Flavonoids-Rich Orthosiphon stamineus Extract as New Candidate for Angiotensin I-Converting Enzyme Inhibition: A Molecular Docking Study
Abstract
:1. Introduction
2. Results
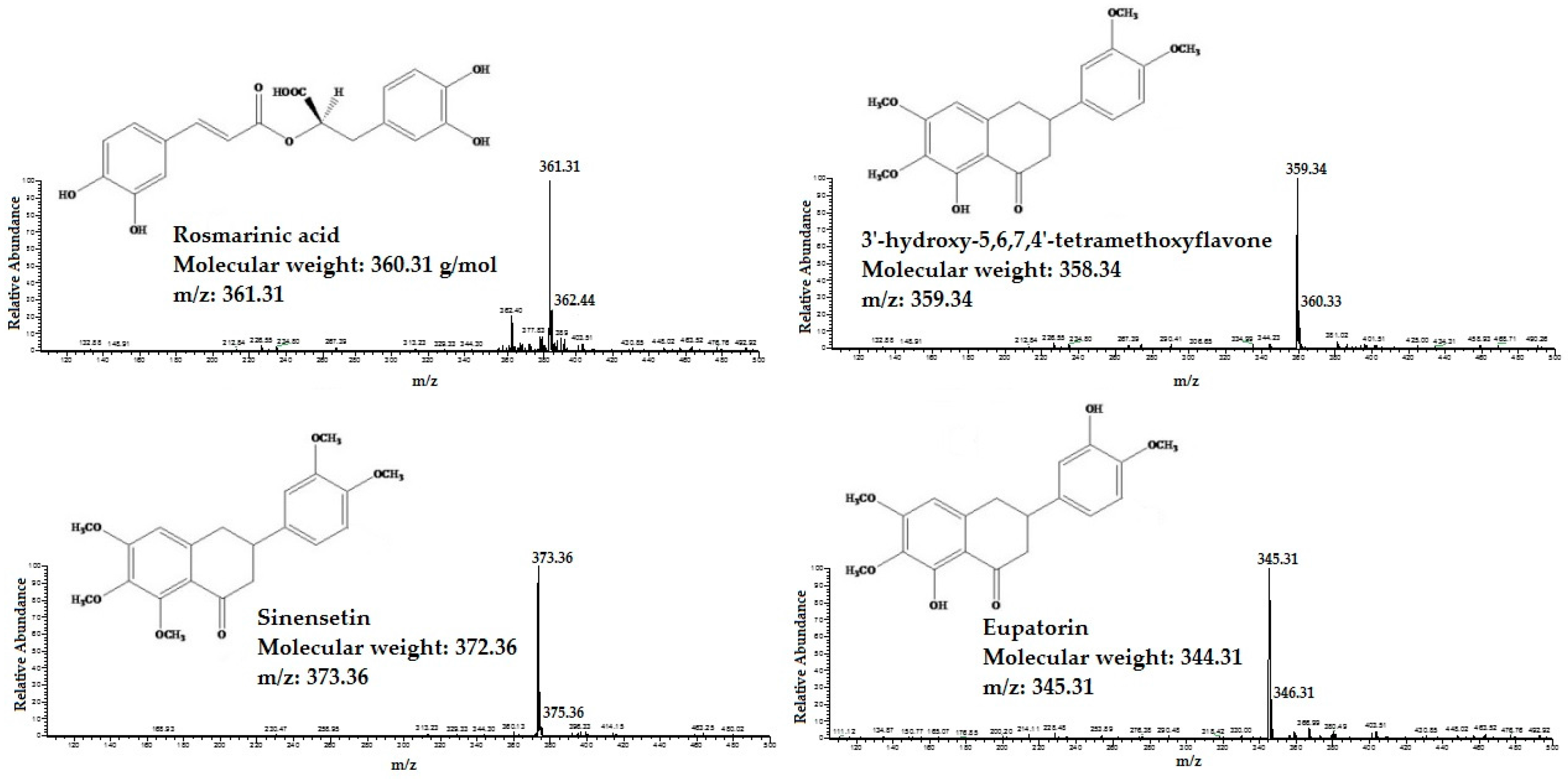
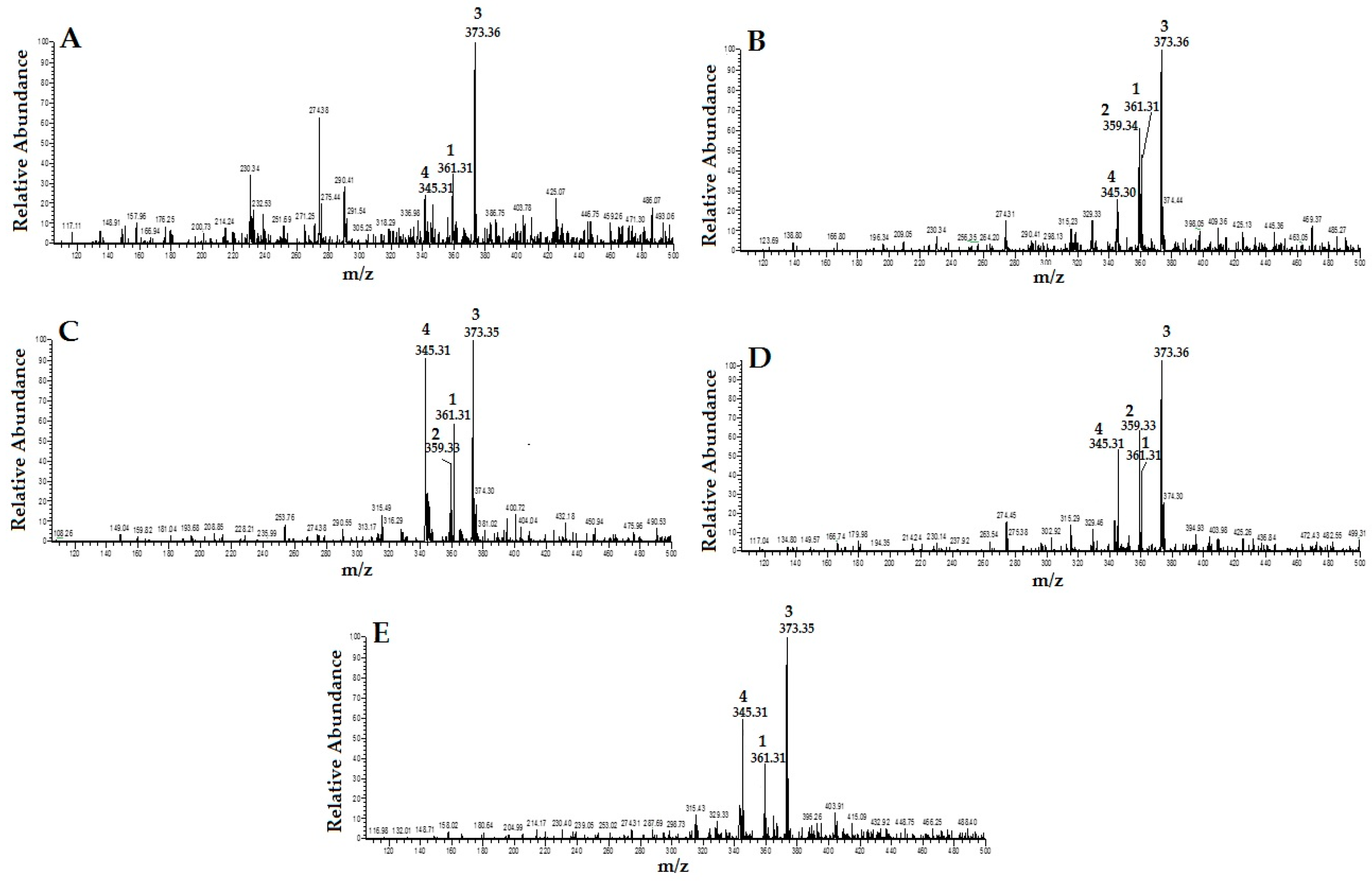
2.1. Identification and Quantification of RA, TMF, SIN and EUP in OS Extracts
2.2. Validation of HPLC Method for Measurement of ACE Inhibition Activity
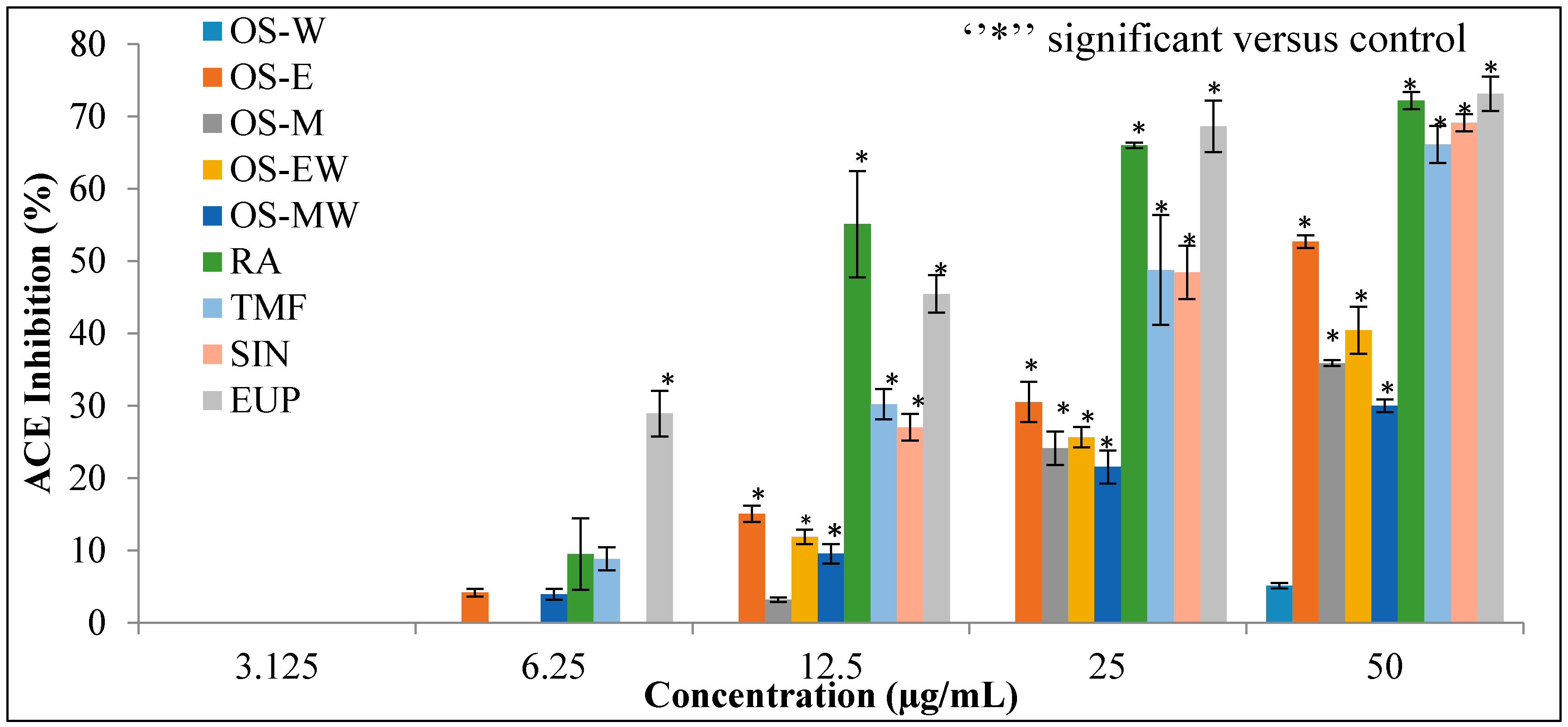
2.3. In Vitro ACE Inhibition Activity
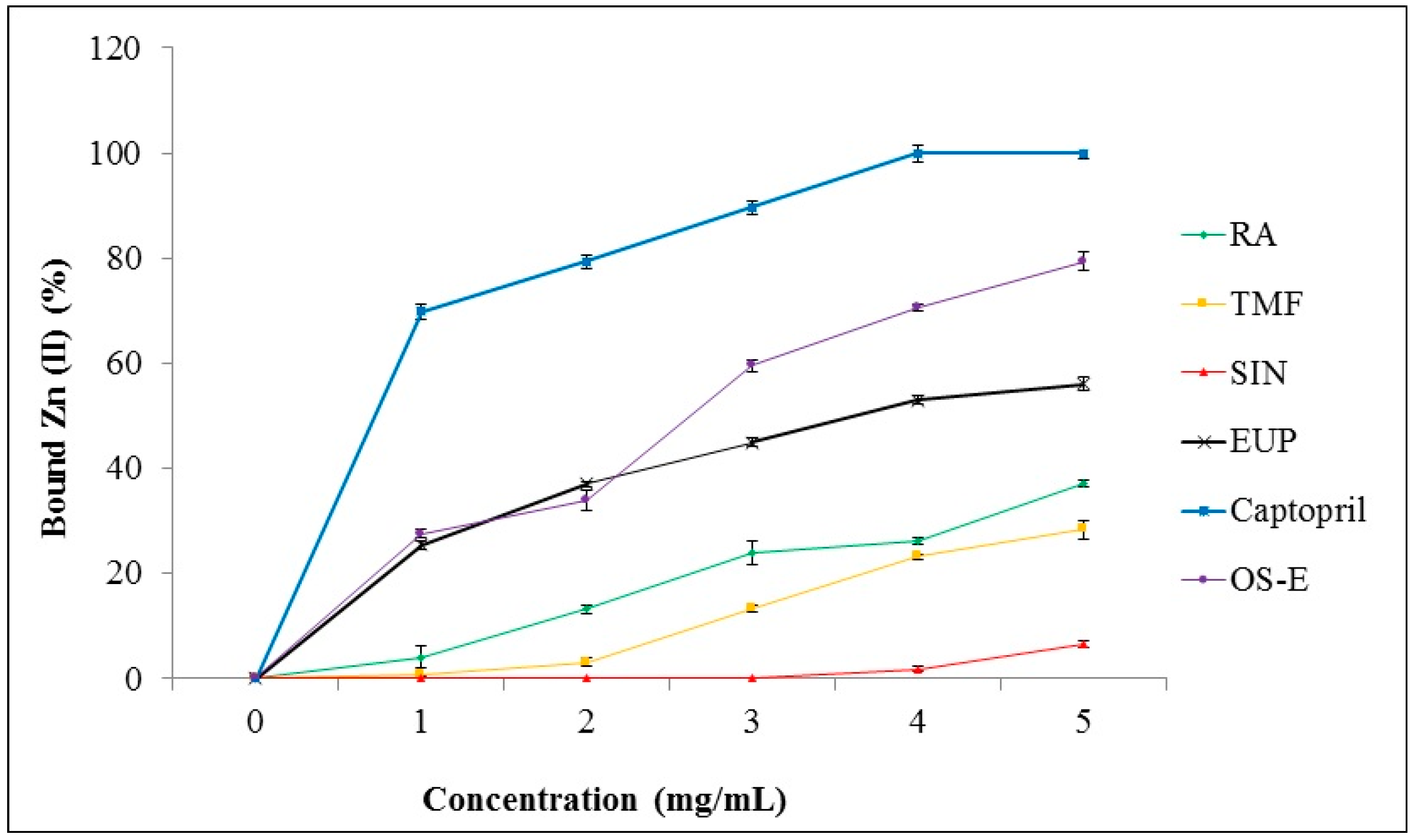
2.4. Chelation of Zinc (II) Ion by OS-E, RA, TMF, SIN, EUP and Captopril
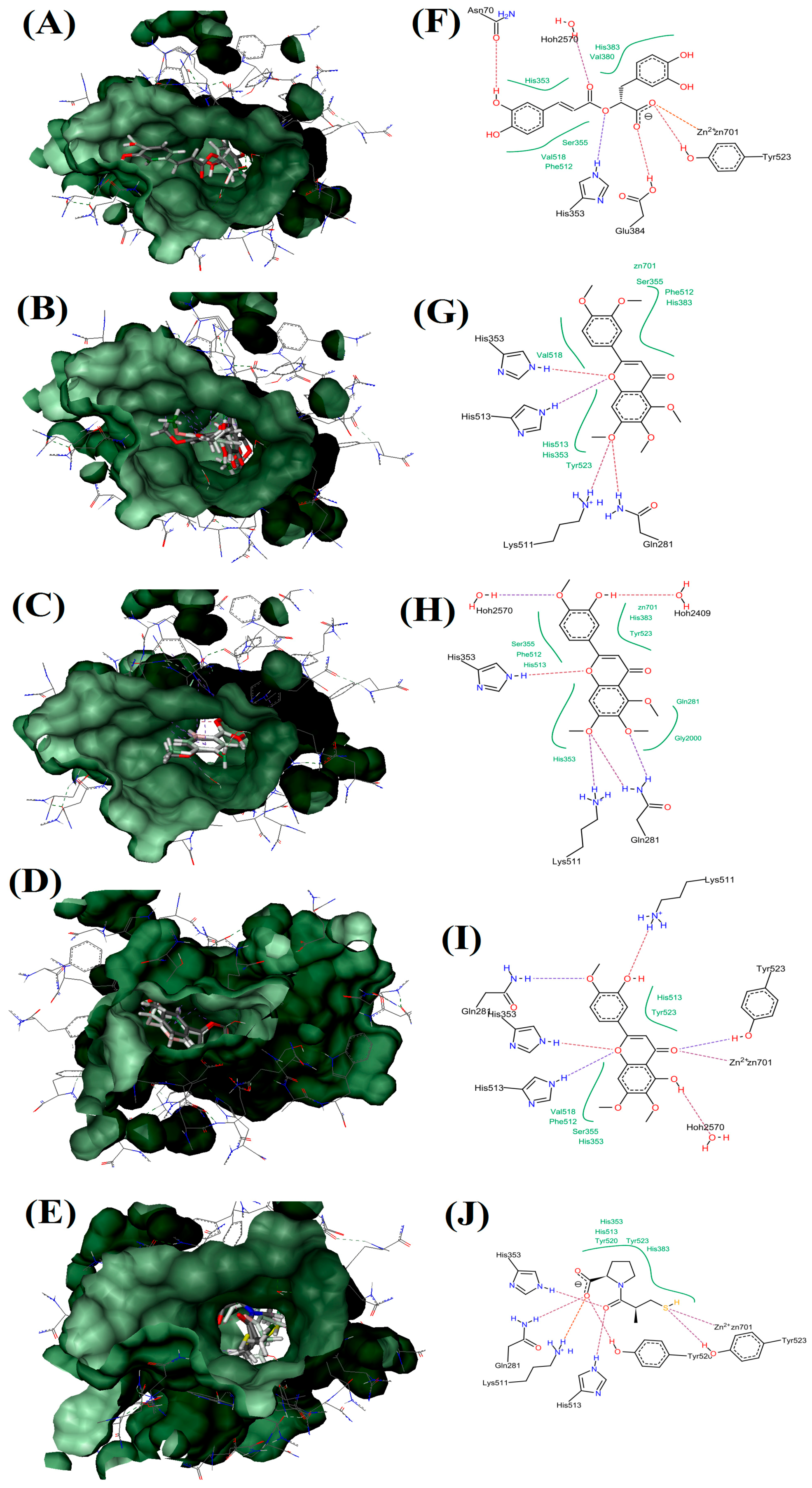
2.5. Molecular Docking Study
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material and Extraction
4.3. Identification and Quantification of RA, TMF, SIN and EUP in OS Extracts
4.3.1. HPLC Mass Spectrometry
4.3.2. HPLC
4.4. In Vitro ACE Inhibition Activity
4.4.1. HPLC Method for Measurement of ACE Inhibition Activity
Chromatographic Condition
HA Calibration Curve
ACE Calibration Curve
Validation of HPLC Method
4.5. In Vitro ACE Inhibition Activity
Sample Preparation with the Purified Rabbit Lung ACE
4.6. Chelation of Zinc Ion (II) by OS-E, RA, TMF, SIN, EUP and Captopril
4.7. Molecular Docking Study
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kearney, P.M.; Whelton, M.; Reynolds, K.; Muntner, P.; Whelton, P.K.; He, J. Global burden of hypertension: Analysis of worldwide data. Lancet 2005, 365, 217–223. [Google Scholar] [CrossRef]
- Hartmann, R.; Meisel, H. Food-derived peptides with biological activity: From research to food applications. Curr. Opin. Biotechnol. 2007, 18, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Mittal, B.V.; Singh, A.K. Hypertension in the developing world: Challenges and opportunities. Am. J. Kidney Dis. 2010, 55, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Maruthur, N.M.; Wang, N.-Y.; Appel, L.J. Lifestyle interventions reduce coronary heart disease risk results from the premier trial. Circulation 2009, 119, 2026–2031. [Google Scholar] [CrossRef] [PubMed]
- Coates, D. The angiotensin converting enzyme (ACE). Int. J. Biochem. Cell Biol. 2003, 35, 769–773. [Google Scholar] [CrossRef]
- Al Shukor, N.; Van Camp, J.; Gonzales, G.B.; Staljanssens, D.; Struijs, K.; Zotti, M.J.; Raes, K.; Smagghe, G. Angiotensin-converting enzyme inhibitory effects by plant phenolic compounds: A study of structure activity relationships. J. Agric. Food Chem. 2013, 61, 11832–11839. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.A.; Frohlich, E.D. Improvements in clinical outcomes with the use of angiotensin-converting enzyme inhibitors: Cross-fertilization between clinical and basic investigation. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H2021–H2025. [Google Scholar] [CrossRef] [PubMed]
- Israili, Z.H.; Hall, W.D. Cough and angioneurotic edema associated with angiotensin-converting enzyme inhibitor therapy: A review of the literature and pathophysiology. Ann. Intern. Med. 1992, 117, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Opie, L. Ace inhibitors in pregnancy: How to avoid the sting in the tail. S. Afr. Med. J. 1996, 86, 326–327. [Google Scholar] [PubMed]
- Nyman, U.; Joshi, P.; Madsen, L.; Pedersen, T.; Pinstrup, M.; Rajasekharan, S.; George, V.; Pushpangadan, P. Ethnomedical information and in vitro screening for angiotensin-converting enzyme inhibition of plants utilized as traditional medicines in gujarat, rajasthan and kerala (india). J. Ethnopharmacol. 1998, 60, 247–263. [Google Scholar] [CrossRef]
- Park, P.-J.; Je, J.-Y.; Kim, S.-K. Angiotensin i converting enzyme (ace) inhibitory activity of hetero-chitooligosaccharides prepared from partially different deacetylated chitosans. J. Agric. Food Chem. 2003, 51, 4930–4934. [Google Scholar] [CrossRef] [PubMed]
- Heran, B.S.; Wong, M.M.; Heran, I.K.; Wright, J.M. Blood pressure lowering efficacy of angiotensin converting enzyme (ace) inhibitors for primary hypertension. Cochrane Libr. 2008. [Google Scholar] [CrossRef]
- Cinq-Mars, C.D.; Li-Chan, E.C. Optimizing angiotensin I-converting enzyme inhibitory activity of pacific hake (merluccius productus) fillet hydrolysate using response surface methodology and ultrafiltration. J. Agric. Food Chem. 2007, 55, 9380–9388. [Google Scholar] [CrossRef] [PubMed]
- Vercruysse, L.; Van Camp, J.; Smagghe, G. Ace inhibitory peptides derived from enzymatic hydrolysates of animal muscle protein: A review. J. Agric. Food Chem. 2005, 53, 8106–8115. [Google Scholar] [CrossRef] [PubMed]
- Somova, L.; Nadar, A.; Rammanan, P.; Shode, F. Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine 2003, 10, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, L.; Castillo, J.; Quiñones, M.; Garcia-Vallvé, S.; Arola, L.; Pujadas, G.; Muguerza, B. Inhibition of angiotensin-converting enzyme activity by flavonoids: Structure-activity relationship studies. PLoS ONE 2012, 7, e49493. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Xu, X.; Liang, Y.; Head, R.; Bennett, L. Inhibition of angiotensin converting enzyme (ace) activity by polyphenols from tea (Camellia sinensis) and links to processing method. Food Funct. 2011, 2, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, D.; Jiménez-Ferrer, E.; Zamilpa, A.; Herrera-Arellano, A.; Tortoriello, J.; Alvarez, L. Inhibition of angiotensin convertin enzyme (ACE) activity by the anthocyanins delphinidin-and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa. J. Ethnopharmacol. 2010, 127, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Said, A.; Tundis, R.; Rashed, K.; Statti, G.A.; Hufner, A.; Menichini, F. Inhibition of angiotensin converting enzyme (ACE) by flavonoids isolated from Ailanthus excelsa (Roxb) (Simaroubaceae). Phytother. Res. 2007, 21, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Häckl, L.; Cuttle, G.; Sanches Dovichi, S.; Lima-Landman, M.; Nicolau, M. Inhibition of angiotensin-converting enzyme by quercetin alters the vascular response to bradykinin and angiotensin I. Pharmacology 2002, 65, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Sernia, C.; Brown, L. Ferulic acid improves cardiovascular and kidney structure and function in hypertensive rats. J. Cardiovasc. Pharmacol. 2013, 61, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Actis-Goretta, L.; Ottaviani, J.I.; Keen, C.L.; Fraga, C.G. Inhibition of angiotensin converting enzyme (ACE) activity by flavan-3-ols and procyanidins. FEBS Lett. 2003, 555, 597–600. [Google Scholar] [CrossRef]
- Kwon, E.-K.; Lee, D.-Y.; Lee, H.; Kim, D.-O.; Baek, N.-I.; Kim, Y.-E.; Kim, H.-Y. Flavonoids from the buds of Rosa damascena inhibit the activity of 3-hydroxy-3-methylglutaryl-coenzyme a reductase and angiotensin I-converting enzyme. J. Agric. Food Chem. 2009, 58, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Bormann, H.; Melzig, M. Inhibition of metallopeptidases by flavonoids and related compounds. Die Pharm. 2000, 55, 129–132. [Google Scholar]
- Yesudas, R.; Gumaste, U.; Snyder, R.; Thekkumkara, T. Tannic acid down-regulates the angiotensin type 1 receptor through a mapk-dependent mechanism. Mol. Endocrinol. 2012, 26, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Balasuriya, B.N.; Rupasinghe, H.V. Plant flavonoids as angiotensin converting enzyme inhibitors in regulation of hypertension. Funct. Foods Health Dis. 2011, 1, 172–188. [Google Scholar]
- Quiñones, M.; Sánchez, D.; Muguerza, B.; Miguel, M.; Aleixandre, A. Mechanisms for antihypertensive effect of cocoanox, a polyphenol-rich cocoa powder, in spontaneously hypertensive rats. Food Res. Int. 2011, 44, 1203–1208. [Google Scholar] [CrossRef]
- Junior, A.G.; Gasparotto, F.M.; Lourenço, E.L.B.; Crestani, S.; Stefanello, M.E.A.; Salvador, M.J.; da Silva-Santos, J.E.; Marques, M.C.A.; Kassuya, C.A.L. Antihypertensive effects of isoquercitrin and extracts from Tropaeolum majus L.: Evidence for the inhibition of angiotensin converting enzyme. J. Ethnopharmacol. 2011, 134, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Lahogue, V.; Réhel, K.; Taupin, L.; Haras, D.; Allaume, P. A HPLC-UV method for the determination of angiotensin I-converting enzyme (ACE) inhibitory activity. Food Chem. 2010, 118, 870–875. [Google Scholar] [CrossRef]
- Alves, M.; Araujo, M.d.C.; Juliano, M.A.; Oliveira, E.M.d.; Krieger, J.E.; Casarini, D.E.; Juliano, L.; Carmona, A.K. A continuous fluorescent assay for the determination of plasma and tissue angiotensin I-converting enzyme activity. Braz. J. Med. Biol. Res. 2005, 38, 861–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sentandreu, M.Á.; Toldrá, F. A rapid, simple and sensitive fluorescence method for the assay of angiotensin-I converting enzyme. Food Chem. 2006, 97, 546–554. [Google Scholar] [CrossRef]
- Cushman, D.; Cheung, H. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem. Pharmacol. 1971, 20, 1637–1648. [Google Scholar] [CrossRef]
- Inoue, K.; Kitade, M.; Hino, T.; Oka, H. Screening assay of angiotensin-converting enzyme inhibitory activity from complex natural colourants and foods using high-throughput LC-MS/MS. Food Chem. 2011, 126, 1909–1915. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.; Manso, M.A.; Martín-Álvarez, P.J.; Aleixandre, A.; López-Fandiño, R. Angiotensin-converting enzyme activity in plasma and tissues of spontaneously hypertensive rats after the short-and long-term intake of hydrolysed egg white. Mol. Nutr. Food Res. 2007, 51, 555–563. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.A.H.; Mohamed, A.J.; Asmawi, M.Z.; Sadikun, A.; Ebrika, O.S.; Yam, M.F. Antihyperglycemic effect of orthosiphon stamineus benth leaves extract and its bioassay-guided fractions. Molecules 2011, 16, 3787–3801. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Bohgaki, T.; Watarai, M.; Suzuki, H.; Ohashi, K.; Shibuya, H. Antihypertensive actions of methylripariochromene a from Orthosiphon aristatus, an Indonesian traditional medicinal plant. Biol. Pharm. Bull. 1999, 22, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Arafat, O.; Tham, S.; Sadikun, A.; Zhari, I.; Haughton, P.; Asmawi, M. Studies on diuretic and hypouricemic effects of Orthosiphon stamineus methanol extracts in rats. J. Ethnopharmacol. 2008, 118, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Dolečková, I.; Rárová, L.; Grúz, J.; Vondrusová, M.; Strnad, M.; Kryštof, V. Antiproliferative and antiangiogenic effects of flavone eupatorin, an active constituent of chloroform extract of Orthosiphon stamineus leaves. Fitoterapia 2012, 83, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Akowuah, G.; Zhari, I.; Norhayati, I.; Sadikun, A.; Khamsah, S. Sinensetin, eupatorin, 3′-hydroxy-5,6,7,4′-tetramethoxyflavone and rosmarinic acid contents and antioxidative effect of Orthosiphon stamineus from Malaysia. Food Chem. 2004, 87, 559–566. [Google Scholar] [CrossRef]
- Akowuah, G.; Ismail, Z.; Norhayati, I.; Sadikun, A. The effects of different extraction solvents of varying polarities on polyphenols of orthosiphon stamineus and evaluation of the free radical-scavenging activity. Food Chem. 2005, 93, 311–317. [Google Scholar] [CrossRef]
- Corradini, E.; Foglia, P.; Giansanti, P.; Gubbiotti, R.; Samperi, R.; Laganà, A. Flavonoids: Chemical properties and analytical methodologies of identification and quantitation in foods and plants. Natl. Prod. Res. 2011, 25, 469–495. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.J.; Croley, T.R.; Metcalfe, C.D.; March, R.E. A tandem mass spectrometric study of selected characteristic flavonoids. Int. J. Mass Spectrom. 2001, 210, 371–385. [Google Scholar] [CrossRef]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Nunez, L.; Castillo, J.; Lozano, M.L.; Martinez, C.; Benavente-García, O.; Vicente, V.; Rivera, J. Thromboxane a2 receptor antagonism by flavonoids: Structure−activity relationships. J. Agric. Food Chem. 2009, 57, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Benavente-Garcıa, O.; Castillo, J.; Lorente, J.; Ortuno, A.; Del Rio, J. Antioxidant activity of phenolics extracted from Olea europaea L. Leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Parellada, J.; Suárez, G.; Guinea, M. Inhibition of zinc metallopeptidases by flavonoids and related phenolic compounds: Structure-activity relationships. J. Enzym. Inhib. 1998, 13, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Ende, C.; Gebhardt, R. Inhibition of matrix metalloproteinase-2 and-9 activities by selected flavonoids. Planta Med. 2004, 70, 1006–1008. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.J.A.; Ismail, Z. Simultaneous analysis of bioactive markers from Orthosiphon stamineus benth leaves extracts by reverse phase high performance liquid chromatography. Trop. J. Pharm. Res. 2011, 10, 97–103. [Google Scholar] [CrossRef]
- International Conference on Harmonization. Validation of Analytical Procedures: Methodology (Q2B); FDA: Rockville, MD, USA, 1996.
- Karamać, M. Chelation of Cu (II), Zn (II), and Fe (II) by tannin constituents of selected edible nuts. Int. J. Mol. Sci. 2009, 10, 5485–5497. [Google Scholar] [CrossRef] [PubMed]
- Böhm, H.-J. Prediction of binding constants of protein ligands: A fast method for the prioritization of hits obtained from de novo design or 3D database search programs. J. Comput. Aided Mol. Des. 1998, 12, 309–309. [Google Scholar] [CrossRef] [PubMed]
- Rarey, M.; Kramer, B.; Lengauer, T.; Klebe, G. A fast flexible docking method using an incremental construction algorithm. J. Mol. Biol. 1996, 261, 470–489. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds RA, TMF, SIN and EUP are available from the authors.
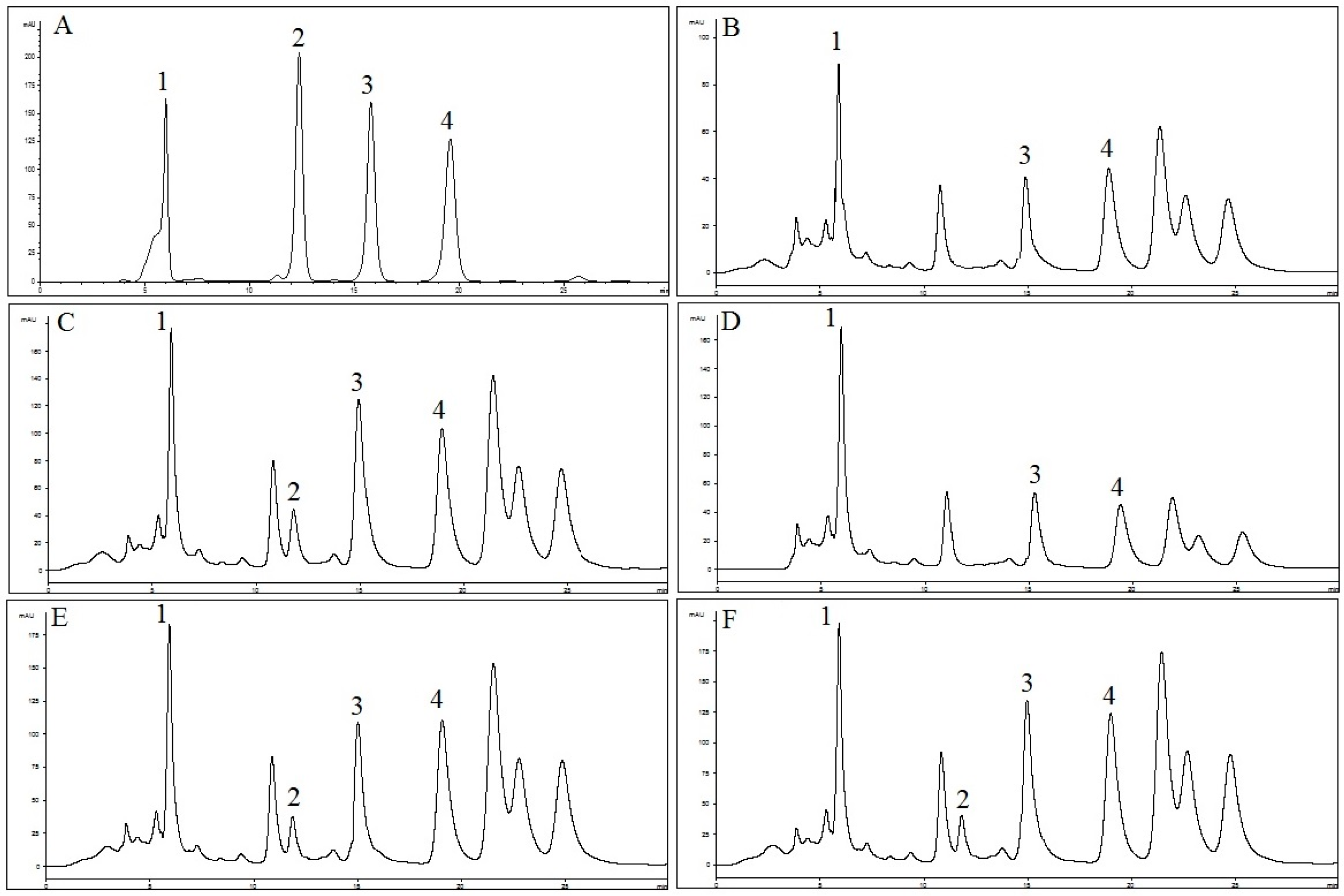
| Component | Water Extract (OS-W) | Ethanolic Extract (OS-E) | Methanolic Extract (OS-M) | 50% Ethanolic Extract (OS-EW) | 50% Methanolic Extract (OS-MW) |
|---|---|---|---|---|---|
| RA | 31.0 ± 0.2 | 219.8 ± 0.6 | 200.8 ± 1.5 | 235.5 ± 0.2 | 212.3 ± 0.01 |
| TMF | 0.0 ± 0.7 | 11.5 ± 1.0 | 0.0 ± 0.2 | 4.3 ± 0.01 | 0.9 ± 0.01 |
| SIN | 3.2 ± 0.01 | 23.2 ± 0.1 | 6.1 ± 0.01 | 10.5 ± 0.01 | 8.0 ± 0.3 |
| EUP | 2.3 ± 0.01 | 166.9 ± 3.5 | 22.4 ± 0.3 | 66.8 ± 0.9 | 30.6 ± 0.1 |
| Components | IC50 | Unit |
|---|---|---|
| OS-W | 358.8 ± 24.2 | µg/mL |
| OS-E | 45.8 ± 1.2 | µg/mL |
| OS-M | 63.7 ± 1.1 | µg/mL |
| OS-EW | 58.1 ± 2.0 | µg/mL |
| OS-MW | 78.2 ± 7.9 | µg/mL |
| Captopril | 0.002 ± 0.001 | µM |
| RA | 18.8 ± 0.2 | µg/mL |
| TMF | 27.9 ± 2.4 | µg/mL |
| SIN | 29.5 ± 0.3 | µg/mL |
| EUP | 15.4 ± 4.5 | µg/mL |
| Receptor-Ligand Complex (LEADIT) | Binding Affinity Energy, ΔG (kJ/mol) | Ligand Efficiency (kcal/mol Per Heavy Atom) | Residue Interactions | Hydrogen Bonds |
|---|---|---|---|---|
| ACE-RA | −17 | 0.16 | Zn701, Tyr523, Glu384, His353, Ser355, Val516, Phe512, Asn70, His353, Hoh2570, His383 and Val380 | 6 |
| ACE-SIN | 10 | 0 | Zn701, Ser355, Phe512, His383, Gln281, Lys511, Tyr523, His353, His513 and Val518 | 4 |
| ACE-TMF | −6 | 0.06 | Zn701, His383, Tyr523, Hoh2409, Gln281, Gly2000, Lys511, His353, Ser355, Phe512, His513 and Hoh2570. | 5 |
| ACE-EUP | −29 | 0.28 | Zn701, Hoh2570, Ser355, His353, Val518, Phe512, His513, Gln281, Lys511, His513 and Tyr523 | 7 |
| ACE-Captopril | −38 | 0.64 | Zn701, Tyr523, Tyr520, His513, Lys511, Gln281, His353 and His383 | 7 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shafaei, A.; Sultan Khan, M.S.; Aisha, A.F.A.; Abdul Majid, A.M.S.; Hamdan, M.R.; Mordi, M.N.; Ismail, Z. Flavonoids-Rich Orthosiphon stamineus Extract as New Candidate for Angiotensin I-Converting Enzyme Inhibition: A Molecular Docking Study. Molecules 2016, 21, 1500. https://doi.org/10.3390/molecules21111500
Shafaei A, Sultan Khan MS, Aisha AFA, Abdul Majid AMS, Hamdan MR, Mordi MN, Ismail Z. Flavonoids-Rich Orthosiphon stamineus Extract as New Candidate for Angiotensin I-Converting Enzyme Inhibition: A Molecular Docking Study. Molecules. 2016; 21(11):1500. https://doi.org/10.3390/molecules21111500
Chicago/Turabian StyleShafaei, Armaghan, Md Shamsuddin Sultan Khan, Abdalrahim F. A. Aisha, Amin Malik Shah Abdul Majid, Mohammad Razak Hamdan, Mohd Nizam Mordi, and Zhari Ismail. 2016. "Flavonoids-Rich Orthosiphon stamineus Extract as New Candidate for Angiotensin I-Converting Enzyme Inhibition: A Molecular Docking Study" Molecules 21, no. 11: 1500. https://doi.org/10.3390/molecules21111500





