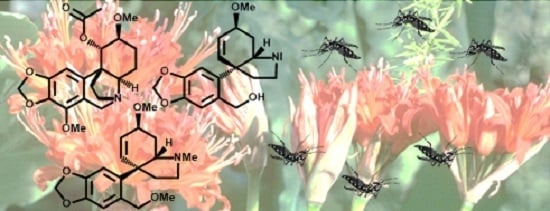
Alkaloids with Activity against the Zika Virus Vector Aedes aegypti (L.)—Crinsarnine and Sarniensinol, Two New Crinine and Mesembrine Type Alkaloids Isolated from the South African Plant Nerine sarniensis
Abstract
:1. Introduction
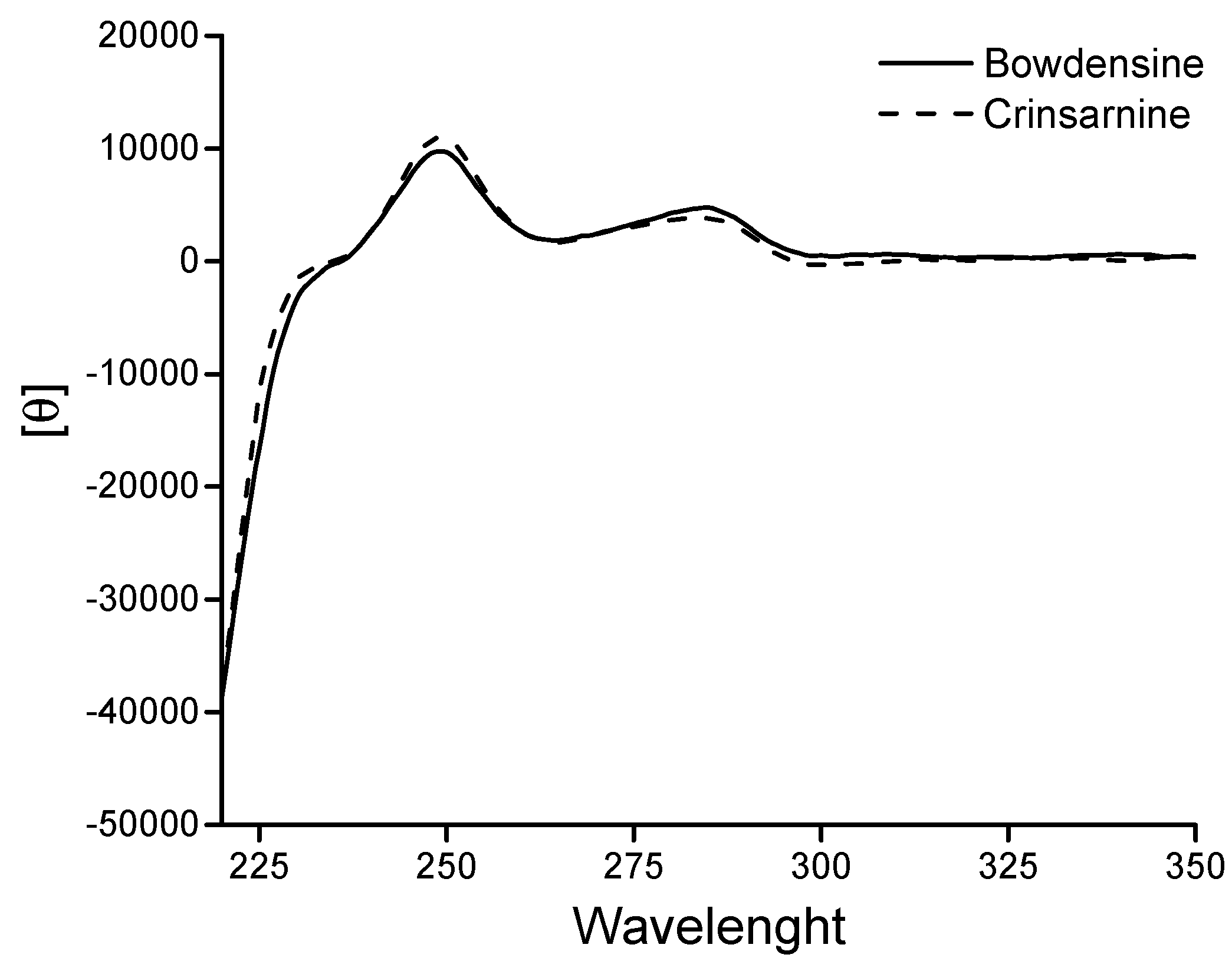
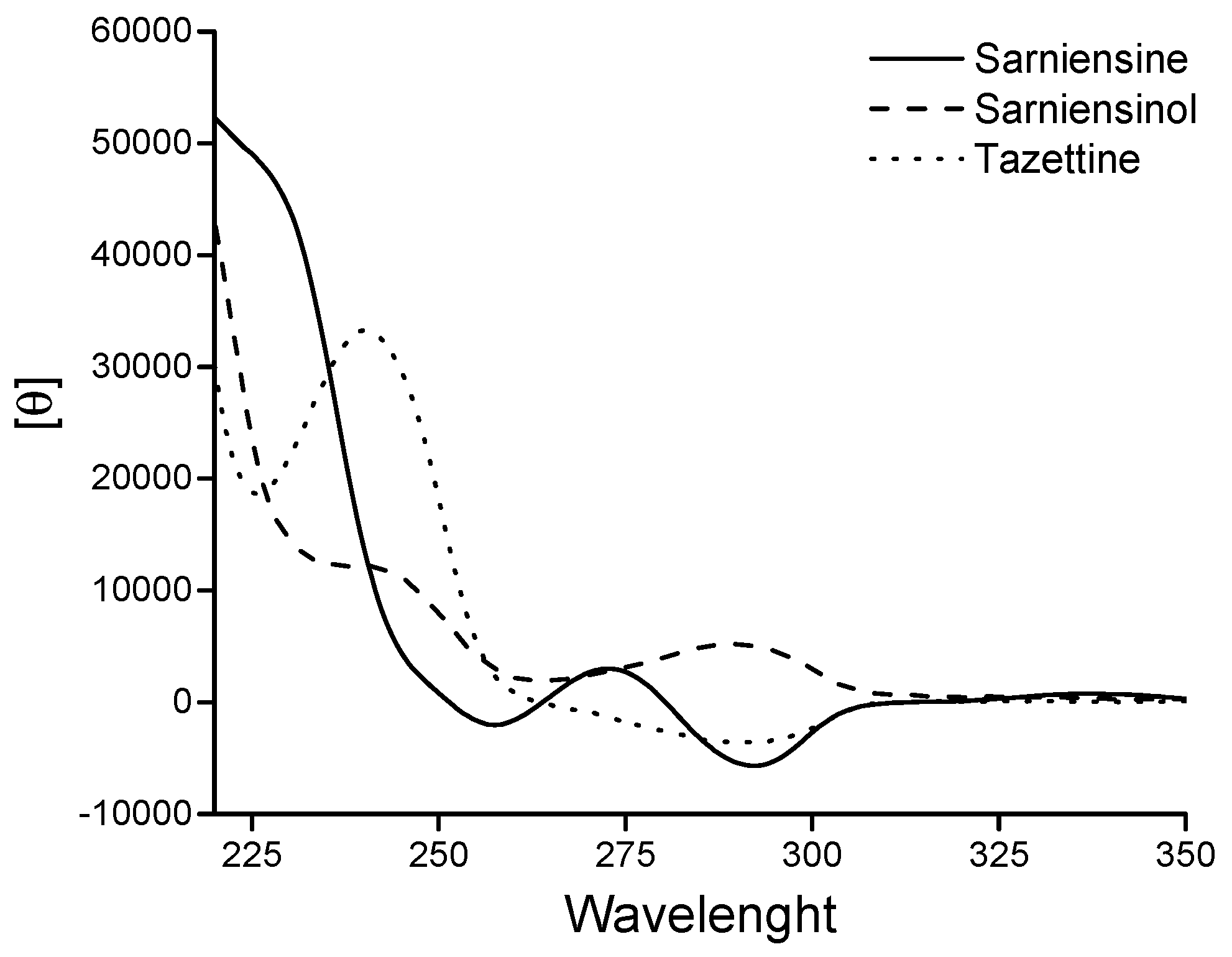
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Plant Material
3.3. Extraction and Purification of N. sarniensis Bulbs Extract
3.4. Characterization of Compounds
3.5. Mosquitoes and Bioassay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rasmussen, S.A.; Jamieson, D.J.; Honein, M.A.; Petersen, L.R. Zika virus and birth defects—Reviewing the evidence for causality. N. Engl. J. Med. 2016, 374, 1981–1987. [Google Scholar] [CrossRef] [PubMed]
- Orsborne, J.; DeRaedt Banks, S.; Hendy, A.; Gezan, S.A.; Kaur, H.; Wilder-Smith, A.; Lindsay, S.W.; Logan, J.G. Personal protection of permethrin-treated clothing against Aedes aegypti, the vector of dengue and zika virus, in the laboratory. PLoS ONE 2016, 11, e0152805. [Google Scholar] [CrossRef] [PubMed]
- De Azambuja García, G.; dos Santos, L.M.B.; Villela, D.A.M.V.; Maciel-de-Freitas, R. Using Wolbachia releases to estimate Aedes aegypti (Diptera: Culicidae) population size and survival. PLoS ONE 2016, 11, e0160196. [Google Scholar]
- Han, W.W.; Lazaro, A.; McCall, P.J.; Runge-Ranzinger, G.L.; Toledo, J.; Velayudhan, R.; Horstick, O. Efficacy and community effectiveness of larvivorous fish for dengue vector control. Trop. Med. Int. Health 2015, 20, 1239–1256. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G. Plant-borne ovicides in the fight against mosquito vectors of medical and veterinary importance: A systematic review. Parasitol. Res. 2015, 114, 3201–3212. [Google Scholar] [CrossRef] [PubMed]
- Kornienko, A.; Evidente, E. Chemistry, biology and medicinal potential of narciclasine and its congeners. Chem. Rev. 2008, 108, 1982–2014. [Google Scholar] [CrossRef] [PubMed]
- Evidente, A.; Kornienko, A. Anticancer evaluation of structurally diverse Amaryllidaceae alkaloids and their synthetic derivatives. Phytochem. Rev. 2009, 8, 449–459. [Google Scholar] [CrossRef]
- Nair, J.J.; Bastida, J.; Codina, C.; Viladomat, F.; van Staden, J. Alkaloids of South Africa Amaryllidaceae: A review. Natl. Prod. Commun. 2013, 8, 1335–1350. [Google Scholar]
- Nair, J.J.; van Staden, J. Pharmacological and toxicological insight to the South African Amaryllidaceae. Food. Chem. Toxicol. 2013, 62, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Bastida, J.; Viladomat, F.; Codina, C. Narcissus alkaloids. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 1998; pp. 323–405. [Google Scholar]
- Masi, M.; Frolova, L.V.; Yu, X.; Mathieu, V.; Cimmino, A.; De Carvalho, A.; Kiss, R.; Rogelj, S.; Pertsemlidis, A.; Kornienko, A.; et al. Jonquailine, a new pretazettine-type alkaloid isolated from Narcissus jonquilla quail, with activity against drug-resistant cancer. Fitoterapia 2015, 102, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.J.; Ren, Y.; Howes, M.J. Acetylcholinesterase inhibitors from plants and fungi. Natl. Prod. Rep. 2006, 23, 181–199. [Google Scholar] [CrossRef] [PubMed]
- Meerow, A.W.; Snijman, D.A. Amaryllidaceae. In Flowering Plants Monocotyledons; Kubiski, K., Ed.; Springer: Berlin, Germany, 1998; pp. 83–110. [Google Scholar]
- Boit, H.G. Alkaloids of the Amaryllidaceae. VI. The alkaloids of Nerine sarniensis, Crinum moorei, Hippeastrum vittatum and Clivia miniata. Chem. Ber. 1954, 87, 1704–1707. [Google Scholar] [CrossRef]
- Masi, M.; van der Westhuyzen, A.E.; Tabanca, N.; Evidente, M.; Cimmino, A.; Green, I.R.; Bernier, U.R.; Becnel, J.J.; Bloomquist, J.R.; van Otterlo, W.A.L.; et al. Sarniensine, a mesembrine-type alkaloid isolated from Nerine sarniensis an indigenous South African Amaryllidaceae with larvicidal and adulticidal activities against Aedes aegypti. Fitoterapia 2016. submitted. [Google Scholar]
- Lyle, R.E.; Kielar, E.A.; Crowder, J.R.; Wildman, W.C. The alkaloids of Nerine bowdenii W. Wats. and Crinum moorei J.D. Hook. J. Am. Chem. Soc. 1959, 82, 2620–2625. [Google Scholar] [CrossRef]
- Kobayashi, S.; Tokumoto, T.; Kihara, M.; Imakura, Y.; Shingu, T.; Taira, Z. Alkaloidal constituents of Crinum latifolium and Crinum bulbispermum (Amaryllidaceae). Chem. Pharm. Bull. 1984, 8, 3015–3022. [Google Scholar] [CrossRef]
- Ghosal, S.; Rao, P.H.; Jaiswal, D.K.; Kumar, Y.; Frahm, A.W. Alkaloids of Crinum pratense. Phytochemistry 1981, 20, 2003–2007. [Google Scholar] [CrossRef]
- Lamoral-Theys, D.; Andolfi, A.; Van Goietsenoven, G.; Cimmino, A.; Le Calvé, B.; Wauthoz, N.; Mégalizzi, V.; Gras, T.; Bruyère, C.; Dubois, J.; et al. Lycorine, the main phenanthridine Amaryllidaceae alkaloid, exhibits significant antitumor activity in cancer cells that display resistance to proapoptotic stimuli: An investigation of structure—Activity relationship and mechanistic insight. J. Med. Chem. 2009, 52, 6244–6256. [Google Scholar] [CrossRef] [PubMed]
- Sternhell, S. Correlation of interproton spin-spin coupling constants with structure. Quart. Rev. 1969, 23, 236–270. [Google Scholar] [CrossRef]
- Pretsch, E.; Bühlmann, P.; Affolter, C. Structure Determination of Organic Compounds—Tables of Spectral Data; Springer: Berlin, Germany, 2000; pp. 161–243. [Google Scholar]
- Berger, S.; Braun, S. 200 and More Basic NMR Experiments: A Practical Course, 1st ed.; Wiley-VCH: Weinheim, Germany, 2004. [Google Scholar]
- Nakanishi, K.; Solomon, P.H. Infrared Absorption Spectroscopy, 2nd ed.; Holden Day: Oakland, CA, USA, 1977; pp. 17–44. [Google Scholar]
- Breitmaier, E.; Voelter, W. Carbon-13 NMR Spectroscopy; VCH: Weinheim, Germany, 1987; pp. 183–280. [Google Scholar]
- Wagner, J.; Pham, H.L.; Döpke, W. Alkaloids from Hippeastrum equestre Herb.—5 Circular dichroism studies. Tetrahedron 1996, 19, 6591–6600. [Google Scholar] [CrossRef]
- Pham, L.H.; Gründemann, E.; Wagner, J.; Bartoszek, M.; Döpke, W. Two novel Amaryllidaceae alkaloids from Hippeastrum equestres Herb.: 3-O-demetyltazettine and egonine. Phytochemistry 1999, 51, 327–332. [Google Scholar] [CrossRef]
- Al-Massarani, S.; Al-Enzi, F.; Al-Tamimi, M.; Al-Jomaiah, N.; Al-amri, R.; Baser, K.H.C.; Tabanca, N.; Estep, A.S.; Becnel, J.J.; Bloomquist, J.R.; et al. Composition & biological activities of Cyperus rotundus L. tuber volatiles from Saudi Arabia. Natl. Volatiles Essent. Oils 2016, 3, 26–32. [Google Scholar]
- Mann, R.S.; Kaufmann, P.E. Natural product pesticides: Their development, delivery and use against insect vectors. Mini-Rev. Org. Chem. 2012, 9, 185–202. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–8 are available from the authors.

| No. | 1 | 3 | ||||
|---|---|---|---|---|---|---|
| 13C c | 1H (J in Hz) | HMBC | 13C c | 1H (J in Hz) | HMBC | |
| 1 | 76.8 | 5.14 (1H) d (3.7) | H-11 | 74.1 | 5.32 (1H) d (4.4) | H-2, H-3A, H-11A, COOCH3 |
| 2 | 75.8 | 3.96 (1H) br s d | OMe (2) | 68.3 | 5.57 (1H) br s | H-1, H-3B, COOCH3 |
| 3 | 24.4 | 2.09 (1H) br d (13.6) 1.33 (1H) br t (13.6) | H-4B, H-4a | 26.4 | 1.94 (1H) br d (14.4) 1.57 (1H) br dd (14.4, 11.4) | H-4B |
| 4 | 20.4 | 1.66 (1H) td (13.6, 4.8) 1.57 (1H) m | H-3B, H-4a | 21.2 | 2.06 (1H) td (11.4, 5.5) 1.72 (1H) m | H2-3, H-4a |
| 4a | 68.4 | 3.00 (1H) dd (13.6, 4.8) | H-3B, H-4B, H-6B, H-11B, H-12A | 68.2 | 3.03 (1H) dd (5.5, 11.4) | H-3B, H2-4, H-6B, H-11B, H-12A |
| 6 | 58.3 | 4.19 (1H) d (17.3) 3.77 (1H) d (17.3) | 4a, H2-12 | 58.4 | 4.18 (1H) d (17.5) 3.77 (1H) d (17.5) | H-4a, H-10, H-12A, H-12B |
| 6a | 116.7 | H2-6, H-10 | 117.0 | H-6A, H-6B, H-10 | ||
| 7 | 148.1 | H2-6, H-10, OCH2O | 148.1 | H-10 | ||
| 8 | 141.3 | H-1, H-4a, H2-6, H2-11 | 141.0 | H2-6 | ||
| 9 | 140.3 | H2-6, H-10, OMe (7) | 140.3 | H-10, OMe | ||
| 10 | 97.5 | 6.24 (1H) s | 97.4 | 6.18 (1H) s | ||
| 10a | 133.4 | H2-6, H-10, OCH2O | 133.5 | H2-6, H-10, OCH2O | ||
| 10b | 124.5 | 129.7 | ||||
| 11 | 37.3 | 2.03 (1H) ddd (16.0, 9.2, 4.3) 2.92 (1H) ddd (16.0, 11.6, 6.3) | H-1, H-4a,H-6A, H-12 | 37.3 | 2.74 (1H) ddd (13.0, 11.0, 5.5) 2.03 (1H) ddd (13.0, 9.0, 6.0) | H-1, H-4a, H-6A, H-12A |
| 12 | 52.3 | 2.79 (1H) ddd (14.6, 11.6, 4.3) 3.39 (1H) ddd (14.6, 9.2, 6.3) | H-4a, H2-6, H-11A | 52.3 | 3.42 (1H) ddd (12.4, 9.0, 5.5) 2.82 (1H) ddd (12.4, 11.0, 6.0) | H-4a, H2-6, H-11A |
| OCH2O | 100.5 | 5.83 (1H) d (1.3) 5.84 (1H) d (1.3) | 100.5 | 5.84 (1H) d (1.2) 5.83 (1H) d (1.2) | ||
| OMe (7) | 59.1 | 3.96 (3H) s d | H2-6 | 59.1 | 3.97 (3H) s | H2-6 |
| OMe (2) | 57.7 | 3.31 (3H) s | ||||
| COOCH3 (1) | 21.6 | 2.22 (3H) s | 21.3 d | 2.10 (3H) s d | H-1, H-2 | |
| COOCH3 (2) | 21.3 d | 2.10 (3H) s d | H-1, H-2 | |||
| COOCH3 (1) | 170.9 | H-1, COOCH3 | 170.1 | H-1, H-2, COOCH3 (1) | ||
| COOCH3 (2) | 170.4 | H-2, COOCH3 (2) | ||||
| No. | 13C c | 1H (J in Hz) | HMBC |
|---|---|---|---|
| 2 | 55.4 t | 3.21 (1H) ddd (9.4, 9.1, 8.5) 2.50 (1H) ddd (9.4, 18.5, 5.0) | H2-3, N-Me |
| 3 | 39.7 t | 2.35 (1H) ddd (13.5, 8.5, 5.0) 2.14 (1H) ddd (13.5, 9.1, 18.5) | H2-2, N-Me |
| 3a | 50.3 s | H2-2, H2-3, H-4, H-5, H-6 | |
| 4 | 137.1 d | 5.83 (1H) br s d | H2-3 |
| 5 | 124.4 d | 5.83 (1H) br s d | H-6, H2-7 |
| 6 | 72.2 d | 3.89 (1H) dd (5.7, 10.3) | H-5, H2-7, OMe |
| 7 | 26.6 t | 2.20 (1H) dd (11.3, 5.7) 1.56 (1H) dd (10.3, 11.3) | H-5, H-6 |
| 7a | 70.8 d | 2.67 (1H) br s | H2-2, H2-7, N-Me |
| 1′ | 129.7 s | H2-3, H-6′, CH2OH | |
| 2′ | 132.5 s | H-3′, CH2OH | |
| 3′ | 111.1 d | 6.93 (1H) s | CH2OH |
| 4′ | 146.2 s | H-3′, H-6′, OCH2O | |
| 5′ | 147.0 s | H-3′, H-6′, OCH2O | |
| 6′ | 109.1 d | 6.95 (1H) s | H-3′ |
| OCH2O | 101.2 t | 5.93 (2H) br s | |
| CH2OH | 63.0 q | 4.77 (1H) d (12.0) 4.69 (1H) d (12.0) | H-3′ |
| OMe | 55.8 q | 3.39 (3H) s | H-6 |
| N-Me | 40.4 q | 2.39 (3H) s | H2-2 |
| OH | 3.65, br s |
| Compounds | Percent Mortality | |||
|---|---|---|---|---|
| 1 μg/μL | 0.5 μg/μL | 0.25 μg/μL | 0.1 μg/μL | |
| Crinsarnine (1) | 0 | 0 | 0 | 0 |
| Sarniensinol (2) | 7 ± 11 | 0 | 0 | 0 |
| Bowdensine (3) | 13 ± 11 | 0 | 0 | 0 |
| Hippadine (5) | 7 ± 11 | 0 | 0 | 0 |
| 1-O-Acetyl-lycorine (6) | 0 | 0 | 0 | 0 |
| Sarniensine (4) A | 100 ± 0 | 80 ± 0 | 60 ± 0 | 20 ± 0 |
| 3-Epimacronine (7) A | 60 ± 0 | 40 ± 0 | 20 ± 0 | 0 ± 0 |
| Tazettine (8) A | 20 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Compound | Mortality (%) 5 μg/mosquito | LD50 ** ± SE (μg/mosquito) | 95% CI | R2 |
|---|---|---|---|---|
| Crinsarnine (1) | 97 ± 6 | 2.29 ± 0.049 | (2.41–2.17) | 0.9735 |
| Sarniensinol (2) | 33 ± 6 | |||
| Bowdensine (3) | 33 ± 6 | |||
| Hippadine (5) | 33 ± 6 | |||
| 1-O-Acetyl-lycorine (6) | 23 ± 6 | |||
| Sarniensine (4) A | 93 ± 6 | |||
| 3-Epimacronine (7) A | 67 ± 6 | |||
| Tazettine (8) A | 23 ± 6 | |||
| Untreated | 0 | |||
| Solvent Control (acetone) | 0 | |||
| 0.15 ng Permethrin | 37 ± 6 | |||
| 0.23 ng Permethrin | 63 ± 5 | |||
| 2.37 ng Permethrin | 100 ± 0 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masi, M.; Cala, A.; Tabanca, N.; Cimmino, A.; Green, I.R.; Bloomquist, J.R.; Van Otterlo, W.A.L.; Macias, F.A.; Evidente, A. Alkaloids with Activity against the Zika Virus Vector Aedes aegypti (L.)—Crinsarnine and Sarniensinol, Two New Crinine and Mesembrine Type Alkaloids Isolated from the South African Plant Nerine sarniensis. Molecules 2016, 21, 1432. https://doi.org/10.3390/molecules21111432
Masi M, Cala A, Tabanca N, Cimmino A, Green IR, Bloomquist JR, Van Otterlo WAL, Macias FA, Evidente A. Alkaloids with Activity against the Zika Virus Vector Aedes aegypti (L.)—Crinsarnine and Sarniensinol, Two New Crinine and Mesembrine Type Alkaloids Isolated from the South African Plant Nerine sarniensis. Molecules. 2016; 21(11):1432. https://doi.org/10.3390/molecules21111432
Chicago/Turabian StyleMasi, Marco, Antonio Cala, Nurhayat Tabanca, Alessio Cimmino, Ivan R. Green, Jeffrey R. Bloomquist, Willem A. L. Van Otterlo, Francisco A. Macias, and Antonio Evidente. 2016. "Alkaloids with Activity against the Zika Virus Vector Aedes aegypti (L.)—Crinsarnine and Sarniensinol, Two New Crinine and Mesembrine Type Alkaloids Isolated from the South African Plant Nerine sarniensis" Molecules 21, no. 11: 1432. https://doi.org/10.3390/molecules21111432