In Vivo and in Vitro Study on Drug-Drug Interaction of Lovastatin and Berberine from Pharmacokinetic and HepG2 Cell Metabolism Studies
Abstract
:1. Introduction
2. Results
2.1. UPLC-MS/MS Method Development
2.2. Plasma Sample Procedure
2.3. Method Validation
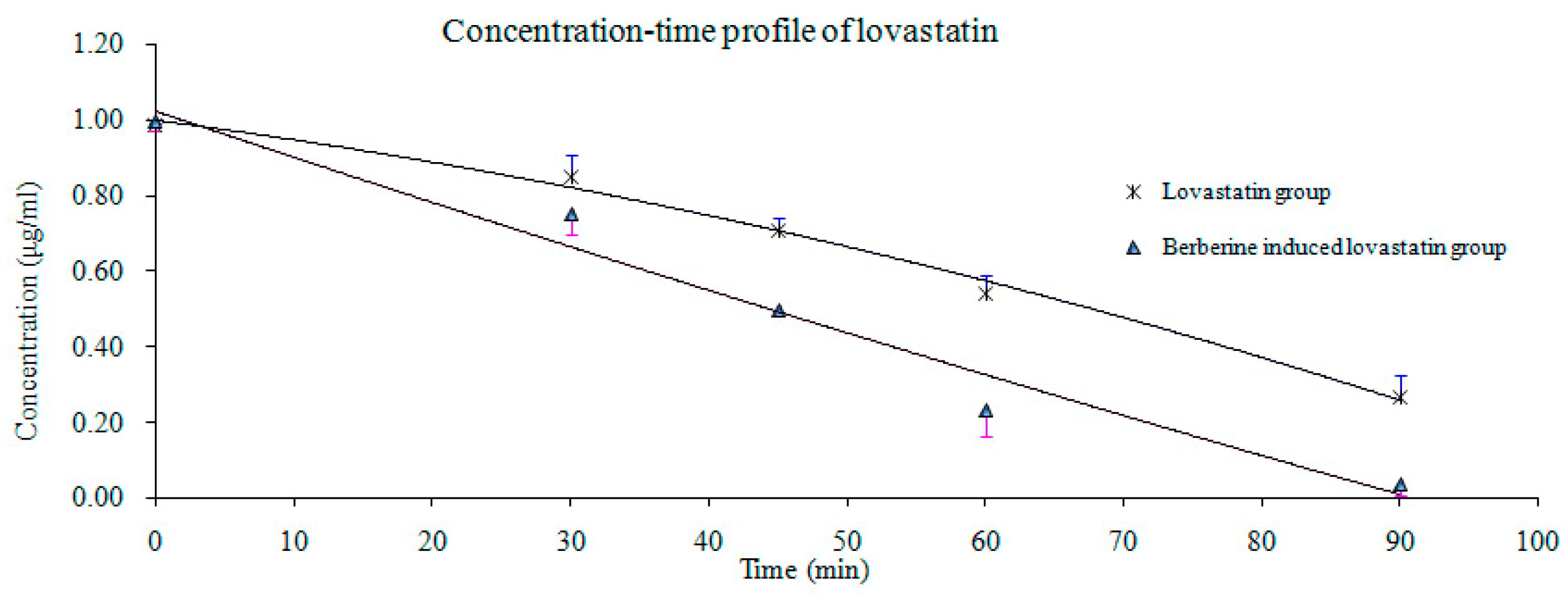
2.4. Pharmacokinetic Study
2.5. Evaluation of Lovastatin Metabolism Induced by Berberine
3. Discussion
4. Experimental Section
4.1. Reagent and Chemicals
4.2. Instrumental
4.3. Preparation of Reference Solution and Working Solutions
4.4. Plasma Sample Procedure
4.5. UPLC-MS/MS Method Optimizing and Validation
4.6. Design of the Study of Lovastatin Pharmacokinetics Induced by Berberine
4.7. Evaluation of the Metabolic Activity of Lovastatin in BER-Induced HepG2 Cells
4.8. Data Analysis
5. Conclusions
Acknowledgment
Authors Contributions
Conflicts of Interest
References
- Xu, Y.; Wang, L.; He, J.; Bi, Y.; Wang, T.; Wang, L.; Jiang, Y.; Dai, M.; Lu, J.; Xu, M.; et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013, 310, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Wang, J.J.; Kuo, S.L.; Pan, T.M. Monascus fermentation of dioscorea for increasing the production of cholesterol-lowering agent-monacolin K and anti-inflammation agent-monascin. Appl. Microbiol. Biotechnol. 2006, 72, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Kong, D.; Iruela-Arispe, L.; Begley, U.; Tang, D.; Galper, J.B. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors interfere with angiogenesis by inhibiting the geranylgeranylation of Rho A. Circ Res. 2002, 91, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, W.; Kirchner, G.; Hallensleben, K.; Mancinelli, L.; Deters, M.; Hackbarth, I.; Benet, L.Z.; Sewing, K.F.; Christians, U. Comparison of cytochrome P450-dependent metabolism and drug interactions of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors lovastatin and pravastatin in the liver. Drug Metab. Dispos. 1999, 27, 173–179. [Google Scholar] [PubMed]
- Mangravite, L.M.; Engelhardt, B.E.; Medina, M.W.; Smith, J.D.; Brown, C.D.; Chasman, D.I.; Mecham, B.H.; Howie, B.; Shim, H.; Naidoo, D.; et al. A statin-dependent QTL for GATM expression is associated with statin-induced myopathy. Nature 2013, 502, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Lodge, J.W.; Fletcher, B.L.; Brown, S.S.; Parham, A.J.; Fernando, R.A.; Collins, B.J. Determination of lovastatin hydroxy acid in female B6C3F1 mouse serum. J. Anal. Toxicol. 2008, 32, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Fan, L.Y.; Zhang, W.; Cao, C.X. Stacking and quantitative analysis of lovastatin in urine samples by the transient moving chemical reaction boundary method in capillary electrophoresis. Anal. Bioanal. Chem. 2007, 387, 2719–2725. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zeng, B.H.; Shang, H.T.; Cen, Y.Y.; Wei, H. Bama miniature pigs (Sus scrofa domestica) as a model for drug evaluation for humans: Comparison of in vitro metabolism and in vivo pharmacokinetics of lovastatin. Comp. Med. 2008, 58, 580–587. [Google Scholar] [PubMed]
- Li, Z.; Seeram, N.P.; Lee, R.; Thames, G.; Minutti, C.; Wang, H.J.; Heber, D. Plasma clearance of lovastatin versus Chinese red yeast rice in healthy volunteers. J. Altern. Complement. Med. 2005, 11, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Donovan, J.M.; Kisicki, J.C.; Stiles, M.R.; Tracewell, W.G.; Burke, S.K. Effect of colesevelamon lovastatin pharmacokinetics. Ann. Pharmacother. 2002, 36, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Prueksaritanont, T.; Zhao, J.J.; Ma, B.; Roadcap, B.A.; Tang, C.; Qiu, Y.; Liu, L.; Lin, J.H.; Pearson, P.G.; Baillie, T.A. Mechanistic studies on metabolic interactions between gemfibrozil and statins. J. Pharmacol. Exp. Ther. 2002, 301, 1042–1051. [Google Scholar] [CrossRef] [PubMed]
- Kivist, K.T.; Kantola, T.; Neuvonen, P.J. Different effects of itraconazole on the pharmacokinetics of fluvastatin and lovastatin. Br. J. Clin. Pharmacol. 1998, 46, 49–53. [Google Scholar] [CrossRef]
- Lilja, J.J.; Neuvonen, M.; Neuvonen, P.J. Effects of regular consumption of grape fruit juice on the pharmacokinetics of simvastatin. Br. J. Clin. Pharmacol. 2004, 58, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.H.; Kim, I.S.; Van Le, T.K.; Jung, I.H.; Yoo, H.H.; Kim, D.H. Gutmicrobiota-mediated drug interactions between lovastatin and antibiotics. Drug Metab. Dispos. 2014, 42, 1508–1513. [Google Scholar] [CrossRef] [PubMed]
- Keskitalo, J.E.; Kurkinen, K.J.; Neuvonen, M.; Backman, J.T.; Neuvonen, P.J.; Niemi, M. No significant effect of ABCB1 haplotypes on the pharmacokinetics of fluvastatin, pravastatin, lovastatin, and rosuvastatin. Br. J. Clin. Pharmacol. 2009, 68, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Lu, F.; Xu, L.J.; Dong, H.; Yi, P.; Wang, F.; Huang, Z.; Zou, X. The anti-diabetic effects and pharmacokinetic profiles of berberine in mice treated with Jiao-Tai-Wan and its compatibility. Phytomedicine 2013, 20, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Zhou, H.; Liu, Z.M.; Ma, Y.; Wang, T.; Liu, Y.; Wang, C. Influence of Coptis Chinensis on pharmacokinetics of flavonoids after oral administration of radix Scutellariae in rats. Biopharm. Drug Dispos. 2009, 30, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Zhang, Y.L.; Dong, Y.; Gong, J.Y.; Cui, H.M. CYP 450 enzyme inhibition of berberine in pooled human liver microsomes by cocktail probe drugs. China J. Chin. Mater. Med. 2013, 38, 2009–2014. [Google Scholar]
- Gurley, B.J.; Swain, A.; Barone, G.W.; Williams, D.K.; Breen, P.; Yates, C.R.; Stuart, L.B.; Hubbard, M.A.; Tong, Y.; Cheboyina, S. Effect of goldenseal (Hydrastiscanadensis) and kavakava (Pipermethysticum) supplementation on digoxin pharmacokinetics inhumans. Drug Metab. Dispos. 2007, 35, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.J.; Wei, J.; Zuo, Z.Y.; Wang, Y.M.; Song, D.Q.; You, X.F.; Zhao, L.X.; Pan, H.N.; Jiang, J.D. Combination of simvastatin with berberine improves the lipid-lowering efficacy. Metabolism 2008, 57, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Tong, N.; Zhang, J.; Chen, Y.; Li, Z.; Luo, Y.; Zuo, H.; Zhao, X. Berberine sensitizes multiple human cancer cells to the anticancer effects of doxorubicin in vitro. Oncol. Lett. 2012, 3, 1263–1267. [Google Scholar] [PubMed]
- Musumeci, R.; Speciale, A.; Costanzo, R.; Annino, A.; Ragusa, S.; Rapisarda, A.; Pappalardo, M.S.; Iauk, L. Berberisaetnensis C. Presl. extracts: Antimicrobial properties and interaction with ciprofloxacin. Int. J. Antimicrob. Agents 2003, 22, 48–53. [Google Scholar] [CrossRef]
- Quan, H.; Cao, Y.Y.; Xu, Z.; Zhao, J.X.; Gao, P.H.; Qin, X.F.; Jiang, Y.Y. Potent In vitro synergism of fluconazole and berberine chloride against clinicalisolates of Candidaalbicans resistant to fluconazole. Antimicrob. Agents Chemother. 2006, 50, 1096–1099. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.L.; Tsai, T.H. Measurement of unbound pravastatin in rat blood and bile on the perspective of hepatobiliary excretion and its interaction with cyclosporin A and berberine. Anal. Chim. Acta 2005, 548, 33–40. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, H.; Chen, F.; Lan, K.; Wang, A. Reduced system exposures of total rhein and baicalin after combinatory oral administration of rhein, baicalin and berberine to beagle dogs and rats. J. Ethnopharmacol. 2013, 145, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.C.; Li, Q.; Xin, H.; Yu, A.; Zhong, M. Effects of berberine on the blood concentration of cyclosporine A in renal transplanted recipients: Clinical and pharmacokinetic study. Eur. J. Clin. Pharmacol. 2005, 61, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.W.; Wu, X.C.; Li, Q.; Yu, A.R.; Zhong, M.Y.; Liu, Y.Y. The effects of berberine on the pharmacokinetics of ciclosporin A in healthy volunteers. Methods Find Exp. Clin. Pharmacol. 2006, 28, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.M.; He, P.; Liu, A.M.; Zhang, L.; Liu, Y.; Dai, R. Drug-drug interactions between ketoconazole and berberine in rats: Pharmacokinetic effects benefit pharmacodynamic synergism. Phytother. Res. 2011, 26, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Chi, L.; Peng, L.; Hu, X.; Pan, N.; Zhang, Y. Berberine combined with atorvastatin downregulates LOX1 expression through the ET1 receptor in monocyte macrophages. Int. J. Mol. Med. 2014, 34, 283–90. [Google Scholar] [PubMed]
- Cui, H.M.; Zhang, Q.Y.; Wang, J.L.; Chen, J.L.; Zhang, Y.L.; Tong, X.L. In vitro studies of berberine metabolism and its effect of enzyme induction on HepG2 cells. J. Ethnopharmacol. 2014, 158, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lin, J.; Smolarek, T.; Tremaine, L. P-glycoprotein has differential effects on the disposition of statin acid and lactone form sinMDR1A/B knockout and wild-typemice. Drug Metab. Dispos. 2007, 5, 1725–1729. [Google Scholar] [CrossRef] [PubMed]
- Berta, E.; Harangi, M.; Zsíros, N.; Nagy, E.V.; Paragh, G.; Bodor, M. Effect of thyroidhormone status and concomitant medication on statin induced adverse effects in hyperlipidemic patients. Pharmazie 2014, 69, 420–423. [Google Scholar] [PubMed]
- Li, J.; Volpe, D.A.; Wang, Y.; Zhang, W.; Bode, C.; Owen, A.; Hidalgo, I.J. Use of transporter knockdown Caco-2 cells to investigate the in vitro efflux of statin drugs. Drug Metab. Dispos. 2011, 39, 1196–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Luzum, J.A.; Phelps, M.A. Liquid chromatography-tandem mass spectrometry assay for the simultaneous quantification of simvastatin, lovastatin, atorvastatin, and their major metabolites in human plasma. J. Chromatogr. B 2015, 983–984, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Guidance for Industry: Bioanalytical Method Validation. Available online: http://www.fda.gov/downloads/Drugs/Guidances/ucm070107.pdf (accessed on 18 December 2014).
- Drug Bank Database DB0022. Available online: http://www.drugbank.ca/drugs/DB00227 (accessed on 16 November 2014).
- FDA guidance. Drug Development and Drug Interactions: Table of Substrates, Inhibitors and Inducers. Available online: http://www.fda.gov/drugs/developmentapprovalprocess/developmentresources/druginteractionslabeling/ucm080499.htm (accessed on 3 December 2014).
- Liu, M.; Su, X.; Li, G.; Zhao, G.; Zhao, L. Validated UPLC-MS/MS method for simultaneous determination of simvastatin, simvastatin hydroxyl acid and berberine in rat plasma: Application to the drug-drug pharmacokinetic interaction study of simvastatin combined with berberine after oral administration in rats. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 1006, 8–15. [Google Scholar]
- Ju, W.J.; Peng, K.W.; Yang, S.Y.; Huijing, S.; Mario, S.; Zhuo, W.M. A simple protein precipitation-based simultaneous quantification of lovastatin and its active metabolite lovastatin acid in human plasma by ultra-performance liquid chromatography-tandem mass spectrometry using polarity switching. J. Chromatogr. Sep. Technol. 2015, 6, 268. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds areavailable from the authors.
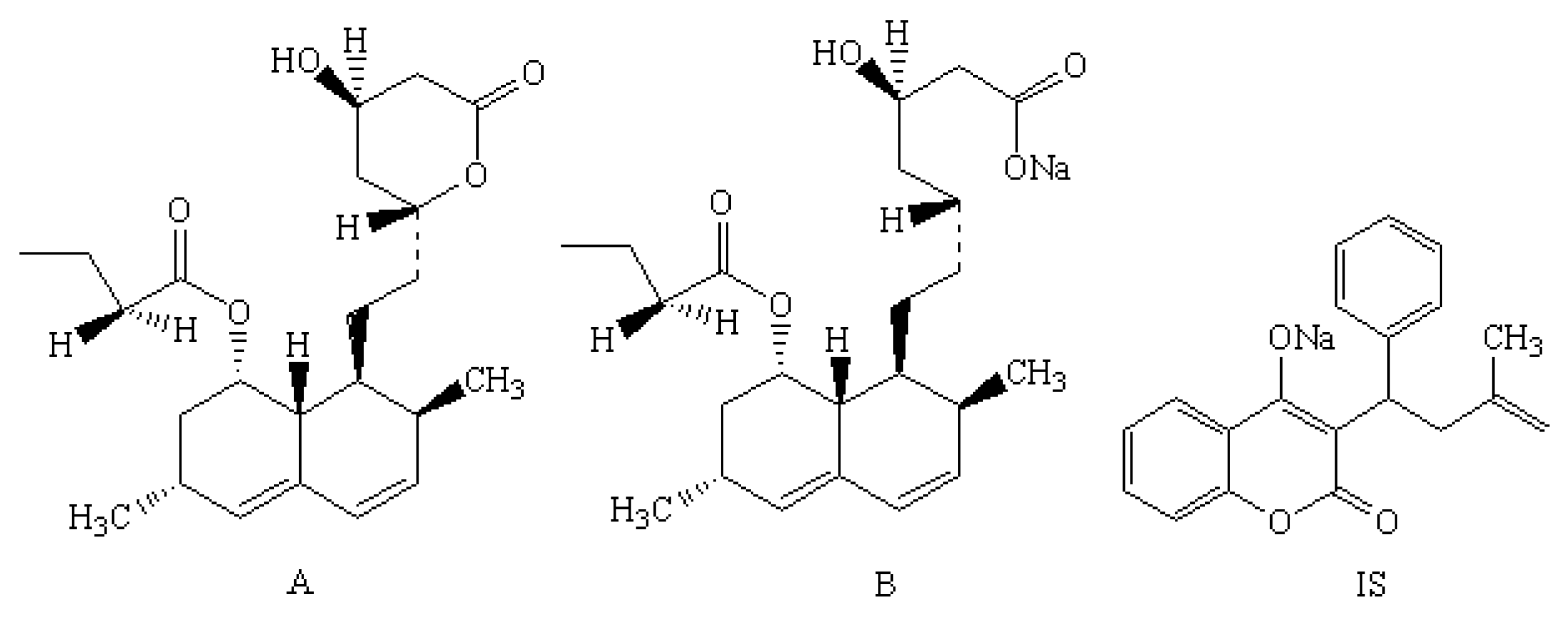
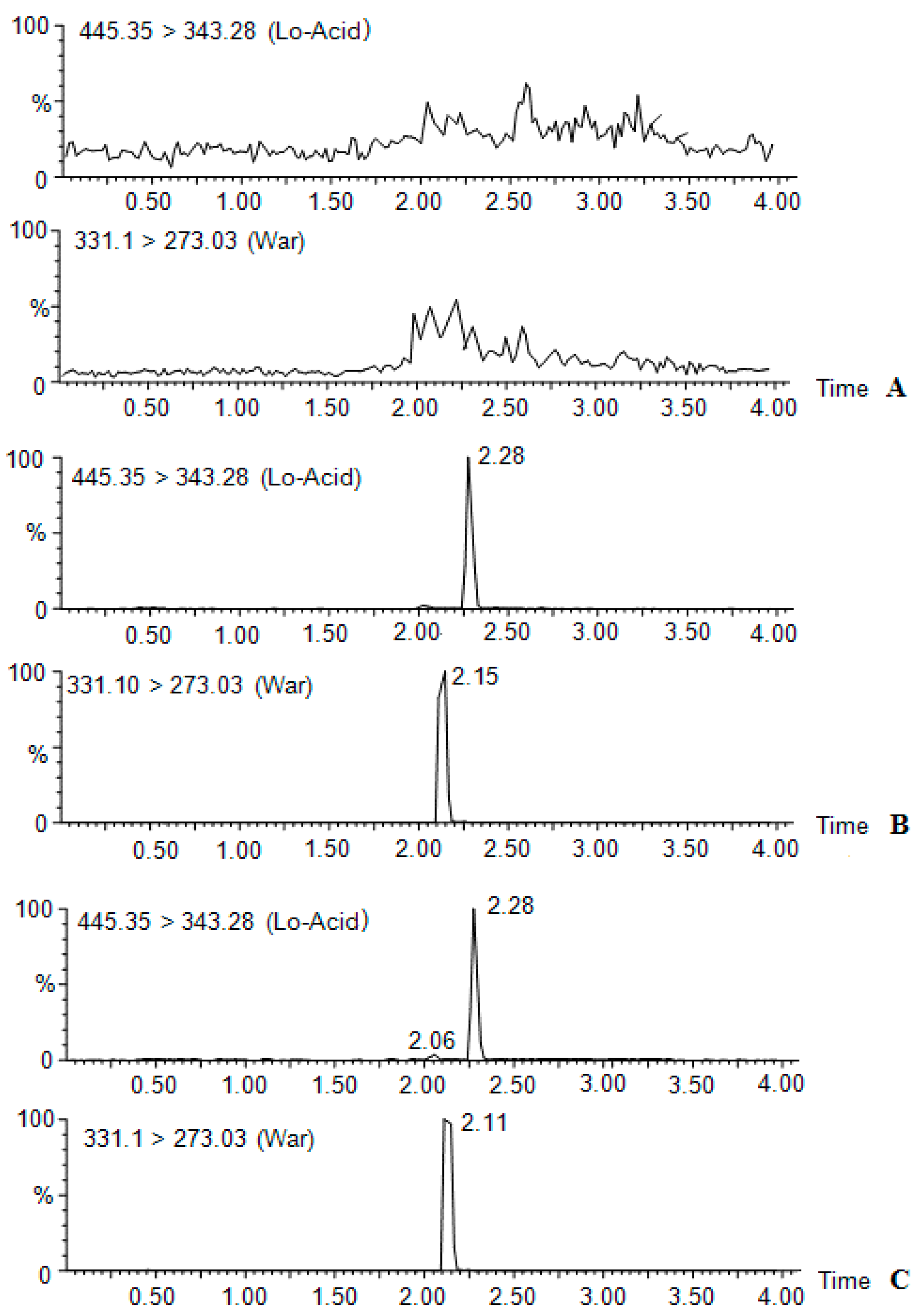

| Compound | Molecular Weight | Precursor Ion | Product Ion (m/z) | Cone (V) | Collision (V) | Retention Time (min) |
|---|---|---|---|---|---|---|
| Lovastatina cid | 444.5 | 445.35 | 343.26 | 36 | 18 | 2.3 |
| Warfarin | 330.10 | 331.30 | 273.03 | 44 | 22 | 2.1 |
| Content | Concentration (ng·mL−1) | ||||
|---|---|---|---|---|---|
| Nominal | 1 | 10 | 100 | ||
| Recovery (%) | Accuracy | 89.2 | 92.5 | 94.6 | |
| RSD | 6.8 | 5.7 | 4.3 | ||
| Matrixeffect (%) | Accuracy | 95.8 | 96.4 | 102.7 | |
| RSD | 6.4 | 4.1 | 6.9 | ||
| Precision (%) | Inter-day | Accuracy | 92.5 | 94.6 | 101.5 |
| RSD | 9.6 | 6.1 | 4.8 | ||
| Inner-day | Accuracy | 89.6 | 98.3 | 104.2 | |
| RSD | 6.5 | 7.2 | 4.4 | ||
| Stability (%) | 1cycle | Accuracy | 92.2 | 96.8 | 102.3 |
| RSD | 8.2 | 4.6 | 3.5 | ||
| 3cycles | Accuracy | 90.6 | 93.1 | 95.9 | |
| RSD | 7.8 | 6.3 | 5.4 | ||
| 12 h post-procedure | Accuracy | 95.3 | 97.9 | 103.4 | |
| RSD | 5.9 | 4.8 | 3.3 | ||
| 12 h under sampler | Accuracy | 94.6 | 97.5 | 101.6 | |
| RSD | 6.8 | 4.2 | 4.1 | ||
| Parameter | Berberine Induced Prior to Lovastatin | Lovastatin | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| t1/2α (h) | 3.75 * | 2.19 | 2.20 | 0.41 |
| t1/2β (h) | 16.60 ** | 5.46 | 2.93 | 1.32 |
| V (L) | 0.24 | 0.09 | 0.09 | 0.05 |
| CL/F (L/h) | 0.05 | 0.02 | 0.03 | 0.01 |
| AUC0–12 (ng/mL·h) | 37.28 ** | 14.85 | 91.97 | 55.01 |
| AUC0-∞ (ng/mL·h) | 41.17 ** | 15.98 | 93.66 | 55.49 |
| Lagtime (h) | 0.03 | 0.05 | 0.02 | 0.05 |
| MRT0–12 (h) | 7.42 | 1.41 | 4.37 | 0.97 |
| tmax (h) | 1.03 | 0.59 | 0.92 | 0.20 |
| Cmax (ng/mL) | 11.13 ** | 4.32 | 47.40 | 14.39 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, H.; Wang, J.; Zhang, Q.; Dang, M.; Liu, H.; Dong, Y.; Zhang, L.; Yang, F.; Wu, J.; Tong, X. In Vivo and in Vitro Study on Drug-Drug Interaction of Lovastatin and Berberine from Pharmacokinetic and HepG2 Cell Metabolism Studies. Molecules 2016, 21, 464. https://doi.org/10.3390/molecules21040464
Cui H, Wang J, Zhang Q, Dang M, Liu H, Dong Y, Zhang L, Yang F, Wu J, Tong X. In Vivo and in Vitro Study on Drug-Drug Interaction of Lovastatin and Berberine from Pharmacokinetic and HepG2 Cell Metabolism Studies. Molecules. 2016; 21(4):464. https://doi.org/10.3390/molecules21040464
Chicago/Turabian StyleCui, Hanming, Jialong Wang, Qiuyan Zhang, Mengmeng Dang, Hui Liu, Yu Dong, Lu Zhang, Fang Yang, Jianhua Wu, and Xiaolin Tong. 2016. "In Vivo and in Vitro Study on Drug-Drug Interaction of Lovastatin and Berberine from Pharmacokinetic and HepG2 Cell Metabolism Studies" Molecules 21, no. 4: 464. https://doi.org/10.3390/molecules21040464





