Imidazole Alkaloids from the South China Sea Sponge Pericharax heteroraphis and Their Cytotoxic and Antiviral Activities
Abstract
:1. Introduction
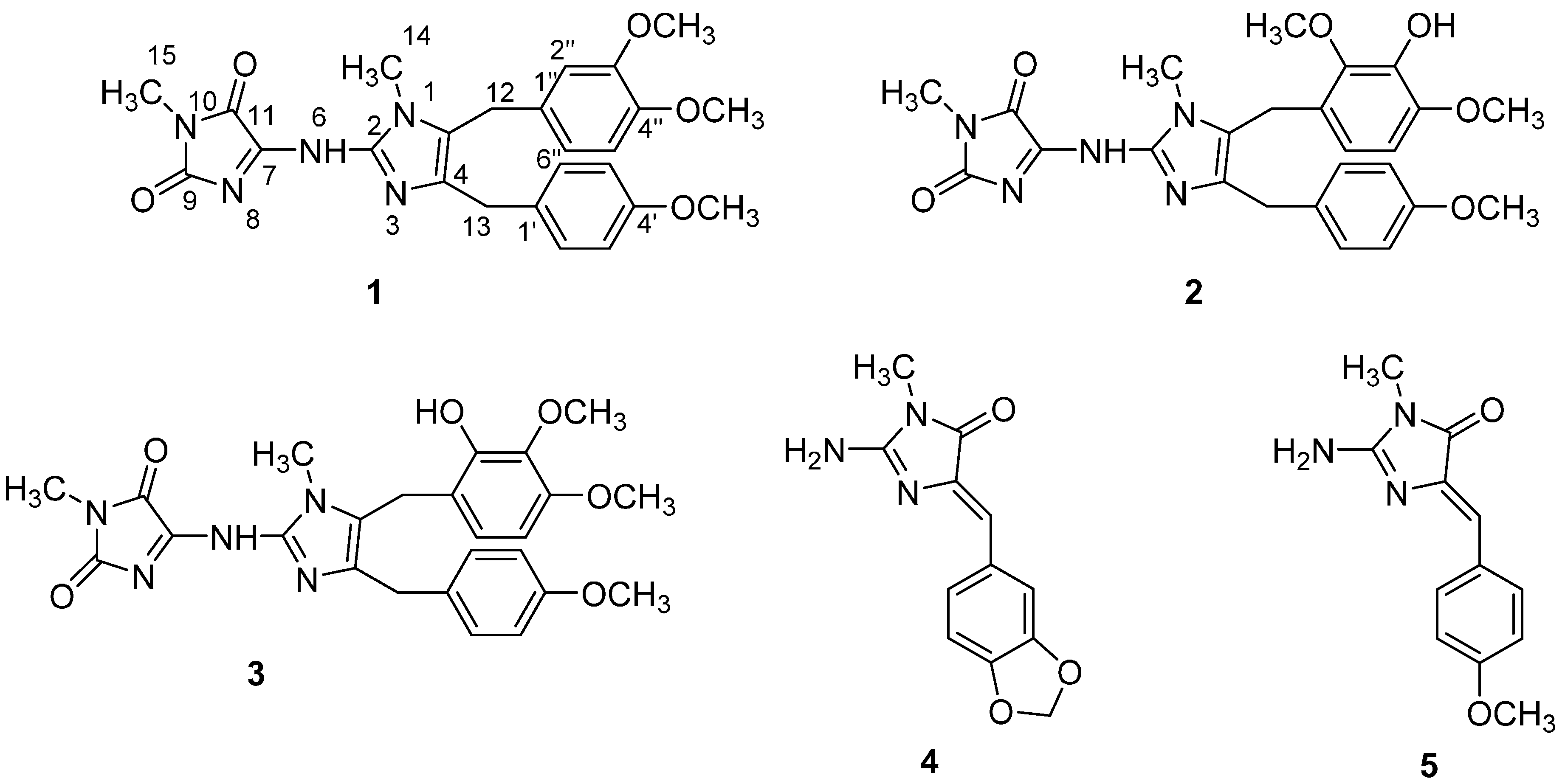
2. Results and Discussion
2.1. Structure Elucidation


| Position | 1 | Naamidine A | ||||
|---|---|---|---|---|---|---|
| δH, mult. (J in Hz) | δC | δH, mult. (J in Hz) | δC | |||
| 1 | - | - | - | - | ||
| 2 | - | 146.6 | (s) | - | 145.7 | (s) |
| 3 | - | - | - | - | ||
| 4 | - | 127.0 | (s) | - | 133.3 | (s) |
| 5 | - | 129.7 | (s) | - | 126.9 | (s) |
| 6 | - | - | - | - | ||
| 7 | - | 144.7 | (s) | - | 148.4 | (s) |
| 8 | - | - | (s) | - | - | (s) |
| 9 | - | 155.5 | (s) | - | 157.3 | (s) |
| 10 | - | - | - | - | ||
| 11 | - | 162.3 | (s) | - | 162.5 | (s) |
| 12 | 3.91 (s) | 29.2 | (t) | 3.91 (s) | 27.7 | (t) |
| 13 | 3.89 (s) | 32.3 | (t) | 3.93 (s) | 30.8 | (t) |
| 14 | 3.49 (s) | 30.1 | (q) | 3.39 (s) | 29.6 | (q) |
| 15 | 3.17 (s) | 24. 8 | (q) | 2.95 (s) | 24.4 | (q) |
| 1′ | - | 131.7 | (s) | - | 131.9 | (s) |
| 2′ 6′ | 7.13 (d J = 8.4 Hz) | 129.5 | (d) | 7.15 (d J = 8.6 Hz) | 129.5 | (d) |
| 3′ 5′ | 6.80 (d J = 8.4 Hz) | 114.2 | (d) | 6.81 (d J = 8.6 Hz) | 113.9 | (d) |
| 4′ | - | 158.3 | (s) | - | 157.8 | (s) |
| 1′′ | - | 127.0 | (s) | - | 127.9 | (s) |
| 2′′ | 6.42 (s) | 111.2 | (d) | 6.84 (d J = 8.6 Hz) | 129.1 | (d) |
| 3′′ | - | 148.1 | (s) | 6.63 (d J = 8.6 Hz) | 115.5 | (d) |
| 4′′ | - | 149.5 | (s) | - | 156.0 | (s) |
| 5′′ | 6.74 (d J = 8.1 Hz) | 111.5 | (d) | 6.63 (d J = 8.6 Hz) | 115.5 | (d) |
| 6′′ | 6.54 (d J = 8.1 Hz) | 120.1 | (d) | 6.84 (d J = 8.6 Hz) | 129.1 | (d) |
| 4′ OCH3 | 3.77 (s) | 55.3 | (q) | 3.69 (s) | 55.1 | (q) |
| 3′′ OCH3 | 3.84 (s) | 55.9 | (q) | - | - | (q) |
| 4′′ OCH3 | 3.67 (s) | 55.7 | (q) | - | - | (q) |
| Position | δH, mult. (J in Hz) | δC | HMBC(H to C) | NOESY | |
|---|---|---|---|---|---|
| 1 | - | - | |||
| 2 | - | 146.6 | (s) | ||
| 3 | - | - | |||
| 4 | - | 127.0 | (s) | ||
| 5 | - | 129.7 | (s) | ||
| 6 | - | - | |||
| 7 | - | 144.7 | (s) | ||
| 8 | - | - | (s) | ||
| 9 | - | 155.5 | (s) | ||
| 10 | - | - | |||
| 11 | - | 162.3 | (s) | ||
| 12 | 3.91 (s) | 29.2 | (t) | C-1′′, 2′′, 6′′, 4, 5 | H-2′′, 6′′ |
| 13 | 3.89 (s) | 32.3 | (t) | C-1′, 2′, 6′, 4, 5 | H- 2′, 6′ |
| 14 | 3.49 (s) | 30.1 | (q) | C-2, 5 | H-12, 2′′, 6′′ |
| 15 | 3.17 (s) | 24. 8 | (q) | C-9, 11 | |
| 1′ | - | 131.7 | |||
| 2′ 6′ | 7.13 (d J = 8.4 Hz) | 129.5 | (d) | C-1′, 4′, 13 | |
| 3′ 5′ | 6.80 (d J = 8.4 Hz) | 114.2 | (d) | C-1′, 4′ | |
| 4′ | - | 158.3 | (s) | ||
| 1′′ | - | 127.0 | (s) | ||
| 2′′ | 6.42 (s) | 111.2 | (d) | C-12, 6′′, 4′′ | |
| 3′′ | - | 148.1 | (s) | ||
| 4′′ | - | 149.5 | (s) | ||
| 5′′ | 6.74 (d J = 8.1 Hz) | 111.5 | (d) | C-4′′ | |
| 6′′ | 6.54 (d J = 8.1 Hz) | 120.1 | (d) | C-13, 5′′, 4′′ | |
| 4′ OCH3 | 3.77 (s) | 55.3 | (q) | C-4′ | H-3′, 5′ |
| 3′′ OCH3 | 3.84 (s) | 55.9 | (q) | C-3′′ | H-5′′ |
| 4′′ OCH3 | 3.67 (s) | 55.7 | (q) | C-4′′ | H-2′′ |
2.2. Biological Evaluations
| Compounds | IC50 (μM) | |||
|---|---|---|---|---|
| HeLa a | P388 b | A549 a | K562 b | |
| 1 | NA | NA | NA | 11.3 |
| 2 | 21.4 | NA | 22.1 | 9.4 |
| 3 | NA | NA | NA | NA |
| 4 | NA | NA | NA | NA |
| 5 | NA | NA | NA | NA |
| ADM(Adriamycin) c | 0.2 | 0.02 | 0.6 | 0.2 |
3. Experimental Section
3.1. General Methods
3.2. Animal Material
3.3. Extraction and Isolation

3.4. Cytotoxic Assay
3.5. Anti-H1N1 Virus Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Koswatta, P.B.; Lovely, C.J. Structure and Synthesis of 2-aminoimidazole Alkaloids from Leucetta and Clathrina Sponges. Nat. Prod. Rep. 2011, 28, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Gross, H.; Kehraus, S.; König, G.M.; Woerheide, G.; Wright, A.D. New and Biologically Active Imidazole Alkaloids from Two Sponges of the Genus Leucetta. J. Nat. Prod. 2002, 65, 1190–1193. [Google Scholar] [CrossRef] [PubMed]
- Plubrukarn, A.; Smith, D.W.; Cramer, R.E.; Davidson, B.S. (2E,9E)-Pyronaamidine 9-(N-Methylimine), a New Imidazole Alkaloid from the Northern Mariana Islands Sponge Leucetta sp. cf. chagosensis. J. Nat. Prod. 1997, 60, 712–715. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Kawabata, T.; Kato, H.; Ohta, T.; Rotinsulu, H.; Mangindaan, R.E.P.; van Soest, R.W.M.; Ukai, K.; Kobayashi, H.; Namikoshi, M. Naamidines H and I, Cytotoxic Imidazole Alkaloids from the Indonesian Marine Sponge Leucetta chagosensis. J. Nat. Prod. 2007, 70, 1658–1660. [Google Scholar] [CrossRef] [PubMed]
- Ralifo, P.; Crews, P. A New Structural Theme in the Imidazole-Containing Alkaloids from a Calcareous Leucetta Sponge. J. Org. Chem. 2004, 69, 9025–9029. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.; Hassanean, H.A.; Elkhayat, E.S.; Edrada, R.A.; Ebel, R.; Proksch, P. Imidazole Alkaloids from the Indopacific Sponge Pericharax heteroraphis. Bull. Pharm. Sci. 2007, 30, 149–157. [Google Scholar]
- Fu, X.; Schmitz, F.J.; Tanner, R.S.; Kelly-Borges, M. New Imidazole Alkaloids and Zinc Complexes from the Micronesian Sponge Leucetta cf. chagosensis. J. Nat. Prod. 1998, 61, 384–386. [Google Scholar]
- Chan, G.W.; Mong, S.; Hemling, M.E.; Freyer, A.J.; Offen, P.H.; Debrosse, C.W.; Sarau, H.M.; Westley, J.W. New Leukotriene B, Receptor Antagonist: Leucettamine A and Related Imidazole Alkaloids from the Marine Sponge Leucetta microraphis. J. Nat. Prod. 1993, 56, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Copp, B.R.; Fairchild, C.R.; Cornell, L.; Casazza, A.M.; Robinson, S.; Ireland, C.M. Naamidine A Is an Antagonist of the Epidermal Growth Factor Receptor and an in Vivo Active Antitumor Agent. J. Med. Chem. 1998, 41, 3909–3911. [Google Scholar] [CrossRef] [PubMed]
- Beedessee, G.; Ramanjooloo, A.; Aubert, G.; Eloyc, L.; Surnam-Boodhuna, R.; van Soest, R.W.M.; Cresteil, T.; Marie, D.E.P. Cytotoxic activities of hexane, ethyl acetate and butanol extracts of marine sponges from Mauritian Waters on human cancer cell lines. Environ. Toxicol. Pharmacol. 2012, 34, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Girish, B.; Avin, R.; Rashmee, S.B.; van Soest, R.W.M.; Marie, D.E.P. Acetylcholinesterase-Inhibitory Activities of the Extracts from Sponges Collected in Mauritius Waters. Chem. Biodivers. 2013, 10, 442–451. [Google Scholar]
- Carmely, S.; Kashman, Y. Naamines and naamidines, novel imidazole alkaloids from the calcareous sponge Leucetta chagosensis. Tetrahedron Lett. 1987, 28, 3003–3006. [Google Scholar] [CrossRef]
- Akee, R.K.; Carroll, T.R.; Yoshida, W.Y.; Scheuer, P.J. Two Imidazole Alkaloids from a Sponge. J. Org. Chem. 1990, 55, 1944–1946. [Google Scholar] [CrossRef]
- Crews, P.; Clark, D.P.; Tenney, K. Variation in the Alkaloids among Indo-Pacific Leucetta Sponges. J. Nat. Prod. 2003, 66, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Alley, M.C.; Scudiere, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of Drug Screening with Panels of Human Tumor Cell Lines Using a Microculture Tetrazolium Assay. Cancer. Res. 1988, 48, 589–601. [Google Scholar] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, P.; Yu, G.L.; Li, C.X.; Hao, C.; Qi, X.; Zhang, L.J.; Guan, H.S. Preparation and Anti-Influenza A Virus Activity of k-Carrageenan Oligosaccharide and Its Sulphated Derivatives. Food Chem. 2012, 133, 880–888. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–5 are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, K.-K.; Tang, X.-L.; Liu, Y.-S.; Li, P.-L.; Li, G.-Q. Imidazole Alkaloids from the South China Sea Sponge Pericharax heteroraphis and Their Cytotoxic and Antiviral Activities. Molecules 2016, 21, 150. https://doi.org/10.3390/molecules21020150
Gong K-K, Tang X-L, Liu Y-S, Li P-L, Li G-Q. Imidazole Alkaloids from the South China Sea Sponge Pericharax heteroraphis and Their Cytotoxic and Antiviral Activities. Molecules. 2016; 21(2):150. https://doi.org/10.3390/molecules21020150
Chicago/Turabian StyleGong, Kai-Kai, Xu-Li Tang, Yi-Sheng Liu, Ping-Lin Li, and Guo-Qiang Li. 2016. "Imidazole Alkaloids from the South China Sea Sponge Pericharax heteroraphis and Their Cytotoxic and Antiviral Activities" Molecules 21, no. 2: 150. https://doi.org/10.3390/molecules21020150