Metabolite Profiling Reveals the Effect of Dietary Rubus coreanus Vinegar on Ovariectomy-Induced Osteoporosis in a Rat Model
Abstract
:1. Introduction
2. Results
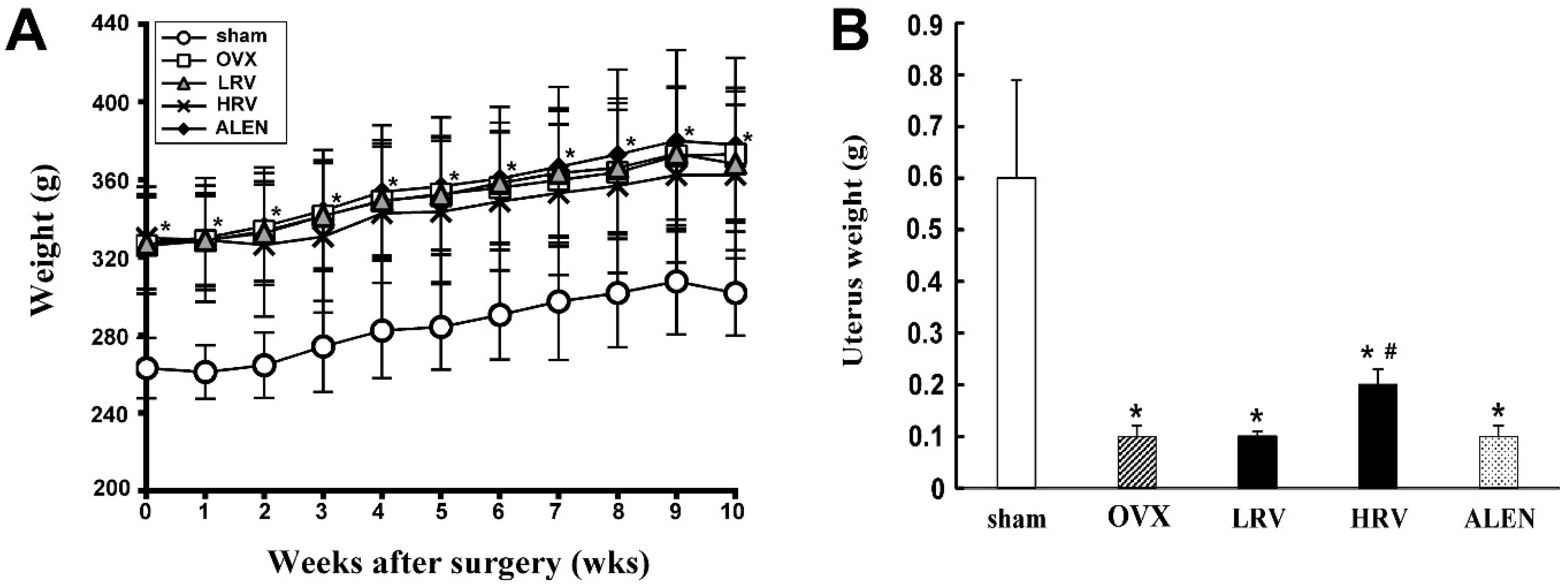
2.1. Body and Uterus Weight
2.2. Serum and Urinary Biochemistry
| Parameters | Sham | OVX | LRV | HRV | ALEN |
|---|---|---|---|---|---|
| Lipid profile | |||||
| Serum AST (U/L) | 127.5 ± 25.97 | 136.7 ± 26.09 | 134.6 ± 22.81 | 129.6 ± 27.97 | 112.5 ± 23.53 |
| Serum ALT (U/L) | 26.4 ± 5.24 | 29.1 ± 4.53 | 26.9 ± 8.68 | 25.9 ± 5.01 | 26.4 ± 4.63 |
| Bone formation | |||||
| Serum ALP (U/L) | 34.0 ± 6.80 | 45.7 ± 7.85 * | 44.9 ± 5.22 * | 42.6 ± 4.24 * | 45.1 ± 7.66 * |
| Serum OC (ng/mL) | 17.9 ± 3.77 | 21.6 ± 7.65 | 20.8 ± 6.47 | 20.0 ± 3.86 | 17.7 ± 5.46 |
| Bone resorption | |||||
| Urinary DPD (nM BCE/mM Cr) | 90.9 ± 47.04 | 70.2 ± 52.14 | 78.9 ± 53.90 | 92.8 ± 30.18 | 43.1 ± 13.35 |
| Urinary NTx (nM BCE/mM Cr) | 5.9 ± 2.19 | 5.9 ± 1.36 | 6.3 ± 1.06 | 7.5 ± 2.04 | 7.2 ± 2.57 |
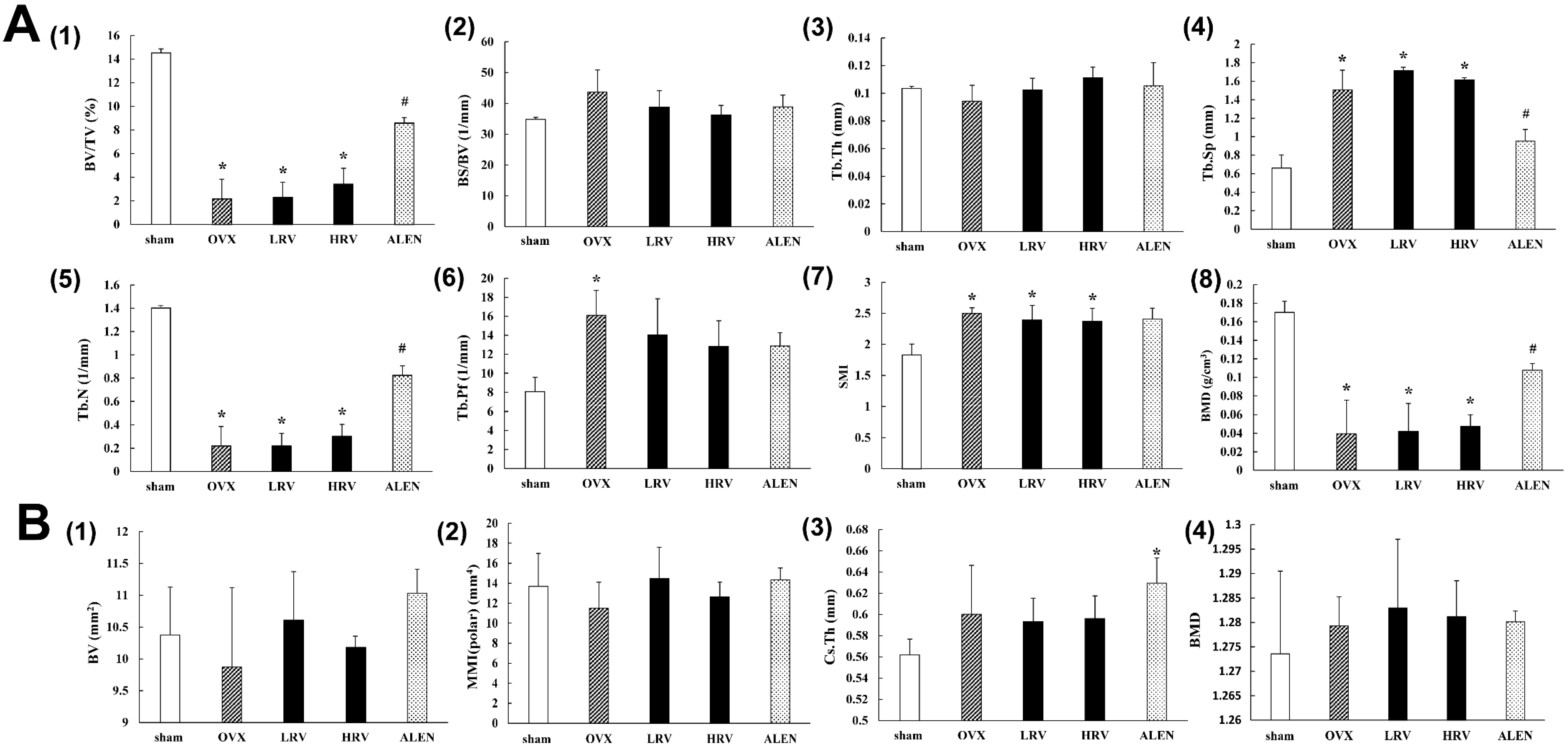
2.3. Micro-Computed Tomography Analysis
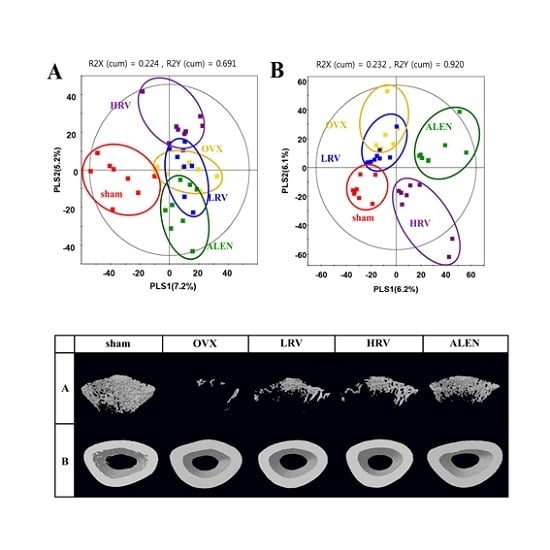
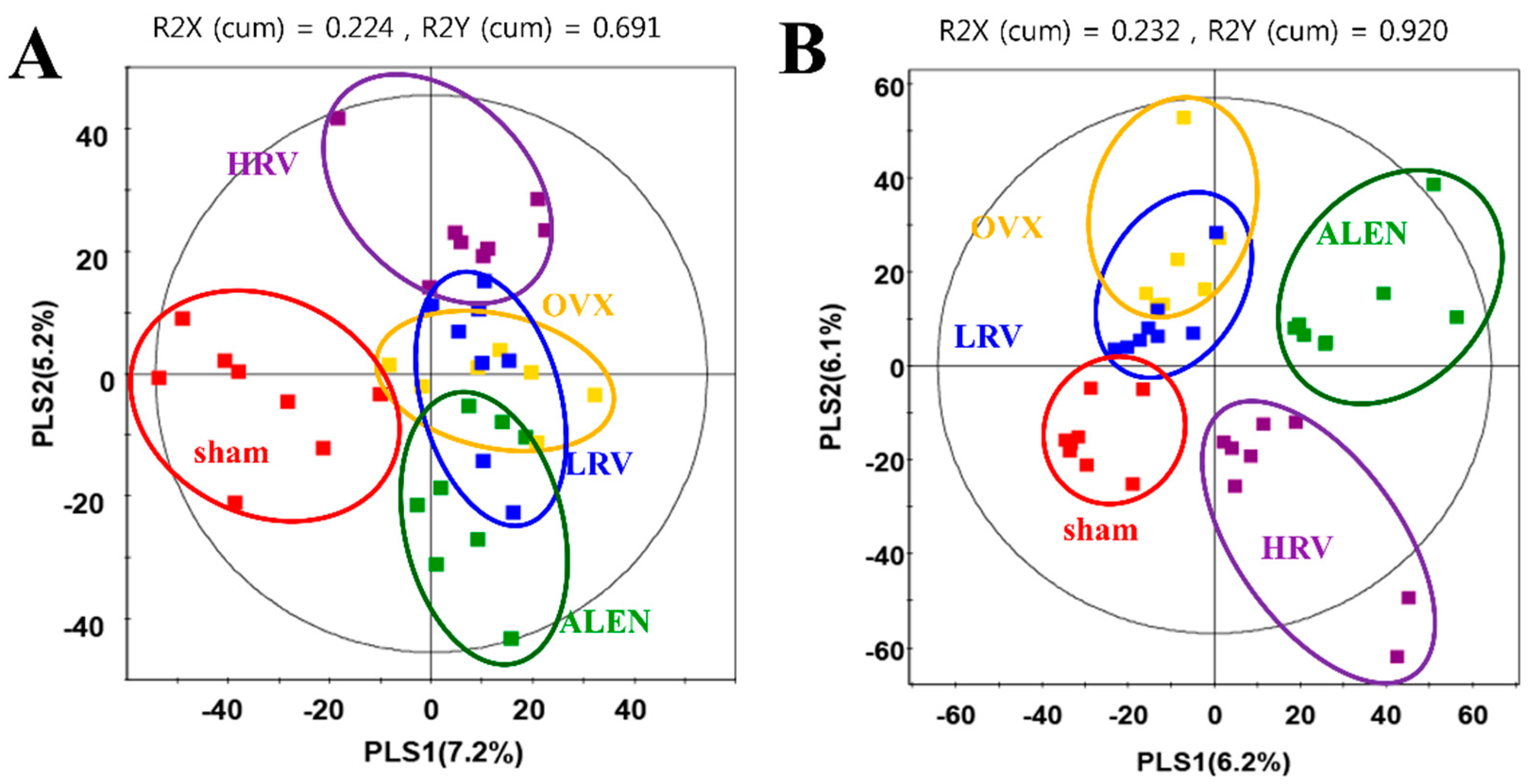
2.4. Analysis of Plasma Metabolite Profiles
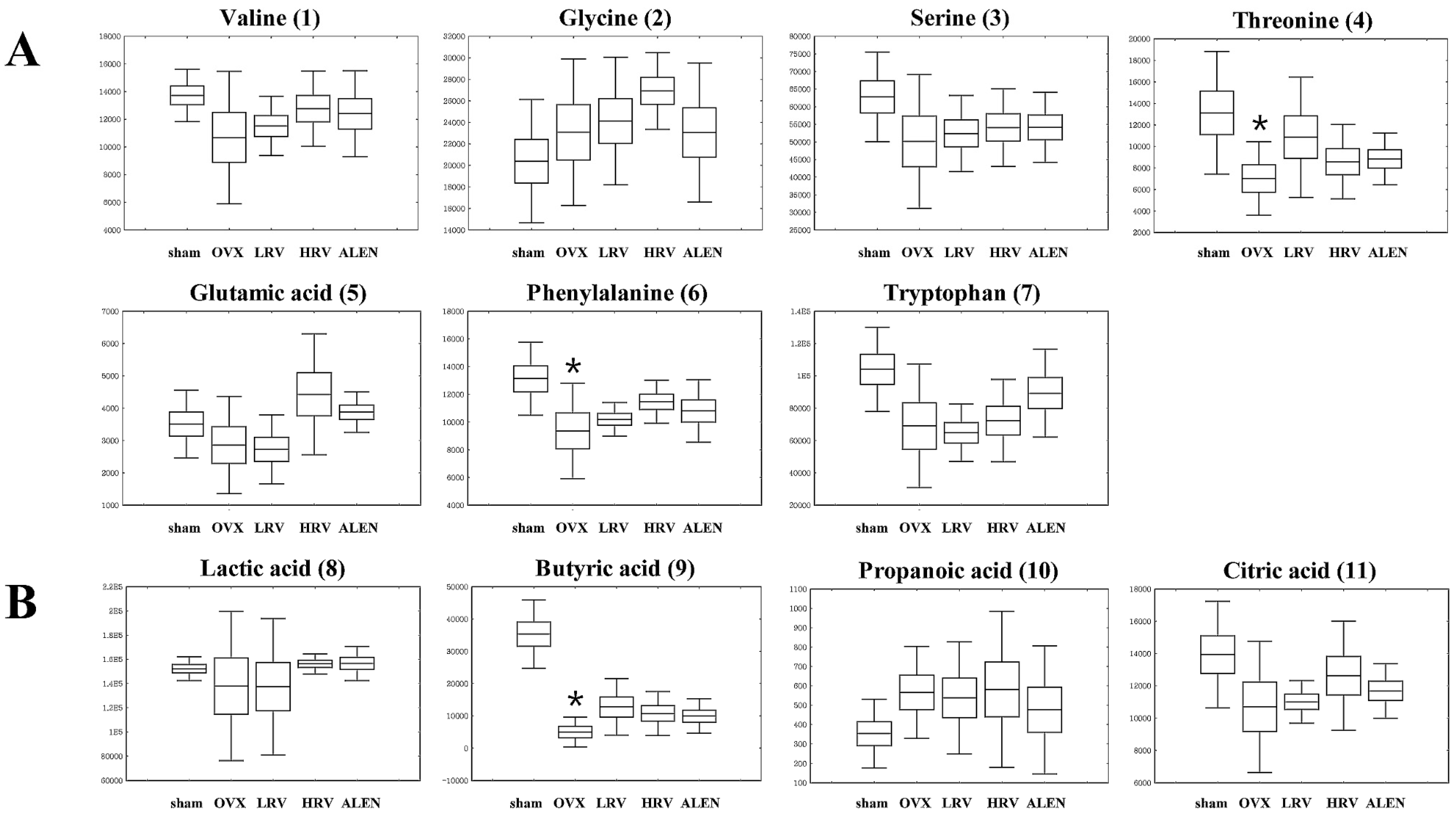
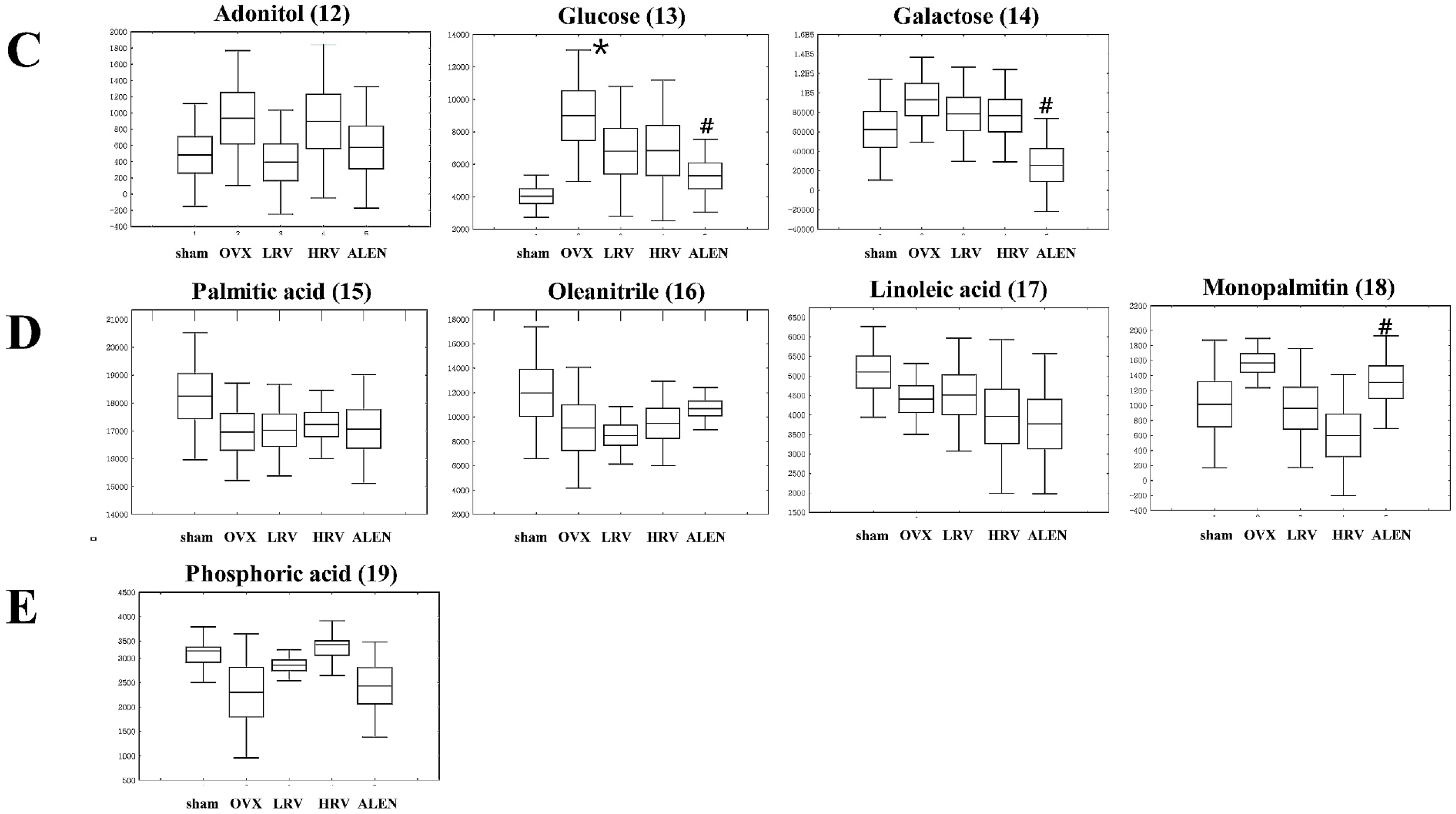
2.5. Identification of Significantly-Discriminable Metabolites in Each Group
| No. | RT (min) | Tentative Metabolites a | MS Fragment b | p-Value | Derivatized | ID c |
|---|---|---|---|---|---|---|
| Amino acids | ||||||
| 1 | 6.43 | Valine | 59, 100, 156, 218, 246 | 0.365 | (TMS)2 | STD |
| 2 | 7.30 | Glycine | 86, 133, 174, 249, 276 | 0.280 | (TMS)3 | STD |
| 3 | 7.79 | Serine | 100, 188, 204, 218, 278 | 0.385 | (TMS)2 | STD |
| 4 | 8.04 | Threonine | 57, 86, 129, 219, 291 | 0.083 | (TMS)3 | STD |
| 5 | 9.94 | Glutamic acid | 56, 84, 128, 246, 348 | 0.073 | (TMS)3 | STD |
| 6 | 10.04 | Phenylalanine | 100, 192, 218, 266, 294 | 0.036 | (TMS)3 | STD |
| 7 | 14.03 | Tryptophan | 59, 100, 202, 218, 291 | 0.039 | (TMS)3 | STD |
| Organic acids | ||||||
| 8 | 4.92 | Lactic acid | 45, 88, 117, 191, 219 | 0.724 | (TMS)2 | STD |
| 9 | 5.55 | Butyric acid | 59, 115, 131, 205, 233 | 0.000 | (TMS)2 | MS |
| 10 | 7.53 | Propanoic acid | 59, 133, 189, 205, 292 | 0.556 | (TMS)3 | MS |
| 11 | 11.46 | Citric acid | 99, 133, 211, 273, 465 | 0.203 | (TMS)4 | STD |
| Sugars and sugar derivatives | ||||||
| 12 | 10.77 | Adonitol | 59, 103, 129, 205, 217 | 0.541 | (TMS)2 | STD |
| 13 | 11.87 | Glucose | 59, 89, 103, 217, 307 | 0.081 | (TMS)4 | STD |
| 14 | 12.03 | Galactose | 59, 103, 129, 205, 319 | 0.090 | (TMS)5 | STD |
| Fatty acids | ||||||
| 15 | 12.84 | Palmitic acid | 55, 95, 201, 269, 313 | 0.609 | TMS | STD |
| 16 | 13.13 | Oleanitrile | 55, 122, 136, 220, 263 | 0.397 | TMS | MS |
| 17 | 13.85 | Linoleic acid | 55, 129, 178, 262, 337 | 0.455 | TMS | STD |
| 18 | 15.90 | Monopalmitin | 57, 129, 203, 239, 371 | 0.121 | (TMS)2 | MS |
| Other | ||||||
| 19 | 11.60 | Phosphoric acid | 59, 211, 299, 357, 445 | 0.130 | (TMS)5 | STD |
| No. | RT (min) | Tentative Metabolites a | Measured MS (m/z) | HMDB Formula | Error b (mDa) | Adduct | p-Value | |
|---|---|---|---|---|---|---|---|---|
| Negative | Positive | |||||||
| 1 | 5.43 | Taurocholic acid | 514.2847 | 538.2816 | C26H45NO7S | 0.9 | [M + Na]+ | 0.378 |
| 2 | 5.91 | Glycocholic acid | 464.3002 | 488.2986 | C26H43NO6 | −0.5 | [M + Na]+ | 0.467 |
| 3 | 7.13 | N. I. (1) | - | 288.2910 | - | - | - | 0.015 |
| 4 | 7.63 | N. I. (2) | - | 357.2797 | - | - | - | 0.035 |
| 5 | 7.72 | LysoPC 14:0 | 512.2922 | 468.3075 | C22H46NO7P | 0.0 | [M + H]+ | 0.115 |
| 6 | 7.78 | LysoPC 20:5 | 586.3132 | 542.3254 | C28H48NO7P | 0.7 | [M + H]+ | 0.655 |
| 7 | 7.86 | LysoPC 18:3 | 562.3149 | 518.3243 | C26H48NO7P | −0.4 | [M + H]+ | 0.099 |
| 8 | 7.98 | LysoPC 16:1 | 538.3101 | 494.3202 | C24H48NO7P | −0.3 | [M + H]+ | 0.174 |
| 9 | 8.09 | LysoPC 22:6 | 612.3307 | 568.3358 | C30H50NO7P | −1.7 | [M + H]+ | 0.035 |
| 10 | 8.27 | LysoPC 20:4 | 588.3356 | 544.3422 | C28H50NO7P | −1.0 | [M + H]+ | 0.018 |
| 11 | 8.47 | LysoPC 22:5 | 614.3473 | 570.355 | C30H52NO7P | −1.0 | [M + H]+ | 0.349 |
| 12 | 8.75 | LysoPC 16:0 | 540.3259 | 496.3415 | C24H50NO7P | 0.1 | [M + H]+ | 0.049 |
| 13 | 9.22 | N. I. (3) | - | 508.3765 | - | - | - | 0.383 |
| 14 | 9.33 | N. I. (4) | - | 228.2317 | - | - | - | 0.234 |
| 15 | 9.85 | LysoPC 18:0 | 568.3609 | 524.3718 | C26H54NO7P | −0.3 | [M + H]+ | 0.580 |
| 16 | 10.44 | N. I. (5) | - | 282.2787 | - | - | - | 0.246 |

3. Discussion
4. Experimental Section
4.1. Reagents
4.2. Experimental Design and Diet
4.3. Analysis of Serum and Urine Parameters
4.4. Determination of Architectural and Mineralization Parameters by Using Micro-Computed Tomography
4.5. Instrumental Analysis
4.5.1. Sample Preparation for MS Analysis
4.5.2. GC-TOF-MS Analysis
4.5.3. UPLC-Q-TOF-MS Analysis
4.6. Data Processing and Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rosen, C.J.; Bouxsein, M.L. Mechanisms of disease: Is osteoporosis the obesity of bone? Nat. Clin. Pract. Rheumatol. 2006, 2, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Galsworthy, T.D. Osteoporosis: Statistics, intervention, and prevention. Ann. N. Y. Acad. Sci. 1994, 736, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Deal, C. Osteoporosis in elderly: Prevention and treatment. Clin. Geriatr. Med. 2002, 18, 529–555. [Google Scholar] [CrossRef]
- Adler, R.A. Osteoporosis in men: A review. Bone Res. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Meniru, G.I.; Hecht, B.R. Prevention of Infertility and Complications in Women; iUniverse: New York, NY, USA, 2003. [Google Scholar]
- Balch, P.A. Prescription for Nutritional Healing, 4th ed.; Penguin: New York, NY, USA, 2006. [Google Scholar]
- Miyauchi, Y.; Sato, Y.; Kobayashi, T.; Yoshida, S.; Mori, T.; Kanagawa, H.; Katsuyama, E.; Fujie, A.; Hao, W.; Miyamoto, K.; et al. HIF1α is required for osteoclast activation by estrogen deficiency in postmenopausal osteoporosis. Proc. Natl. Acad. Sci. USA 2013, 110, 16568–16573. [Google Scholar] [CrossRef] [PubMed]
- Hmamouchi, I.; Allali, F.; Khazzani, H.; Bennani, L.; El Mansouri, L.; Ichchou, L.; Cherkaoui, M.; Abouqal, R.; Hajjaj-Hassouni, N. Low bone mineral density is related to atherosclerosis in postmenopausal Moroccan women. BMC Public Health 2009, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Watts, N.B.; Diab, D.L. Long-term use of bisphosphonates in osteoporosis. J. Clin. Endocrinol. Metab. 2010, 95, 1555–1565. [Google Scholar] [CrossRef] [PubMed]
- Whelan, A.M.; Jurgens, T.M.; Bowles, S.K. Natural health products in the prevention and treatment of osteoporosis: Systematic review of randomized controlled trials. Ann. Pharmacother. 2006, 40, 836–849. [Google Scholar] [CrossRef] [PubMed]
- Michaelsson, K.; Wolk, A.; Langenskiold, S.; Basu, S.; Lemming, E.W.; Melhus, H.; Byberg, L. Milk intake and risk of mortality and fractures in women and men: Cohort studies. BMJ 2014, 27, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.S.; Lee, S.S. Effect of soybeans and sword beans on bone metabolism in a rat model of osteoporosis. Ann. Nutr. Metab. 2010, 56, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Lhotta, K.; Hofle, G.; Gasser, R.; Finkensted, G. Hypokalemia, hyperreninemia and osteoporosis in a patient ingesting large amounts of cider vinegar. Nephron 1998, 80, 242–243. [Google Scholar] [CrossRef] [PubMed]
- Chung, L.; Chung, S.J.; Kim, J.Y.; Kim, K.O.; O’Mahony, M.; Vickers, Z.; Cha, S.M.; Ishii, R.; Baures, K.; Kim, H.R. Comparing the liking for Korean style salad dressings and beverages between U.S. and Korean consumers: Effects of sensory and non-sensory factors. Food Qual. Prefer. 2012, 26, 105–118. [Google Scholar] [CrossRef]
- Honsho, S.; Sugiyama, A.; Takahara, A.; Satoh, Y.; Nakamura, Y.; Hashimoto, K. A red wine vinegar beverage can inhibit the renin-angiotensin system: Experimental evidence in vivo. Biol. Pharm. Bull. 2005, 28, 1208–1210. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.I.; Lee, J.; Choi, R.Y.; Lee, H.I.; Lee, J.H.; Jeong, J.K.; Kim, M.J.; Lee, M.K. Anti-obesity and anti-insulin resistance effects of tomato vinegar beverage in diet-induced obese mice. Food Funct. 2014, 5, 1579–1586. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.Y.; Baek, C.H.; Jeong, K.H.; Park, N.Y.; Jeong, Y.J. Effect of vinegar on the solubility of calcium. Korean J. Food Preserv. 2005, 12, 112–116. [Google Scholar]
- Sakanaka, S.; Ishihara, Y. Comparison of antioxidant properties of persimmon vinegar and some other commercial vinegars in radical-scavenging assays and on lipid oxidation in tuna homogenates. Food Chem. 2008, 107, 739–744. [Google Scholar] [CrossRef]
- Ye, X.J.; Morimura, S.; Han, L.S.; Shigematsu, T.; Kida, K. In vitro evaluation of physiological activity of vinegar produced from barley-, sweet potato-, and rice-shochu post-distillation slurry. Biosci. Biotechnol. Biochem. 2004, 68, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.K.; Lee, M.Y.; Kim, H.Y.; Lee, S.; Yeo, S.H.; Baek, S.Y.; Lee, C.H. Comparison of traditional and commercial vinegars based on metabolite profiling and antioxidant activity. J. Microbiol. Biotechnol. 2015, 25, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Bae, S.Y.; Lee, J.H.; Cho, K.J.; Kim, K.H.; Chung, M. Antiviral effects of black raspberry (Rubus coreanus) juice on foodborne viral surrogates. Foodborne Pathog. Dis. 2012, 9, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Son, M.Y.; Jung, S.; Nam, P.; Sung, J.S.; Lee, K.G. Antioxidant properties of Korean black raspberry wines and their apoptotic effects on cancer cells. J. Sci. Food Agric. 2009, 89, 970–977. [Google Scholar] [CrossRef]
- Jung, M.A.; Cho, S.H.; Lee, S.Y.; Kim, J.H.; Kim, Y.S.; Oh, K.; Yoo, G.S.; Lee, D.W.; Kim, S. Anti-obesity effects on unripe Rubus coreanus Miquel extract in high fat diet-induced obese mice. Int. J. Biochem. Res. Rev. 2015, 5, 20–26. [Google Scholar]
- Do, S.H.; Lee, J.W.; Jeong, W.I.; Chung, J.Y.; Park, S.J.; Hong, I.H.; Jeon, S.K.; Lee, I.S.; Jeong, K.S. Bone-protecting effect of Rubus coreanus by dual regulation of osteoblasts and osteoclasts. Menopause 2008, 15, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Choi, E.M. Rubus coreanus Miq. extract promotes osteoblast differentiation and inhibits bone-resorbing mediators in MC3T3-E1 Cells. Am. J. Chin. Med. 2006, 34, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.J.; Sohn, E.H.; Kang, S.C. Combinatory mixture of Rubus coreanus and Astragalus membranaceus attenuates bone loss through RANK signal pathway in ovariectomized mice (1034.9). FASEB J. 2014, 28, 1034–1039. [Google Scholar]
- Lim, H.K.; Lee, H.R.; Do, S.H. Stimulation of cannabinoid receptors by using Rubus coreanus extracts to control osteoporosis in aged male rats. Aging Male 2015, 18, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Lindon, J.C. Systems biology: Metabonomics. Nature 2008, 455, 1054–1056. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Lee, M.Y.; Park, H.M.; Park, Y.K.; Shon, J.C.; Liu, K.H.; Lee, C.H. Urine and serum metabolite profiling of rats fed a high-fat diet and the anti-obesity effects of caffeine consumption. Molecules 2015, 20, 3107–3128. [Google Scholar] [CrossRef] [PubMed]
- Park, H.M.; Shon, J.C.; Lee, M.Y.; Liu, K.H.; Kim, J.K.; Lee, S.J.; Lee, C.H. Mass spectrometry-based metabolite profiling in the mouse liver following exposure to ultraviolet B radiation. PLoS ONE 2014, 9, e109479. [Google Scholar]
- Dunn, W.B.; Ellis, D.I. Metabolomics: Current analytical platforms and methodologies. Trends Anal. Chem. 2005, 24, 285–294. [Google Scholar]
- Zhao, D.; Han, L.; He, Z.; Zhang, J.; Zhang, Y. Identification of the plasma metabolomics as early diagnostic markers between biliary atresia and neonatal hepatitis syndrome. PLoS ONE 2014, 9, e85694. [Google Scholar] [CrossRef] [PubMed]
- Hartke, J.R. Preclinical development of agents for the treatment of osteoporosis. Toxicol. Pathol. 1999, 27, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wu, Z.X.; Zhang, Y.; Yan, Y.B.; He, Q.; Cao, P.C.; Lei, W. Anti-osteoporosis activity of Cibotium barometz extract on ovariectomy-induced bone loss in rats. J. Ethnopharmacol. 2011, 137, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- The North American Menopause Society (NAMS). The Menopause Guidebook, 7th ed.; NAMS: New York, NY, USA, 2012. [Google Scholar]
- Ryan, K.J. Biochemistry of aromatase: Significance to female reproductive physiology. Cancer Res. 1982, 42, 3342s–3344s. [Google Scholar] [PubMed]
- Wegorzewska, I.N.; Walters, K.; Weiser, M.J.; Cruthirds, D.F.; Ewell, E.; Larco, D.O.; Handa, R.J.; Wu, T.J. Postovariectomy weight gain in female rats is reversed by estrogen receptor α agonist, propylpyrazoletriol. Am. J. Obstet. Gynecol. 2008, 199, e1–e5. [Google Scholar] [CrossRef] [PubMed]
- Kumagai, S.; Holmang, A.; Bjorntorp, P. The effects of oestrogen and progesterone on insulin sensitivity in female rats. Acta Physiol. Scand. 1993, 149, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; McElrath, T.; Tong, W.; Pollard, J.W. The molecular basis of tamoxifen induction of mouse uterine epithelial cell proliferation. J. Endocrinol. 2005, 184, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, S.; Okamoto, Y.; Miyazaki, K.; Uesugi, T. Evaluation of a soybean product fujiflavone P40 as an antiosteoporotic agent in rats. Phytother. Res. 2003, 17, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Pollard, J.W. Estradiol-17beta regulates mouse uterine epithelial cell proliferation through insulin-like growth factor 1 signalling. Proc. Natl. Acad. Sci. USA 2007, 104, 15847–15851. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.L.; Redmer, D.A.; Reynolds, L.P. Effects of ovarian steroids on uterine growth, morphology, and cell proliferation in ovariectomized, steroid-treated ewes. Biol. Reprod. 1997, 57, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Baek, B.K.; Lim, C.W.; Choi, Y.G.; Lee, S.I.; Jeon, B.H. Effects of black raspberry wine on estrogen in Sprague-Dawley rats. Korean J. Orient. Physiol. Pathol. 2005, 19, 426–428. [Google Scholar]
- Saleem, A.N.; Chen, Y.H.; Baek, H.J.; Hsiao, Y.W.; Huang, H.W.; Kao, H.J.; Liu, K.M.; Shen, L.F.; Song, I.W.; Tu, C.P.; et al. Mice with alopecia, osteoporosis, and systemic amyloidosis due to mutation in Zdhhc13, a gene coding for palmitoyl acyltransferase. PLoS Genet. 2010, 10, e1000985. [Google Scholar]
- Bhattarai, T.; Bhattacharya, K.; Chaudhuri, P.; Sengupta, P. Correlation of common biochemical markers for bone turnover, serum calcium, and alkaline phosphatase in post-menopausal women. Malays. J. Med. Sci. 2014, 21, 58–61. [Google Scholar]
- McCormick, R.K. Osteoporosis: Integrating biomarkers and other diagnostic correlates into the management of bone fragility. Altern. Med. Rev. 2007, 12, 113–145. [Google Scholar] [PubMed]
- Chen, S.Y.; Yu, H.T.; Kao, J.P.; Yang, C.C.; Chiang, S.S.; Mishchuk, D.O.; Mau, J.L.; Slupsky, C.M. An NMR metabolomic study on the effect of Alendronate in ovariectomized mice. PLoS ONE 2014, 9, e106559. [Google Scholar]
- Walsh, J.P.; Ward, L.C.; Stewart, G.O.; Will, R.K.; Criddle, R.A.; Prince, R.L.; Stuckey, B.G.; Dhaliwal, S.S.; Bhagat, C.I.; Retallack, R.W.; et al. A randomized clinical trial comparing oral alendronate and intravenous pamidronate for the treatment of Paget’s disease of bone. Bone 2004, 34, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Chesnut, C.H., III; Harris, S.T. Short-term effect of alendronate on bone mass and bone remodeling in postmenopausal women. Osteoporis. Int. 1993, 3, S17–S19. [Google Scholar] [CrossRef]
- Uusi-Rasi, K.; Kannus, P.; Cheng, S.; Sievanen, H.; Pasanen, M.; Heinonen, A.; Nenonen, A.; Halleen, J.; Fuerst, T.; Genant, H.; et al. Effect of alendronate and exercise on bone and physical performance of postmenopausal women: A randomized controlled trial. Bone 2003, 33, 132–143. [Google Scholar] [CrossRef]
- Kondo, S.; Tayama, K.; Tsukamoto, Y.; Ikeda, K.; Yamori, Y. Antihypertensive effects of acetic acid and vinegar on spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 2001, 65, 2690–2694. [Google Scholar] [CrossRef] [PubMed]
- Farnworth, E.R. Handbook of Fermented Functional Foods, 2th ed.; CRC Press: New York, NY, USA, 2008. [Google Scholar]
- Caballero, B.; Allen, L.; Prentice, A. Encyclopedia of Human Nutrition, 2th ed.; Academic Press: San Diego, CA, USA, 2005. [Google Scholar]
- Demirdas, S.; Coakley, K.E.; Bisschop, P.H.; Hollak, C.E.; Bosch, A.M.; Singh, R.H. Bone health in phenylketonuria: A systematic review and meta-analysis. Orphanet J. Rare Dis. 2015, 10, e46. [Google Scholar] [CrossRef] [PubMed]
- Pernow, Y.; Thoren, M.; Saaf, M.; Fernholm, R.; Anderstam, B.; Hauge, E.M.; Hall, K. Associations between amino acids and bone mineral density in men with idiopathic osteoporosis. Bone 2010, 47, 959–965. [Google Scholar] [CrossRef] [PubMed]
- Assadi-Porter, F.; Selen, E.; Shen, C. NMR-based metabolomics analysis in muscle and serum of middle-aged ovariectomized rats supplemented with 6-month green tea polyphenols. FASEB J. 2005, 29, 745.2. [Google Scholar]
- Liu, X.; Zhang, S.; Lu, X.; Zheng, S.; Li, F.; Xiong, Z. Metabonomic study on the anti-osteoporosis effect of Rhizoma Drynariae and its action mechanism using ultra-performance liquid chromatography–tandem mass spectrometry. J. Ethnopharmacol. 2012, 139, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Conigrave, A.D.; Brown, E.M.; Rizzoli, R. Dietary protein and bone health: Roles of amino acid-sensing receptors in the control of calcium metabolism and bone homeostasis. Annu. Rev. Nutr. 2008, 28, 131–155. [Google Scholar] [CrossRef] [PubMed]
- Meijer, K.; de Vos, P.; Priebe, M.G. Butyrate and other short-chain fatty acids as modulators of immunity: What relevance for health? Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Orey, C. The Healing Powers of Vinegar; Kensington Publishing Corp: New York, NY, USA, 2009. [Google Scholar]
- Chau, D.L.; Edelman, S.V. Osteoporosis and diabetes. Clin. Diabetes 2002, 20, 153–157. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kobayashi, T.; Kamata, K. Role of lysophosphatidylcholine (LPC) in atherosclerosis. Curr. Med. Chem. 2007, 14, 3209–3220. [Google Scholar] [CrossRef] [PubMed]
- Allard-Chamard, H.; Dufort, P.; Haroun, S.; de Brum-Fernandes, A.J. Cytosolic phospholipase A2 and eicosanoids modulate life, death and function of human osteoclasts in vitro. Prostaglandins Leukot. Essent. Fatty Acids. 2014, 90, 117–213. [Google Scholar] [CrossRef] [PubMed]
- Rosenbloom, B.E.; Weinreb, N.J. Gaucher disease: A comprehensive review. Crit. Rev. Oncog. 2013, 18, 169–175. [Google Scholar] [CrossRef]
- Mebarek, S.; Abousalham, A.; Magne, D.; Do, L.D.; Bandorowicz-Pikula, J.; Pikula, S.; Buchet, R. Phospholipases of mineralization competent cells and matrix vesicles: Roles in physiological and pathological mineralizations. Int. J. Mol. Sci. 2013, 14, 5036–5129. [Google Scholar] [CrossRef] [PubMed]
- Lommen, A. MetAlign: Interface-driven, versatile metabolomics tool for hyphenated full-scan mass spectrometry data preprocessing. Anal. Chem. 2009, 81, 3079–3086. [Google Scholar] [CrossRef] [PubMed]
- Human Metabolome Database (HMDB). Available online: http://www.hmdb.ca (accessed on 25 January 2016).
- Sample Availability: Samples are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.Y.; Kim, H.Y.; Singh, D.; Yeo, S.H.; Baek, S.Y.; Park, Y.K.; Lee, C.H. Metabolite Profiling Reveals the Effect of Dietary Rubus coreanus Vinegar on Ovariectomy-Induced Osteoporosis in a Rat Model. Molecules 2016, 21, 149. https://doi.org/10.3390/molecules21020149
Lee MY, Kim HY, Singh D, Yeo SH, Baek SY, Park YK, Lee CH. Metabolite Profiling Reveals the Effect of Dietary Rubus coreanus Vinegar on Ovariectomy-Induced Osteoporosis in a Rat Model. Molecules. 2016; 21(2):149. https://doi.org/10.3390/molecules21020149
Chicago/Turabian StyleLee, Mee Youn, Hyang Yeon Kim, Digar Singh, Soo Hwan Yeo, Seong Yeol Baek, Yoo Kyoung Park, and Choong Hwan Lee. 2016. "Metabolite Profiling Reveals the Effect of Dietary Rubus coreanus Vinegar on Ovariectomy-Induced Osteoporosis in a Rat Model" Molecules 21, no. 2: 149. https://doi.org/10.3390/molecules21020149
APA StyleLee, M. Y., Kim, H. Y., Singh, D., Yeo, S. H., Baek, S. Y., Park, Y. K., & Lee, C. H. (2016). Metabolite Profiling Reveals the Effect of Dietary Rubus coreanus Vinegar on Ovariectomy-Induced Osteoporosis in a Rat Model. Molecules, 21(2), 149. https://doi.org/10.3390/molecules21020149