieler, A.; Schwager, J. Effects of resveratrol, piceatannol, tri-acetoxystilbene, and genistein on the inflammatory response of human peripheral blood leukocytes. Mol. Nutr. Food Res. 2005, 49, 431–442. [Google Scholar] [CrossRef] [PubMed]
- D’Acquisto, F.; Cicatiello, L.; Iuvone, T.; Ialenti, A.; Ianaro, A.; Esumi, H.; Weisz, A.; Carnuccio, R. Inhibition of inducible nitric oxide synthase gene expression by glucocorticoid-induced protein(s) in lipopolysaccharide-stimulated J774 cells. Eur. J. Pharmacol. 1997, 339, 87–95. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors (limited stock).
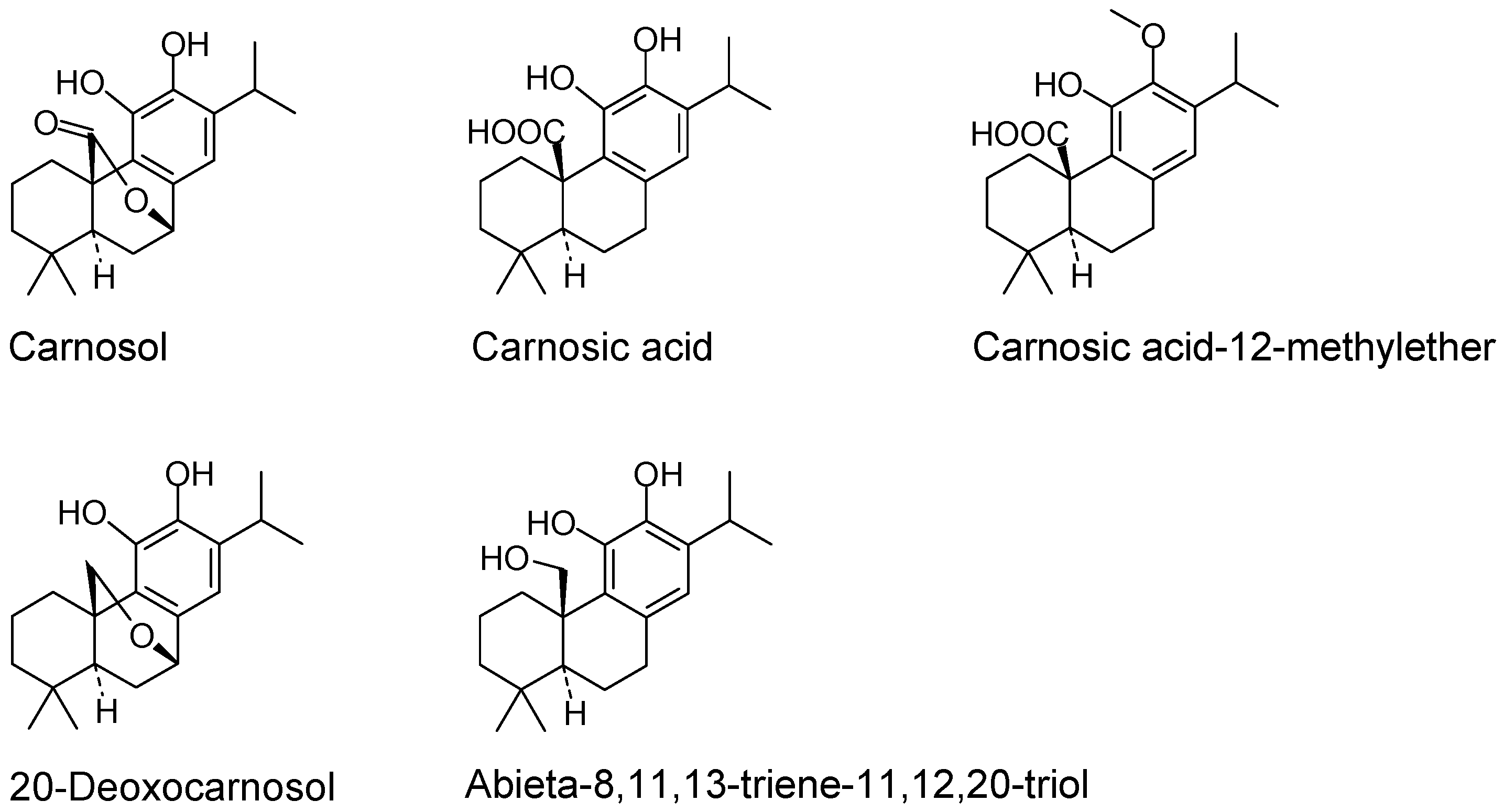
| Carnosol (CL) | Carnosic Acid 12-Methyl Ether (CAME) | 20-Deoxy-Carnosol | Carnosic Acid (CA) | Abieta-8,11,13-Triene-11,12,20 Triol (ABTT) | l-NAME 1 | |
|---|---|---|---|---|---|---|
| NO | 5.0 ± 2.8 | 3.8 ± 0.6 | 11.9 ± 0.6 | 6.9 ± 2.2 | 12.5 ± 4.8 | 150 ± 34 |
| PGE2 | 9.4 ± 2.1 | 44.4 ± 6.1 | 18.8 ± 6.4 | 11.4 ± 0.9 | 7.8 ± 4.6 | >1000 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwager, J.; Richard, N.; Fowler, A.; Seifert, N.; Raederstorff, D. Carnosol and Related Substances Modulate Chemokine and Cytokine Production in Macrophages and Chondrocytes. Molecules 2016, 21, 465. https://doi.org/10.3390/molecules21040465
Schwager J, Richard N, Fowler A, Seifert N, Raederstorff D. Carnosol and Related Substances Modulate Chemokine and Cytokine Production in Macrophages and Chondrocytes. Molecules. 2016; 21(4):465. https://doi.org/10.3390/molecules21040465
Chicago/Turabian StyleSchwager, Joseph, Nathalie Richard, Ann Fowler, Nicole Seifert, and Daniel Raederstorff. 2016. "Carnosol and Related Substances Modulate Chemokine and Cytokine Production in Macrophages and Chondrocytes" Molecules 21, no. 4: 465. https://doi.org/10.3390/molecules21040465





