Regulation of Candida albicans Interaction with Macrophages through the Activation of HOG Pathway by Genistein
Abstract
:1. Introduction
2. Results
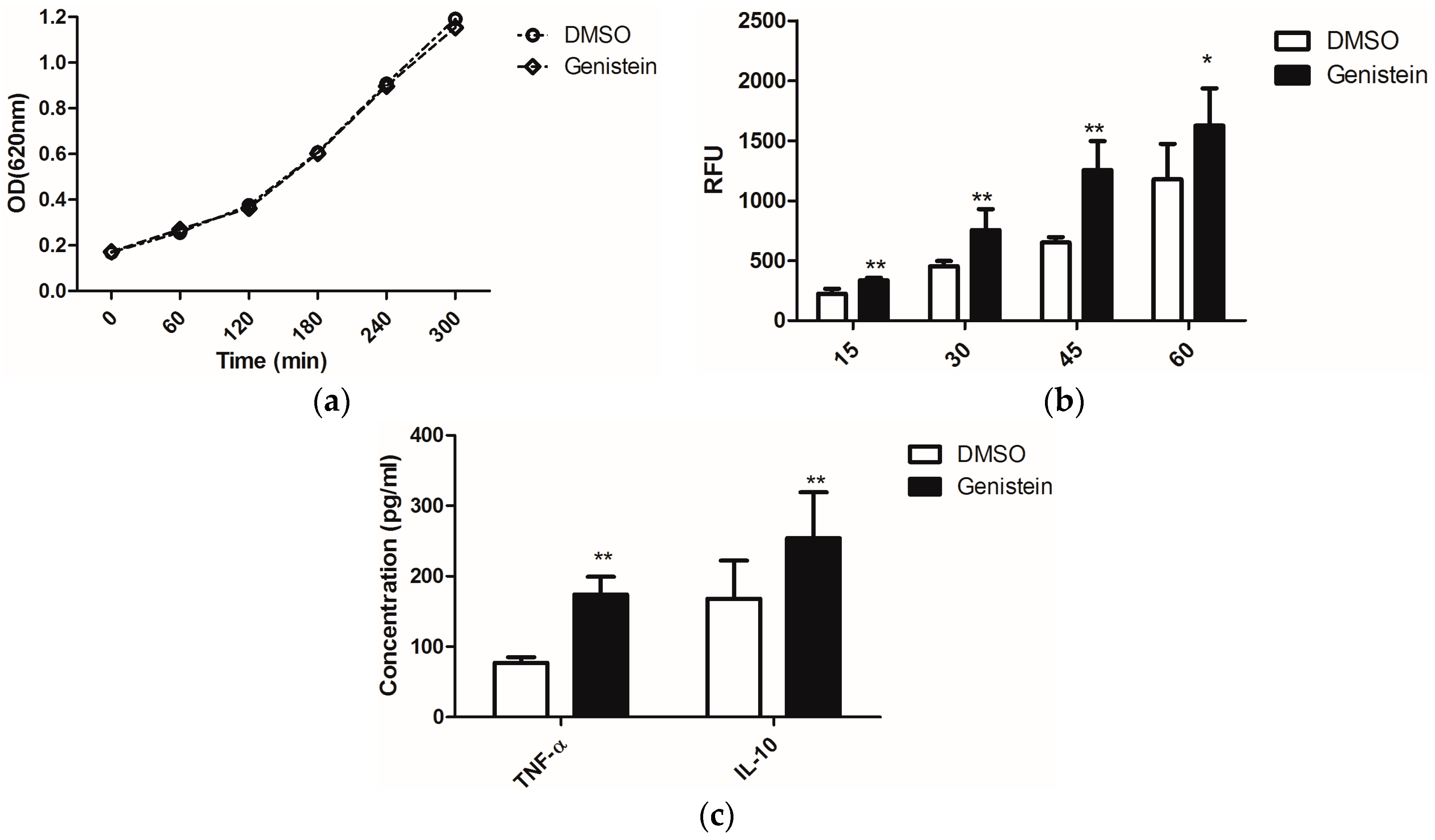
2.1. Genistein Treatment of C. albicans Enhances the Activity of Macrophages
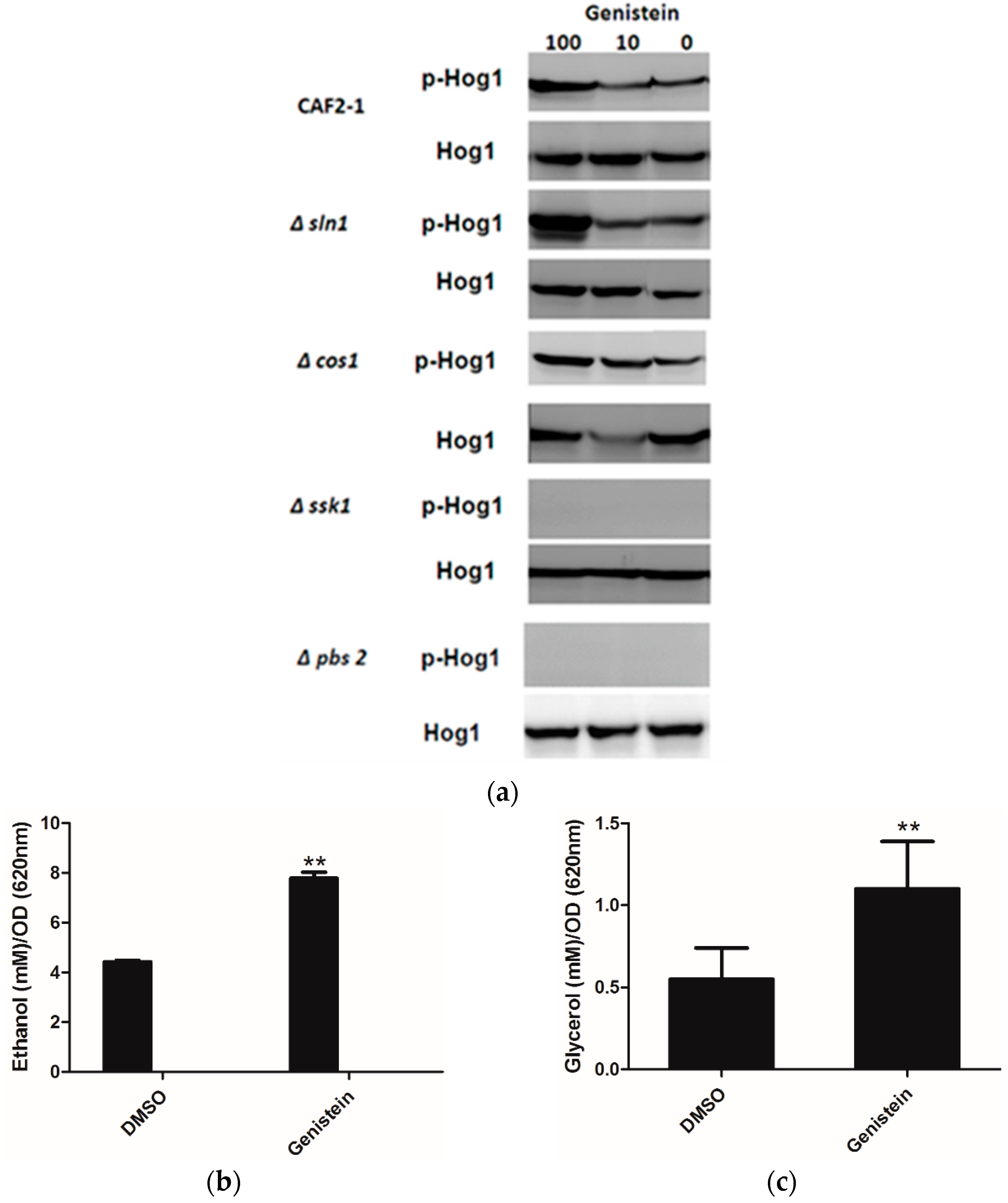
2.2. Genistein Treatment of C. albicans Activates the HOG Pathway of C. albicans
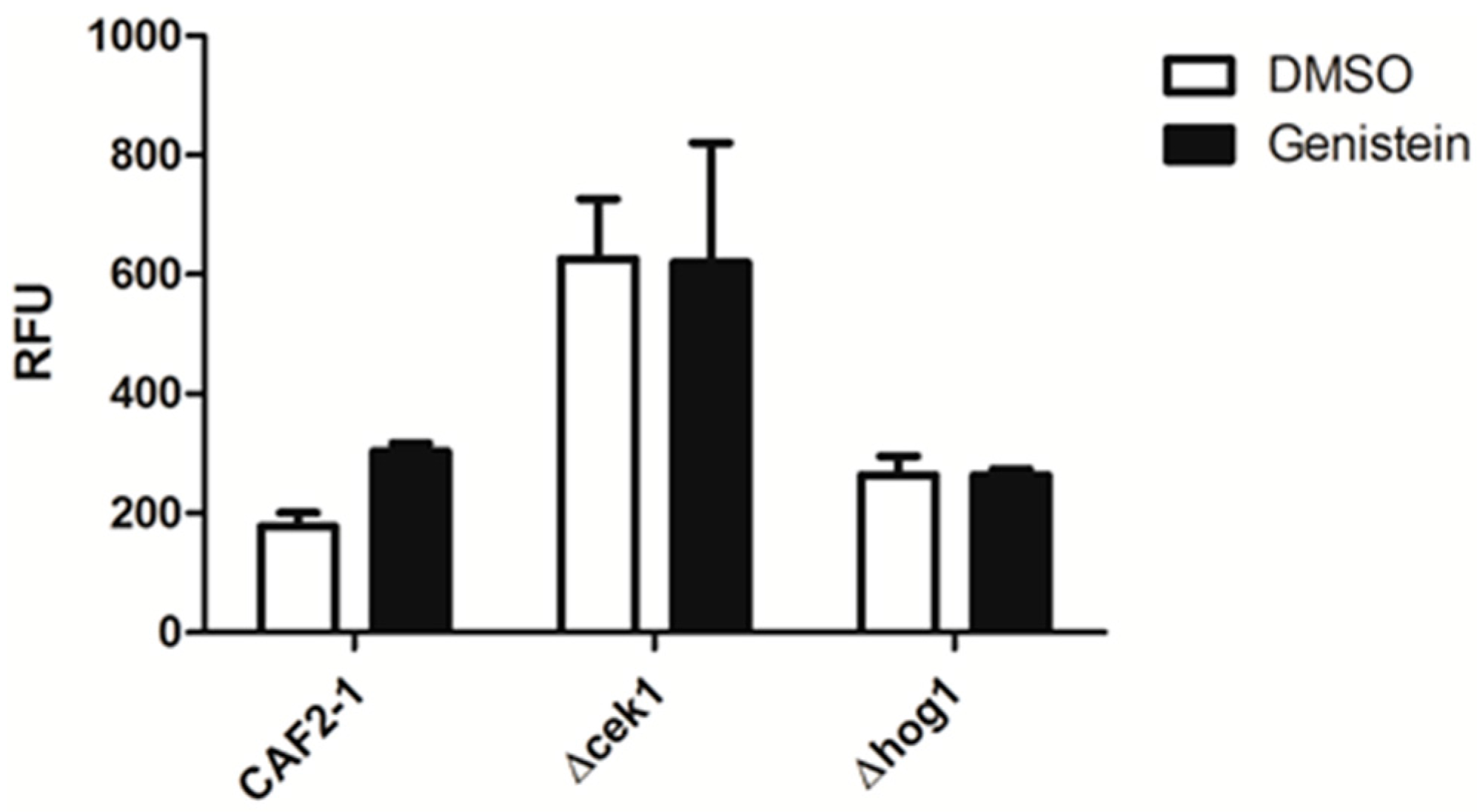
2.3. Enhanced Phagocytosis of Genistein Treated C. albicans Requires an Intact HOG Pathway
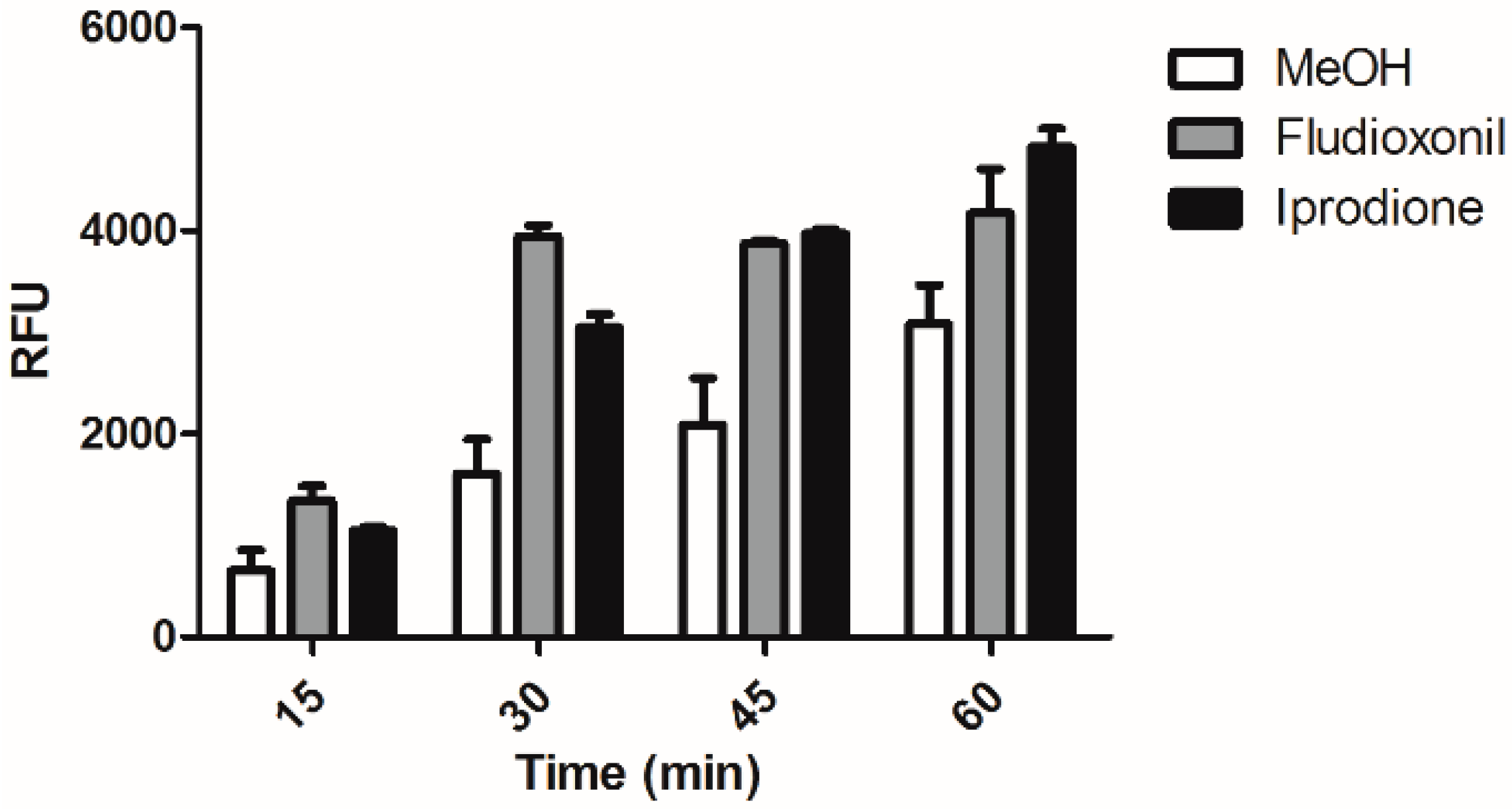
2.4. Fludioxonil and iprodione Treated C. albicans are More Susceptible to Macrophage
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Strains and Culture Condition
| Strains | Synonym | Genotype | Reference |
|---|---|---|---|
| CAF2-1 | ∆ura3::imm434/URA3 | [32] | |
| ∆casln1 | CaSLN1 | ∆ura3::imm434/∆ura3::imm434 ∆casln1::hisG/∆casln1/hisG-URA3-hisG | [8] |
| ∆cos1 | LAC17 | ∆ura3::imm434/∆ura3::imm434 ∆cos1::hisG/∆cos1/hisG-URA3-hisG | [8] |
| ∆pbs2 | BRD3 | ∆ura3::imm434/∆ura3::imm434 ∆his1::hisG/∆his1::hisG∆pbs2::cat/∆pbs2::cat-URA3-cat | [33] |
| ∆ssk1 | CSSK21 | ∆ura3::imm434/∆ura3::imm434 ∆cassk1::hisG/∆cassk1/hisG-URA3-hisG | [34] |
| ∆cek1 | CK43B-16 | ura3/ura3∆cek1::hisG/∆cek1::hisG-URA3-hisG | [35] |
| ∆hog1 | CNC13 | hog1::hisG/hog1::hisG-URA3-hisG ∆his1::hisG/∆his1::hisG | [36] |
4.3. Preparation of Candida albicans for Phagocytosis Assay
4.4. Phagocytosis Assay
4.5. Cytokine Determination
4.6. Protein Analysis
4.7. Ethanol and Glycerol Determination
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MAPKs: | mitogen-activated protein kinases |
| HOG: | high osmotic glycerol |
| RFU: | relative fluorescence units |
References
- Calera, J.A.; Calderone, R. Histidine kinase, two-component signal transduction proteins of Candida albicans and the pathogenesis of candidosis. Mycoses 1999, 42 (Suppl. S2), 49–53. [Google Scholar] [PubMed]
- Romani, L. Innate and adaptive immunity in Candida albicans infections and saprophytism. J. Leukoc. Biol. 2000, 68, 175–179. [Google Scholar] [PubMed]
- Gow, N.A.; Netea, M.G.; Munro, C.A.; Ferwerda, G.; Bates, S.; Mora-Montes, H.M.; Walker, L.; Jansen, T.; Jacobs, L.; Tsoni, V.; et al. Immune recognition of Candida albicans beta-glucan by dectin-1. J. Infect. Dis. 2007, 196, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Kruppa, M.; Goins, T.; Cutler, J.E.; Lowman, D.; Williams, D.; Chauhan, N.; Menon, V.; Singh, P.; Li, D.; Calderone, R. The role of the Candida albicans histidine kinase [CHK1) gene in the regulation of cell wall mannan and glucan biosynthesis. FEMS Yeast Res. 2003, 3, 289–299. [Google Scholar] [PubMed]
- Chauhan, N.; Calderone, R. Two-component signal transduction proteins as potential drug targets in medically important fungi. Infect. Immunity 2008, 76, 4795–4803. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A.; Pirofski, L.A. Adjunctive immune therapy for fungal infections. Clin. Infect. Dis. 2001, 33, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Kruppa, M.; Calderone, R. Two-component signal transduction in human fungal pathogens. FEMS Yeast Res. 2006, 6, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Nagahashi, S.; Mio, T.; Ono, N.; Yamada-Okabe, T.; Arisawa, M.; Bussey, H.; Yamada-Okabe, H. Isolation of CaSLN1 and CaNIK1, the genes for osmosensing histidine kinase homologues, from the pathogenic fungus Candida albicans. Microbiology 1998, 144, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Monge, R.; Carvaihlo, S.; Nombela, C.; Rial, E.; Pla, J. The Hog1 MAP kinase controls respiratory metabolism in the fungal pathogen Candida albicans. Microbiology 2009, 155, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Arana, D.M.; Alonso-Monge, R.; Du, C.; Calderone, R.; Pla, J. Differential susceptibility of mitogen-activated protein kinase pathway mutants to oxidative-mediated killing by phagocytes in the fungal pathogen Candida albicans. Cell. Microb. 2007, 9, 1647–1659. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases; World Health Organization Technical Report Series; WHO: Geneva, Switzerland, 2003; Volume 916, pp. 1–149. [Google Scholar]
- Ogawara, H.; Akiyama, T.; Ishida, J.; Watanabe, S.; Suzuki, K. A specific inhibitor for tyrosine protein kinase from Pseudomonas. J. Antibiot. 1986, 39, 606–608. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, T.; Ishida, J.; Nakagawa, S.; Ogawara, H.; Watanabe, S.; Itoh, N.; Shibuya, M.; Fukami, Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987, 262, 5592–5595. [Google Scholar] [PubMed]
- Akiyama, T.; Ogawara, H. Use and specificity of genistein as inhibitor of protein-tyrosine kinases. Methods Enzymol. 1991, 201, 362–370. [Google Scholar] [PubMed]
- Huang, J.; Nasr, M.; Kim, Y.; Matthews, H.R. Genistein inhibits protein histidine kinase. J. Biol. Chem. 1992, 267, 15511–15515. [Google Scholar] [PubMed]
- Ruetten, H.; Thiemermann, C. Effects of tyrphostins and genistein on the circulatory failure and organ dysfunction caused by endotoxin in the rat: A possible role for protein tyrosine kinase. Br. J. Pharmacol. 1997, 122, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Sasamura, H.; Takahashi, A.; Yuan, J.; Kitamura, H.; Masumori, N.; Miyao, N.; Itoh, N.; Tsukamoto, T. Antiproliferative and antiangiogenic activities of genistein in human renal cell carcinoma. Urology 2004, 64, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Sasamura, H.; Takahashi, A.; Miyao, N.; Yanase, M.; Masumori, N.; Kitamura, H.; Itoh, N.; Tsukamoto, T. Inhibitory effect on expression of angiogenic factors by antiangiogenic agents in renal cell carcinoma. Br. J. Cancer 2002, 86, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kucuk, O.; Hussain, M.; Abrams, J.; Cher, M.L.; Sarkar, F.H. Antitumor and antimetastatic activities of docetaxel are enhanced by genistein through regulation of osteoprotegerin/receptor activator of nuclear factor-kappaB (RANK)/RANK ligand/MMP-9 signaling in prostate cancer. Cancer Res. 2006, 66, 4816–4825. [Google Scholar] [CrossRef] [PubMed]
- Farina, H.G.; Pomies, M.; Alonso, D.F.; Gomez, D.E. Antitumor and antiangiogenic activity of soy isoflavone genistein in mouse models of melanoma and breast cancer. Oncol. Rep. 2006, 16, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Wienhoefer, N.; Bilitewski, U. Genistein induces morphology change and G2/M cell cycle arrest by inducing p38 MAPK activation in macrophages. Int. Immunopharmacol. 2014, 18, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Cho, H.; Park, J.; Cho, C.; Song, Y. Suppressive effects of genistein on oxidative stress and NFkappaB activation in RAW 264.7 macrophages. Biosci. Biotechnol. Biochem. 2003, 67, 1916–1922. [Google Scholar] [CrossRef] [PubMed]
- Batbayar, S.; Lee, D.H.; Kim, H.W. Immunomodulation of fungal beta-Glucan in host defense signaling by dectin-1. Biomol. Ther. 2012, 20, 433–445. [Google Scholar] [CrossRef] [PubMed]
- Buschart, A.; Burakowska, A.; Bilitewski, U. The fungicide fludioxonil antagonizes fluconazole activity in the human fungal pathogen Candida albicans. J. Med. Microb. 2012, 61, 1696–1703. [Google Scholar] [CrossRef] [PubMed]
- Buschart, A.; Gremmer, K.; El-Mowafy, M.; van den Heuvel, J.; Mueller, P.P.; Bilitewski, U. A novel functional assay for fungal histidine kinases group III reveals the role of HAMP domains for fungicide sensitivity. J. Biotechnol. 2012, 157, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.Z.; Kay, J.G.; Sangermani, D.G.; Stow, J.L. A role for the phagosome in cytokine secretion. Science 2005, 310, 1492–1495. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, S.; Xu, L.; Liu, Y.; Deb, D.K.; Platanias, L.C.; Bergan, R.C. Genistein inhibits p38 map kinase activation, matrix metalloproteinase type 2, and cell invasion in human prostate epithelial cells. Cancer Res. 2005, 65, 3470–3478. [Google Scholar] [PubMed]
- Liu, H.; Du, J.; Hu, C.; Qi, H.; Wang, X.; Wang, S.; Liu, Q.; Li, Z. Delayed activation of extracellular-signal-regulated kinase 1/2 is involved in genistein- and equol-induced cell proliferation and estrogen-receptor-alpha-mediated transcription in MCF-7 breast cancer cells. J. Nutr. Biochem. 2009, 21, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Frey, R.S.; Singletary, K.W. Genistein activates p38 mitogen-activated protein kinase, inactivates ERK1/ERK2 and decreases Cdc25C expression in immortalized human mammary epithelial cells. J. Nutr. 2003, 133, 226–231. [Google Scholar] [PubMed]
- Sanchez, Y.; Amran, D.; Fernandez, C.; de Blas, E.; Aller, P. Genistein selectively potentiates arsenic trioxide-induced apoptosis in human leukemia cells via reactive oxygen species generation and activation of reactive oxygen species-inducible protein kinases (p38-MAPK, AMPK). Int. J. Cancer 2008, 123, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, J.; Mo, B.; Hu, C.; Liu, H.; Qi, H.; Wang, X.; Xu, J. Genistein induces G2/M cell cycle arrest via stable activation of ERK1/2 pathway in MDA-MB-231 breast cancer cells. Cell Biol. Toxicol. 2008, 24, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Fonzi, W.A.; Irwin, M.Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics 1993, 134, 717–728. [Google Scholar] [PubMed]
- Arana, D.M.; Nombela, C.; Alonso-Monge, R.; Pla, J. The Pbs2 MAP kinase kinase is essential for the oxidative-stress response in the fungal pathogen Candida albicans. Microbiology 2005, 151, 1033–1049. [Google Scholar] [CrossRef] [PubMed]
- Calera, J.A.; Zhao, X.J.; Calderone, R. Defective hyphal development and avirulence caused by a deletion of the SSK1 response regulator gene in Candida albicans. Infect. Immunity 2000, 68, 518–525. [Google Scholar] [CrossRef]
- Navarro-Garcia, F.; Sanchez, M.; Pla, J.; Nombela, C. Functional characterization of the MKC1 gene of Candida albicans, which encodes a mitogen-activated protein kinase homolog related to cell integrity. Mol. Cell Biol. 1995, 15, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- San Jose, C.; Monge, R.A.; Perez-Diaz, R.; Pla, J.; Nombela, C. The mitogen-activated protein kinase homolog HOG1 gene controls glycerol accumulation in the pathogenic fungus Candida albicans. J. Bacterial. 1996, 178, 5850–5852. [Google Scholar]
- Klippel, N.; Bilitewski, U. Phagocytosis assay based on living Candida albicans for the detection of effects of chemicals on macrophage function. Anal. Lett. 2007, 40, 1400–1411. [Google Scholar] [CrossRef]
- Wesolowski, J.; Hassan, R.Y.; Reinhardt, K.; Hodde, S.; Bilitewski, U. Antifungal compounds redirect metabolic pathways in yeasts: Metabolites as indicators of modes of action. J. Appl. Microbial. 2010, 108, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: All compounds and strains are available through professional sources.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, S.; Hassan, R.Y.A.; Heintz-Buschart, A.; Bilitewski, U. Regulation of Candida albicans Interaction with Macrophages through the Activation of HOG Pathway by Genistein. Molecules 2016, 21, 162. https://doi.org/10.3390/molecules21020162
Cui S, Hassan RYA, Heintz-Buschart A, Bilitewski U. Regulation of Candida albicans Interaction with Macrophages through the Activation of HOG Pathway by Genistein. Molecules. 2016; 21(2):162. https://doi.org/10.3390/molecules21020162
Chicago/Turabian StyleCui, Shuna, Rabeay Y. A. Hassan, Anna Heintz-Buschart, and Ursula Bilitewski. 2016. "Regulation of Candida albicans Interaction with Macrophages through the Activation of HOG Pathway by Genistein" Molecules 21, no. 2: 162. https://doi.org/10.3390/molecules21020162







