Synthesis and Formulation of Thermosensitive Drug Carrier for Temperature Triggered Delivery of Naproxen Sodium
Abstract
:1. Introduction
2. Results
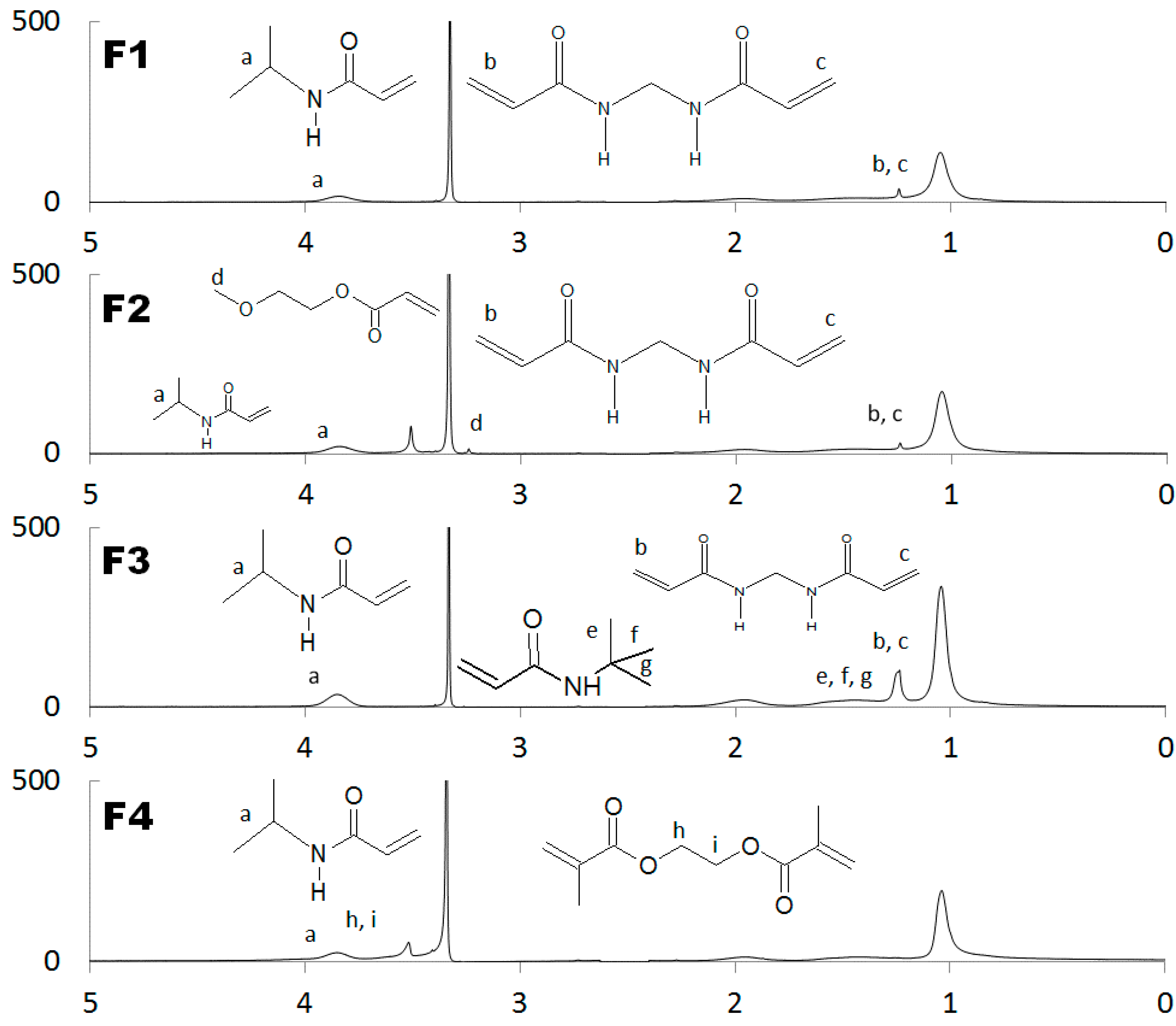
2.1. NMR Spectra
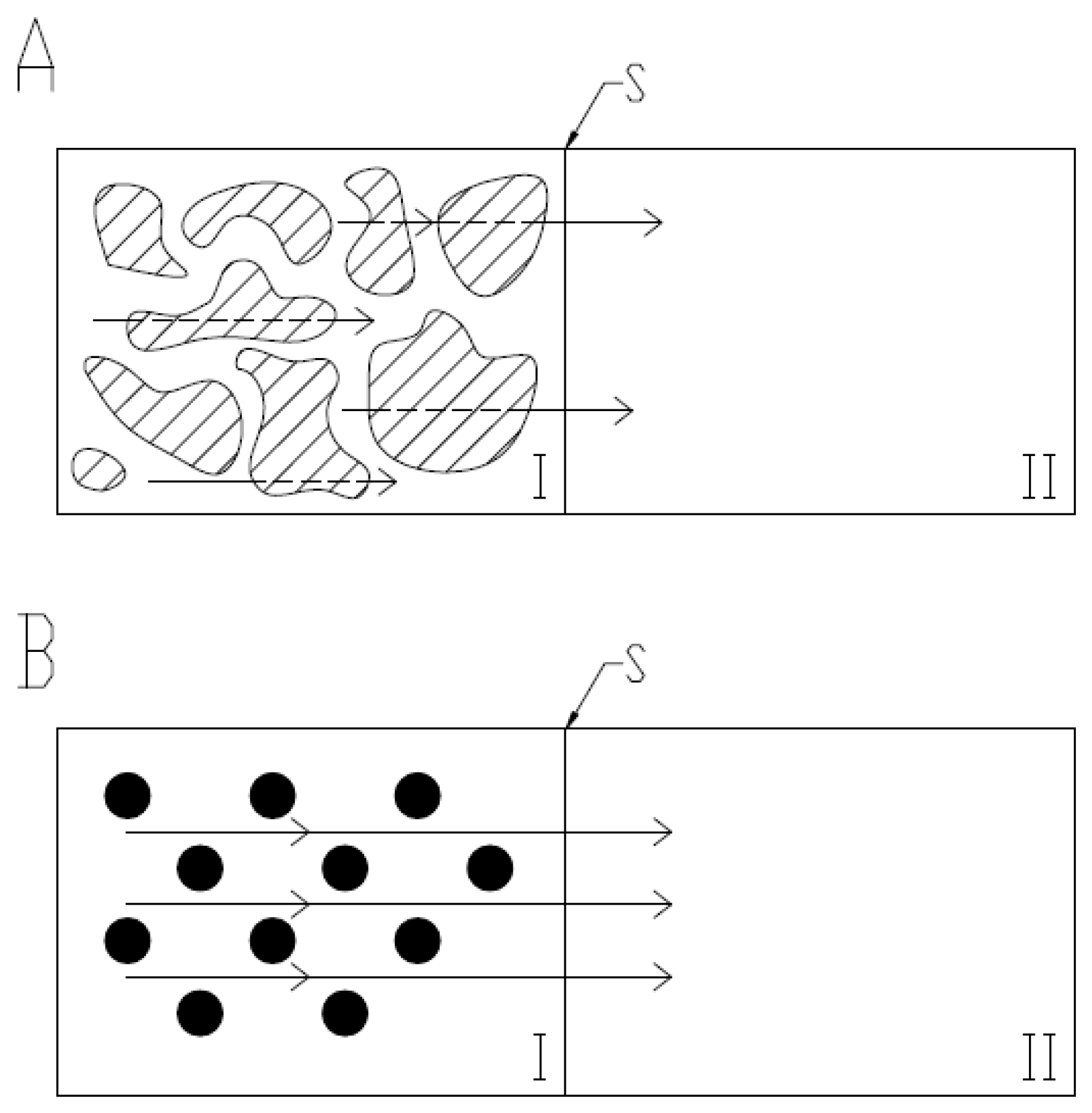
2.2. Hydrodynamic Diameter and VPTT Evaluations
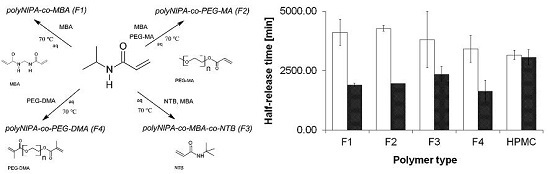
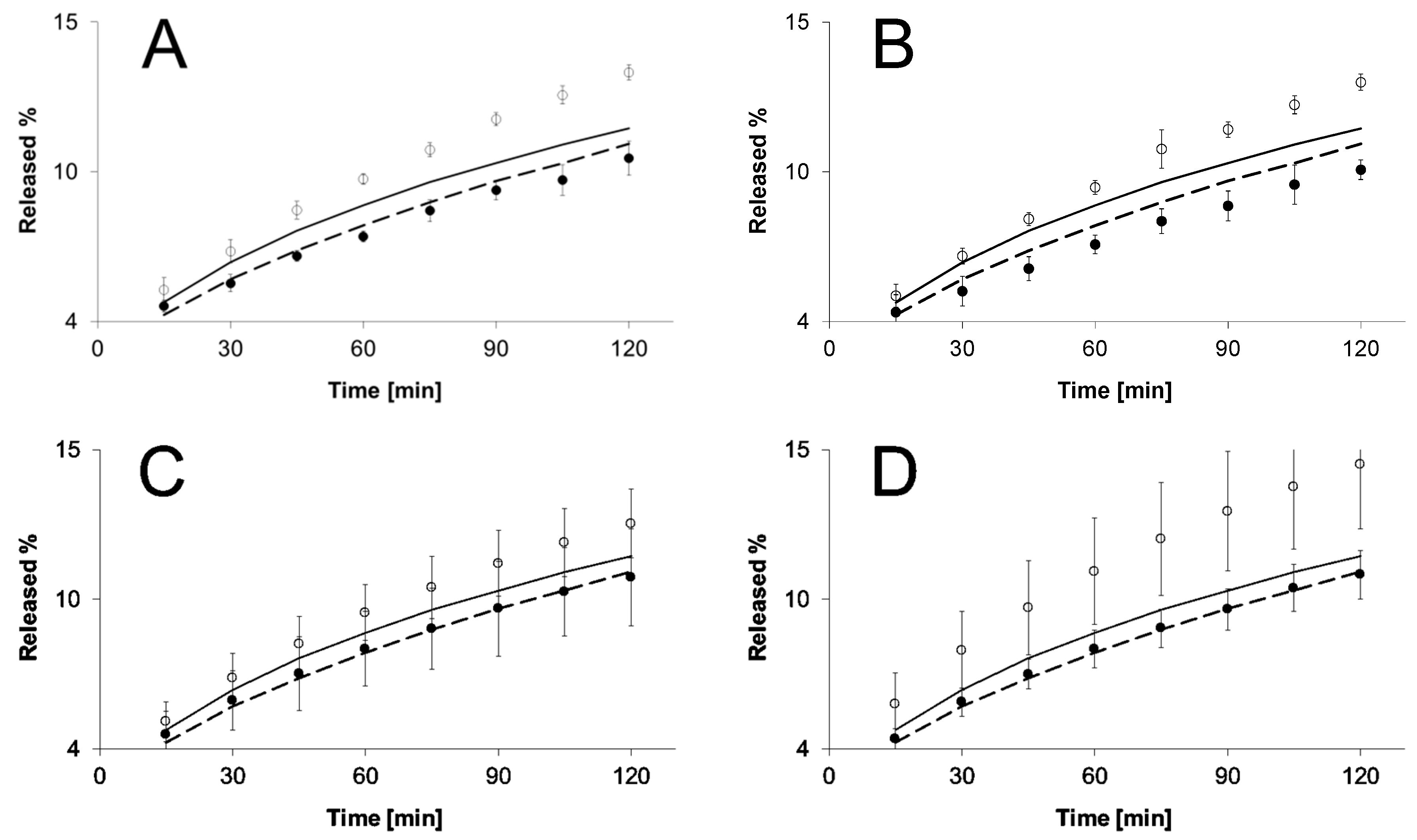
2.3. Release Kinetics Evaluated due to Selected Kinetic Models
3. Discussion
4. Experimental Section
4.1. Materials
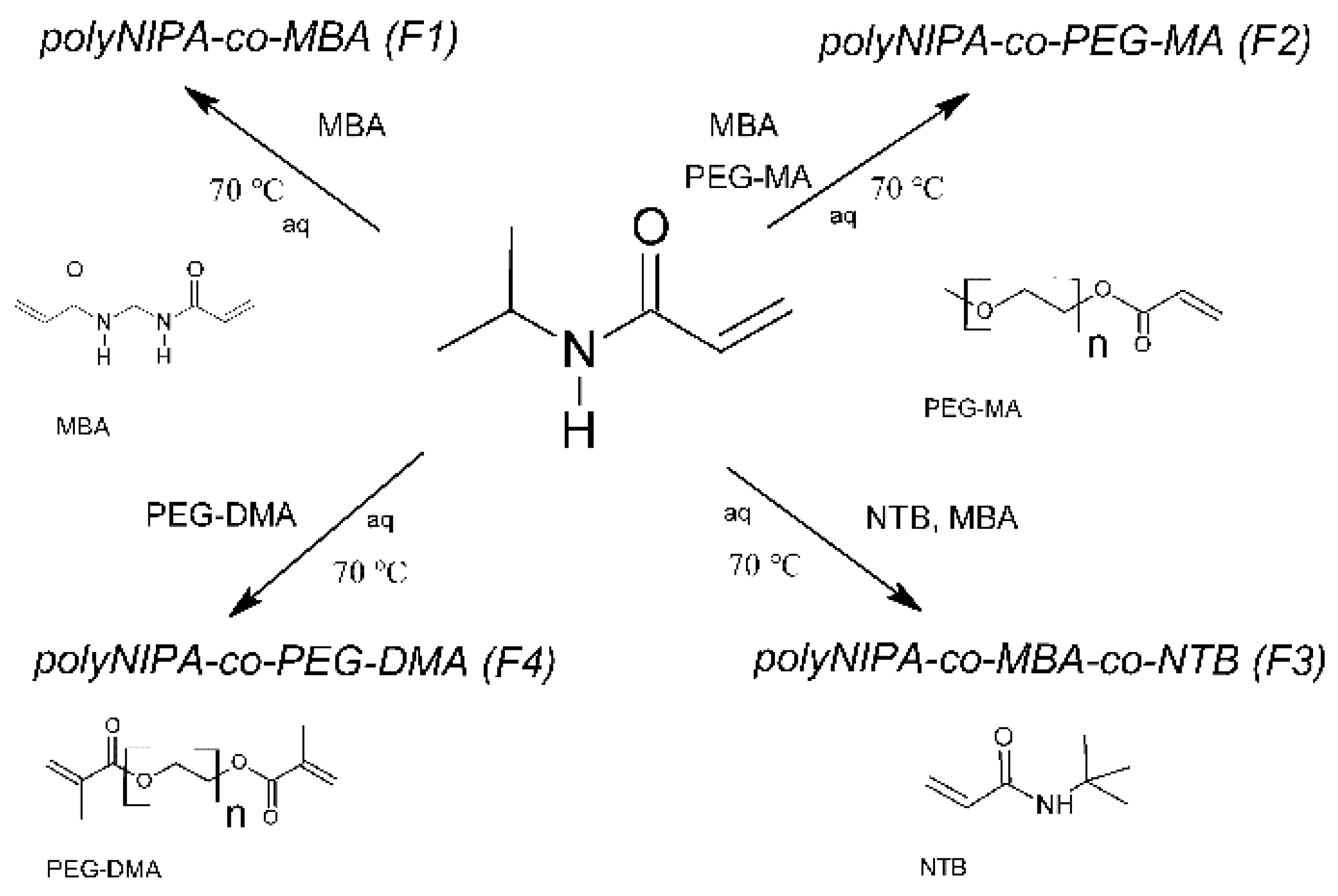
4.2. Synthesis of the Polymers
4.3. NMR Evaluations
4.4. Evaluation of Hydrodynamic Diameter and VPTT
4.5. Preparation of Hydrogels with Naproxen Sodium
4.6. Drug Release from Thermosensitive Hydrogels
5. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Vu, T.-L.; Lovalenti, P.M.; Abdul-Fattah, A.M. Stabilization challenges and formulation strategies associated with oral biologic drug delivery systems. Adv. Drug Deliv. Rev. 2015, 93, 95–108. [Google Scholar]
- Faraji, A.H.; Wipf, P. Nanoparticles in cellular drug delivery. Bioorg. Med. Chem. 2009, 17, 2950–2962. [Google Scholar] [CrossRef] [PubMed]
- Cuggino, J.C.; Contreras, C.B.; Jimenez-Kairuz, A.; Maletto, B.A.; Alvarez Igarzabal, C.I. Novel Poly(NIPA-co-AAc) Functional Hydrogels with Potential Application in Drug Controlled Release. Mol. Pharm. 2014, 11, 2239–2249. [Google Scholar] [CrossRef] [PubMed]
- Chien-Chi, L.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar]
- Chearuil, F.; Corrigan, I.O. Thermosensitivity and release from poly N-isopropylacrylamide—Polylactide copolymers. Int. J. Pharm. 2009, 366, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Bölgen, N.; Aguilar, M.R.; Mar Fernández, M.; Gonzalo-Flores, S.; Villar-Rodil, S.; san Román, J.; Pişkin, E. Thermoresponsive biodegradable HEMA-Lactate-Dextran-co-NIPA cryogels for controlled release of simvastatin, Artifi cial Cells. Nanomed. Biotechnol. 2015, 43, 40–49. [Google Scholar]
- Seddiki, N.; Aliouche, D. Synthesis, rheological, behavior and swelling properties of copolymer hydrogels based on poly(N-isopropylacrylamide) with hydrophilic monomers. Bull. Chem. Soc. Ethiop. 2013, 27, 447–457. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.H.; Jean, O.; Kwon, I.C.; Park, K. Engineerind polymers for advanced drug delivery. Eur. J. Pharm. Biopharm. 2009, 71, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Zhengyang, Z.; Shenmin, Z.; Di, Z. Grafting of thermo-responsive polymer inside mesoporous silica with large pore size using ATRP and investigation of its use in drug release. J. Mater. Chem. 2007, 17, 2428–2433. [Google Scholar]
- Musiał, W.; Voncina, B.; Pluta, J.; Kokol, V. The Study of release of Chlorhexidine from Preparation with Modifield Thermosensitive Poly-N-izopropyloacrylamide Microspheres. Sci. World J. 2012, 12. [Google Scholar] [CrossRef]
- Aguilar, M.R.; Elvira, C.; Gallardo, A.; Vázquez, B.; Román, J.S. Smart Polymers and Their Applications as Biomaterials. Top. Tissue Eng. 2007, 3, 12–16. [Google Scholar]
- Coughlan, D.C.; Quilty, F.P.; Corrigan, O.I. Effect of drug physicochemical properties on swelling/deswellingkinetics and pulsatile drug release from thermoresponsive poly(N-isopropylacrylamide) hydrogels. J. Control Release 2004, 98, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Pelton, R.H.; Chibante, P. Preparation of aqueous lattices with N-isopropylacrylamide. Colloid Surf. 1986, 20, 247–256. [Google Scholar] [CrossRef]
- Fucinos, C.; Fucinos, P.; Miguez, M.; Katime, I.; Pastrana, L.M.; Rua, M.L. Temperature- and pH-Sensitive Nanohydrogels of Poly(N-Isopropylacrylamide) for Food Packaging Applications: Modelling the Swelling-Collapse Behaviour. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Wongsuwarn, S.; Vigolo, D.; Cerbino, R.; Howe, A.M.; Vailati, A.; Piazza, R.; Cicuta, P. Giant thermophoresis of poly(N-isopropylacrylamide) microgel particles. Soft Matter 2012, 8, 5857–5863. [Google Scholar] [CrossRef]
- Costa, R.O.R.; Freitas, R.F.S. Phase behavior of poly(N-isopropylacrylamide) in binary aqueous solutions. Polymer 2002, 43, 5879–5885. [Google Scholar] [CrossRef]
- Aerry, S.; De, A.; Kumar, A.; Saxena, A.; Majumdar, D.K.; Mozumdar, S. Synthesis and characterization of thermoresponsive copolymers for drug delivery. J. Biomed. Mater. Res. 2013, 101A, 2015–2026. [Google Scholar] [CrossRef] [PubMed]
- Constantin, M.; Cristea, M.; Ascenzi, P.; Fundueanu, G. Lower critical solution temperature versus volume phase transition temperature in thermoresponsive drug delivery systems. Express Polym. Lett. 2011, 5, 839–848. [Google Scholar] [CrossRef]
- Musiał, W.; Vincent, B.; Szumny, A.; Voncina, B. Morphological characteristics of modified freeze-dried poly(N-izopropyloacrylamide) microspheres studiem by optical microscopy, SEM, and DLS. Chem. Pap. 2010, 64, 602–612. [Google Scholar] [CrossRef]
- Yang, H.; Yin, W.; Kong, X.; Xu, M.; Yao, Y.; Chen, Q.; Cheng, R. Preparation of the individual compact single-chain globular particulates of poly(N-isopropylacrylamide). Colloid Polym. Sci. 2006, 284, 935–940. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, X.; Xiao, H. Structure and properties of cellulose/poly(N-isopropylacrylamide) hydrogels prepared by SIPN strategy. Carbohydr. Polym. 2013, 94, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Yang, H.; Wang, G.; Zhu, Z.; Song, W.; Zhao, H. Radiation Polymerization and Controlled Drug Release of Polymer Hydrogels with NIPA and NVP. J. Appl. Polym. Sci. 2003, 88, 724–729. [Google Scholar] [CrossRef]
- Gomez, C.; Benito, M.; Katime, I.; Teijón, J.M.; Blanco, M.D. In vitro transdermal and biological evaluation of ALA-loaded poly(N-isopropylacrylamide) and poly(N-isopropylacrylamide-co-acrylic acid) microgels for photodynamic therapy. J. Microencapsul. 2012, 29, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Gasztych, M.; Valh Volmajer, J.; Kokol, V.; Szumny, A.J.; Musiał, W. Synthesis, evaluation and release studies of NIPA nanopolymers presumed for temperature-controlled drug delivery. J. Sol-Gen Sci. Technol. 2016, 79, 466–474. [Google Scholar] [CrossRef]
- Zhang, J.T.; Huang, S.W.; Liu, J.; Zhuo, R.X. Temperature Sensitive Poly[N-isopropylacrylamide-co-(acryloyl β-cyclodextrin)] for Improved Drug Release. Macromol. Biosci. 2005, 5, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Katime, I.; Valderruten, N.; Quintana, J.R. Controlled release of aminophylline from poly(N-isopropylacrylamide-co-itaconic acid) hydrogels. Polym. Int. 2001, 50, 869–874. [Google Scholar] [CrossRef]
- Mallikarjuna, B.; Madhu Sudhana Rao, K.; Prasad, C.V.; Chowdoji Rao, K.; Krishana Rao, K.S.V.; Subha, M.C.S. Synthesis, characterization and use of Poly (N-isopropylacrylamide-co-N-vinylcaprolactam) crosslinked thermoresponsive microspheres for control release of Ciproflaxin hydrochloride drug. J. Appl. Pharm. Sci. 2011, 1, 171–177. [Google Scholar]
- Zhang, L.; Brostowitz, N.R.; Cavicchi, K.A.; Weiss, R.A. Perspective: Ionomer Research and Applications. Molecules 2014, 8, 81–99. [Google Scholar] [CrossRef]
- Prathap, M.; Gulshan, M.D.; Rama Rao, N.; Sathish Kumar, M. Application of Factorial Design for the Development of Site Specific Systems in the Management of Ulcerative Colitis. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1242–1252. [Google Scholar]
- Blanco, M.D.; Guerrero, S.; Teijon, C.; Olmo, R.; Pastrana, L.; Katime, I.; Teijon, J.M. Preparation and characterization of nanoparticulate poly(N-isopropylacrylamide) hydrogel for the controlled release of anti-tumour drugs. Polym. Int. 2008, 57, 1215–1225. [Google Scholar] [CrossRef]
- Cirillo, G.; Spataro, T.; Curcio, M.; Spizzirri, G.; Nicoletta, F.P.; Picci, N.; Iemma, F. Tunable thermo-responsive hydrogels: Synthesis, structural analysis and drug release studies. Mater. Sci. Eng. 2015, 48, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Fan, X.D.; Hu, H.; Tang, Z.H. Release of Chlorambucil from Poly(N-isopropylacrylamide) Hydrogels with β-Cyclodextrin Moieties. Macromol. Biosci. 2004, 4, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds F1–F4 are available from the authors.

| Polymer | DH 22 °C (nm) | SD | DH 42 °C (nm) | SD | VPTT (°C) |
|---|---|---|---|---|---|
| F1 | 798.48 | 9.31 | 413.18 | 3.79 | 35 |
| F2 | 896.58 | 2.12 | 343.96 | 4.48 | • |
| F3 | 332.48 | 6.94 | 100.69 | 0.44 | 26 |
| F4 | 155.46 | 2.75 | 163.62 | 6.22 | • |
| Model | Parameter | Formulation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FP1 | FP2 | FP3 | FP4 | HPMC | |||||||
| Value | SD | Value | SD | Value | SD | Value | SD | Value | SD | ||
| ZO | KZO (% × min−1) | 5.18 × 10−2 | 3.76 × 10−3 | 5.08 × 10−2 | 4.94 × 10−4 | 5.42 × 10−2 | 8.34 × 10−3 | 5.64 × 10−2 | 4.69 × 10−3 | 5.86 × 10−2 | 1.86 × 10−3 |
| r2 | 0.99488 | 0.00377 | 0.99661 | 0.00030 | 0.99150 | 0.00251 | 0.99129 | 0.00014 | 0.99367 | 0.00099 | |
| FO | KFO (min−1) | 5.59 × 10−4 | 4.31 × 10−5 | 5.46 × 10−4 | 2.13 × 10−6 | 5.87 × 10−4 | 9.88 × 10−5 | 6.11 × 10−4 | 5.51 × 10−5 | 6.34 × 10−4 | 2.05 × 10−5 |
| r2 | 0.99543 | 0.00353 | 0.99697 | 0.00006 | 0.99261 | 0.00214 | 0.99248 | 0.00022 | 0.99471 | 0.00094 | |
| SO | KSO (min−1 × %−1) | 6.04 × 10−6 | 4.93 × 10−7 | 5.88 × 10−6 | 1.12 × 10−8 | 6.37 × 10−6 | 1.17 × 10−6 | 6.62 × 10−6 | 6.44 × 10−7 | 6.86 × 10−6 | 2.26 × 10−7 |
| r2 | 0.99591 | 0.00327 | 0.99727 | 0.00366 | 0.99363 | 0.00179 | 0.99358 | 0.00029 | 0.99566 | 0.00087 | |
| H | KH (min0.5) | 7.82 × 10−1 | 5.28 × 10−2 | 7.64 × 10−1 | 1.05 × 10−2 | 8.25 × 10−1 | 1.29 × 10−1 | 8.59 × 10−1 | 7.12 × 10−2 | 8.90 × 10−1 | 2.71 × 10−2 |
| r2 | 0.99540 | 0.00131 | 0.99367 | 0.00366 | 0.99940 | 0.00038 | 0.99977 | 0.00012 | 0.99954 | 0.00023 | |
| BF | SO | SO | H | H | H | ||||||
| Model | Parameter | Formulation | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| FP1 | FP2 | FP3 | FP4 | HPMC | |||||||
| Value | SD | Value | SD | Value | SD | Value | SD | Value | SD | ||
| ZO | KZO (% × min−1) | 7.59 × 10−2 | 1.47 × 10−3 | 7.46 × 10−2 | 6.83 × 10−5 | 6.81 × 10−2 | 4.87 × 10−3 | 8.21 × 10−2 | 1.12 × 10−2 | 5.93 × 10−2 | 3.08 × 10−3 |
| r2 | 0.99518 | 0.00166 | 0.99278 | 0.00252 | 0.99065 | 0.00080 | 0.98943 | 0.00210 | 0.98835 | 0.00110 | |
| FO | KFO (min−1) | 8.38 × 10−4 | 1.29 × 10−5 | 8.20 × 10−4 | 2.31 × 10−6 | 7.48 × 10−4 | 6.20 × 10−5 | 9.18 × 10−4 | 1.44 × 10−4 | 6.46 × 10−4 | 3.91 × 10−5 |
| r2 | 0.99629 | 0.00143 | 0.99396 | 0.00258 | 0.99215 | 0.00060 | 0.99138 | 0.00162 | 0.98984 | 0.00094 | |
| SO | KSO (min−1 × %−1) | 9.25 × 10−6 | 1.07 × 10−7 | 9.03 × 10−6 | 5.91 × 10−8 | 8.22 × 10−6 | 7.75 × 10−7 | 1.03 × 10−5 | 1.82 × 10−6 | 7.03 × 10−6 | 4.86 × 10−7 |
| r2 | 0.99725 | 0.00121 | 0.99500 | 0.00262 | 0.99352 | 0.00043 | 0.99314 | 0.00119 | 0.99123 | 0.00080 | |
| H | KH (min0.5) | 1.15 | 2.48 × 10−2 | 1.13 | 8.79 × 10−4 | 1.04 | 7.51 × 10−2 | 1.25 | 1.74 × 10−1 | 9.05 × 10−1 | 4.80 × 10−2 |
| r2 | 0.99846 | 0.00056 | 0.99763 | 0.00239 | 0.99987 | 0.00009 | 0.99983 | 0.00013 | 0.99984 | 0.00013 | |
| BF | H | H | H | H | H | ||||||
| Substrates (w/w) | Type of Component | |||||||
|---|---|---|---|---|---|---|---|---|
| NIPA | Cross-Linker | Co-Monomer | Cationic Initiator | Solvent Water | ||||
| MBA | PEG-DMA | PEG-MA | NTB | ABAP | ||||
| Type of polymer | F1 | 0.5 | 0.05 | - | - | - | 0.05 | 99.40 |
| F2 | 0.5 | 0.05 | - | 0.05 | - | 0.05 | 99.35 | |
| F3 | 0.5 | 0.05 | - | - | 0.05 | 0.05 | 99.35 | |
| F4 | 0.5 | - | 0.05 | - | - | 0.05 | 99.40 | |
| Composition% | Formulation | ||||
|---|---|---|---|---|---|
| FP1 | FP2 | FP3 | FP4 | REF | |
| NS | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| F1 | 0.5 | ||||
| F2 | 0.5 | ||||
| F3 | 0.5 | ||||
| F4 | 0.5 | ||||
| HPMC | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| H2O | 95.0 | 95.0 | 95.0 | 95.0 | 95.5 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gasztych, M.; Gola, A.; Kobryń, J.; Musiał, W. Synthesis and Formulation of Thermosensitive Drug Carrier for Temperature Triggered Delivery of Naproxen Sodium. Molecules 2016, 21, 1473. https://doi.org/10.3390/molecules21111473
Gasztych M, Gola A, Kobryń J, Musiał W. Synthesis and Formulation of Thermosensitive Drug Carrier for Temperature Triggered Delivery of Naproxen Sodium. Molecules. 2016; 21(11):1473. https://doi.org/10.3390/molecules21111473
Chicago/Turabian StyleGasztych, Monika, Agnieszka Gola, Justyna Kobryń, and Witold Musiał. 2016. "Synthesis and Formulation of Thermosensitive Drug Carrier for Temperature Triggered Delivery of Naproxen Sodium" Molecules 21, no. 11: 1473. https://doi.org/10.3390/molecules21111473