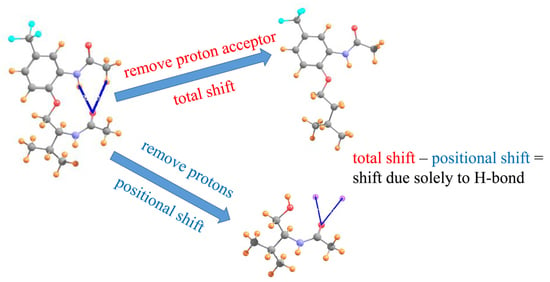
Assessment of the Presence and Strength of H-Bonds by Means of Corrected NMR
Abstract
:1. Introduction
2. Computational Methods
3. Results
3.1. Interamide HBs
3.2. Dipeptide Conformation
3.3. β-Sheet
3.4. Aromatic Amino Acids
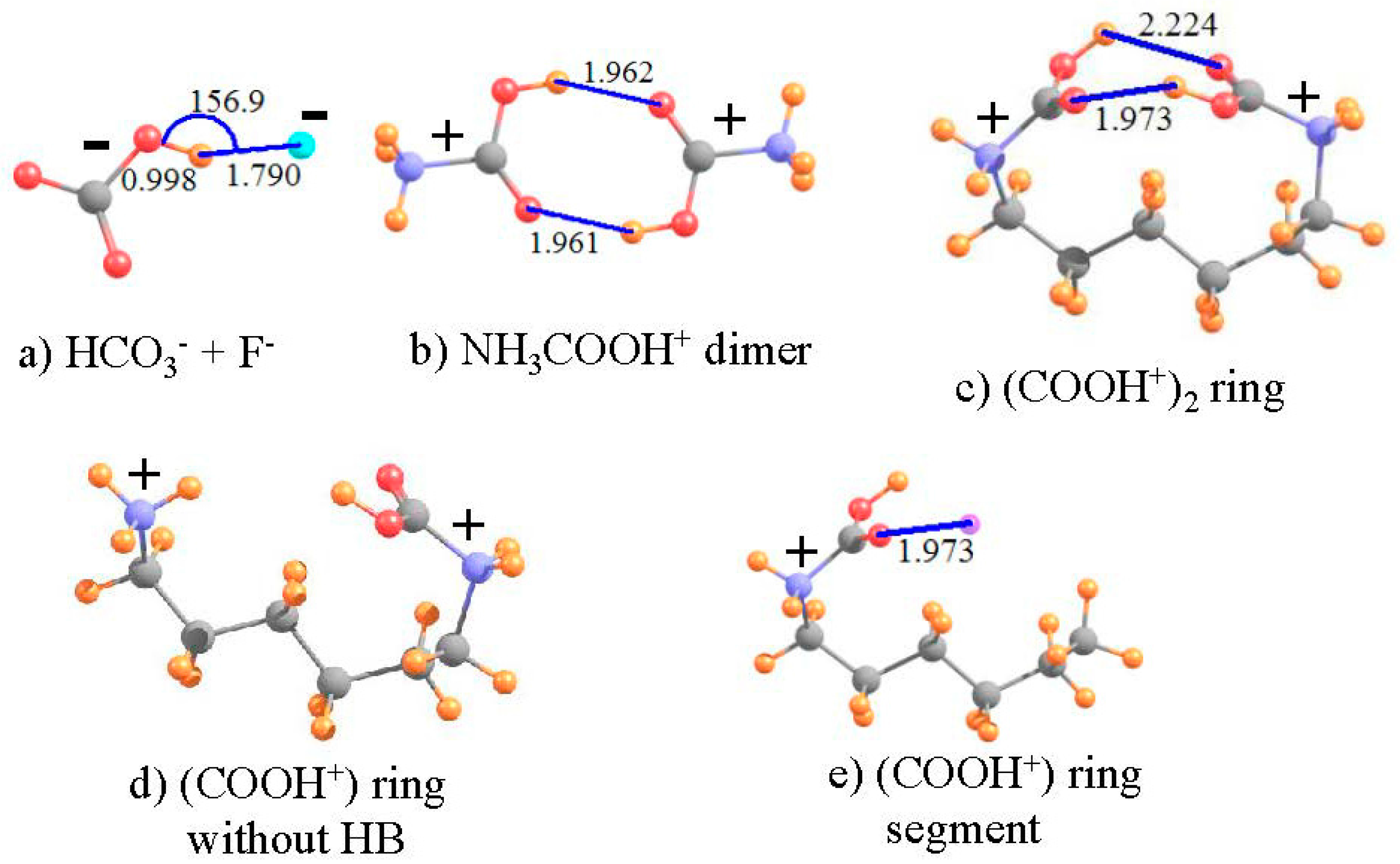
3.5. Anti-Electrostatic HBs
4. Summary and Discussion
Conflicts of Interest
References
- Gilli, G.; Gilli, P. The Nature of the Hydrogen Bond; Oxford University Press: Oxford, UK, 2009; p. 313. [Google Scholar]
- Grabowski, S.J. Hydrogen Bonding—New Insights; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Scheiner, S. Hydrogen Bonding: A Theoretical Perspective; Oxford University Press: New York, NY, USA, 1997; p. 375. [Google Scholar]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Definition of the Hydrogen Bond. Pure Appl. Chem. 2011, 83, 1637–1641. [Google Scholar] [CrossRef]
- Udagawa, T.; Tachikawa, M. H/D Isotope Effect on Charge-Inverted Hydrogen-Bonded Systems: Systematic Classification of Three Different Types in H3XH…YH3 (X = C, Si, or Ge, and Y = B, Al, or Ga) with Multicomponent Calculation. J. Comput. Chem. 2015, 36, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Kozelka, J. Agostic and Hydrogen-Bonding X–H···M Interactions Involving a d8 Metal Center: Recent Advances towards Their Understanding. In Noncovalent Forces; Scheiner, S., Ed.; Springer: Dordrecht, The Netherlands, 2015; Volume 19, pp. 129–158. [Google Scholar]
- Weinhold, F.; Klein, R.A. What Is a Hydrogen Bond? Mutually Consistent Theoretical and Experimental Criteria for Characterizing H-Bonding Interactions. Mol. Phys. 2012, 110, 565–579. [Google Scholar] [CrossRef]
- Li, Q.-Z.; Li, H.-B. Hydrogen Bonds Involving Radical Species. In Noncovalent Forces; Scheiner, S., Ed.; Springer: Dordrecht, The Netherlands, 2015; Volume 19, pp. 107–128. [Google Scholar]
- Cybulski, S.; Scheiner, S. Hydrogen Bonding and Proton Transfers Involving Triply Bonded Atoms Acetylene and Hydrocyanic Acid. J. Am. Chem. Soc. 1987, 109, 4199–4206. [Google Scholar] [CrossRef]
- Biswal, H.S. Hydrogen Bonds Involving Sulfur: New Insights from Ab Initio Calculations and Gas Phase Laser Spectroscopy. In Noncovalent Forces; Scheiner, S., Ed.; Springer: Dordrecht, The Netherlands, 2015; Volume 19, pp. 15–45. [Google Scholar]
- Kryachko, E.; Scheiner, S. CH··F Hydrogen Bonds. Dimers of Fluoromethanes. J. Phys. Chem. A 2004, 108, 2527–2535. [Google Scholar] [CrossRef]
- Domagala, M.; Grabowski, S.J. Hydrocarbons as Proton Donors in C–H···N and C–H···S Hydrogen Bonds. Chem. Phys. 2010, 367, 1–6. [Google Scholar] [CrossRef]
- Gu, Y.; Kar, T.; Scheiner, S. Comparison of the CH···N and CH···O Interactions Involving Substituted Alkanes. J. Mol. Struct. 2000, 552, 17–31. [Google Scholar] [CrossRef]
- Møller, K.H.; Hansen, A.S.; Kjaergaard, H.G. Gas Phase Detection of the NH–P Hydrogen Bond and Importance of Secondary Interactions. J. Phys. Chem. A 2015, 119, 10988–10998. [Google Scholar] [CrossRef] [PubMed]
- Latajka, Z.; Scheiner, S. Structure, Energetics and Vibrational Spectrum of H2O-HCl. J. Chem. Phys. 1987, 87, 5928–5936. [Google Scholar] [CrossRef]
- Mundlapati, V.R.; Ghosh, S.; Bhattacherjee, A.; Tiwari, P.; Biswal, H.S. Critical Assessment of the Strength of Hydrogen Bonds between the Sulfur Atom of Methionine/Cysteine and Backbone Amides in Proteins. J. Phys. Chem. Lett. 2015, 6, 1385–1389. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S.; Kar, T. Effect of Solvent Upon CH··O Hydrogen Bonds with Implications for Protein Folding. J. Phys. Chem. B 2005, 109, 3681–3689. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J.; Lipkowski, P. Characteristics of X-H···π Interactions: Ab Initio and QTAIM Studies. J. Phys. Chem. A 2011, 115, 4765–4773. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J.; Sokalski, W.A.; Leszczynski, J. Can H...σ, π...H+...σ and σ...H+...σ Interactions Be Classified as H-Bonded? Chem. Phys. Lett. 2006, 432, 33–39. [Google Scholar] [CrossRef]
- Schuster, P.; Zundel, G.; Sandorfy, C. The Hydrogen Bond. Recent Developments in Theory and Experiments; North-Holland Publishing Co.: Amsterdam, The Netherlands, 1976. [Google Scholar]
- Hadzi, D.; Bratos, S. Vibrational Spectroscopy of the Hydrogen Bond. In The Hydrogen Bond. Recent Developments in Theory and Experiments; Schuster, P., Zundel, G., Sandorfy, C., Eds.; North-Holland Publishing Co.: Amsterdam, The Netherlands, 1976; Volume 2, pp. 565–611. [Google Scholar]
- Scheiner, S. Dissection of the Factors Affecting Formation of a CH∙∙∙O H-Bond. A Case Study. Crystals 2015, 5, 327–345. [Google Scholar] [CrossRef]
- Verma, K.; Dave, K.; Viswanathan, K.S. Hydrogen-Bonded Complexes of Phenylacetylene–Acetylene: Who Is the Proton Donor? J. Phys. Chem. A 2015, 119, 12656–12664. [Google Scholar] [CrossRef] [PubMed]
- Cormanich, R.A.; Keddie, N.S.; Rittner, R.; O’Hagan, D.; Buhl, M. Particularly Strong C–H···π Interactions between Benzene and All-Cis 1,2,3,4,5,6-Hexafluorocyclohexane. Phys. Chem. Chem. Phys. 2015, 17, 29475–29478. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.S.; Hasan, M.; Burt, M.; Marta, R.A.; Fillion, E.; McMahon, T.B. Persistent Intramolecular C–H···X (X = O or S) Hydrogen-Bonding in Benzyl Meldrum’s Acid Derivatives. J. Phys. Chem. A 2014, 118, 3795–3803. [Google Scholar] [CrossRef] [PubMed]
- Gopi, R.; Ramanathan, N.; Sundararajan, K. Blue-Shift of the C-H Stretching Vibration in CHF3-H2O Complex: Matrix Isolation Infrared Spectroscopy and Ab Initio Computations. Chem. Phys. 2016, 476, 36–45. [Google Scholar] [CrossRef]
- Sandoval-Lira, J.; Fuentes, L.; Quintero, L.; Höpfl, H.; Hernández-Pérez, J.M.; Terán, J.L.; Sartillo-Piscil, F. The Stabilizing Role of the Intramolecular C–H···O Hydrogen Bond in Cyclic Amides Derived from Α-Methylbenzylamine. J. Org. Chem. 2015, 80, 4481–4490. [Google Scholar] [CrossRef] [PubMed]
- Gopi, R.; Ramanathan, N.; Sundararajan, K. Experimental Evidence for Blue-Shifted Hydrogen Bonding in the Fluoroform–Hydrogen Chloride Complex: A Matrix-Isolation Infrared and Ab Initio Study. J. Phys. Chem. A 2014, 118, 5529–5539. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Kar, T.; Scheiner, S. Fundamental Properties of the CH···O Interaction: Is It a True Hydrogen Bond? J. Am. Chem. Soc. 1999, 121, 9411–9422. [Google Scholar] [CrossRef]
- Scheiner, S.; Kar, T. Red Versus Blue-Shifting Hydrogen Bonds: Are There Fundamental Distinctions? J. Phys. Chem. A 2002, 106, 1784–1789. [Google Scholar] [CrossRef]
- Gu, Y.; Kar, T.; Scheiner, S. Evaluation of the H-Bonding Properties of CH···O Interactions Based Upon NMR Spectra. J. Mol. Struct. Theochem 2000, 500, 441–452. [Google Scholar]
- Reed, A.E.; Weinhold, F. Natural Bond Orbital Analysis of near Hartree-Fock Water Dimer. J. Chem. Phys. 1983, 78, 4066–4073. [Google Scholar] [CrossRef]
- Reed, A.E.; Weinhold, F.; Curtiss, L.A.; Pochatko, D.J. Natural Bond Orbital Analysis of Molecular Interactions: Theoretical Studies of Binary Complexes of HF, H2O, NH3, N2, O2, F2, CO and CO2 with HF, H2O, and NH3. J. Chem. Phys. 1986, 84, 5687–5705. [Google Scholar] [CrossRef]
- Bader, R.F.W. Atoms in Molecules, A Quantum Theory; Clarendon Press: Oxford, UK, 1990; Volume 22, p. 438. [Google Scholar]
- Carroll, M.T.; Bader, R.F.W. An Analysis of the Hydrogen Bond in Base-HF Complexes Using the Theory of Atoms in Molecules. Mol. Phys. 1988, 65, 695–722. [Google Scholar] [CrossRef]
- Carroll, M.T.; Chang, C.; Bader, M.F.W. Prediction of the Structures of Hydrogen-Bonded Complexes Using the Laplacian of the Charge Density. Mol. Phys. 1988, 63, 387–405. [Google Scholar] [CrossRef]
- Rusinska-Roszak, D.; Sowinski, G. Estimation of the Intramolecular O–H···O–C Hydrogen Bond Energy via the Molecular Tailoring Approach. Part I: Aliphatic Structures. J. Chem. Inf. Model. 2014, 54, 1963–1977. [Google Scholar] [CrossRef] [PubMed]
- Rusinska-Roszak, D. Intramolecular O–H···O–C Hydrogen Bond Energy Via the Molecular Tailoring Approach to Rahb Structures. J. Phys. Chem. A 2015, 119, 3674–3687. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Sanz, G.; Trujillo, C.; Alkorta, I.; Elguero, J. Intramolecular Pnicogen Interactions in Phosphorus and Arsenic Analogues of Proton Sponges. Phys. Chem. Chem. Phys. 2014, 16, 15900–15909. [Google Scholar] [CrossRef] [PubMed]
- Jabłoński, M. Full vs. Constrain Geometry Optimization in the Open–Closed Method in Estimating the Energy of Intramolecular Charge-Inverted Hydrogen Bonds. Chem. Phys. 2010, 376, 76–83. [Google Scholar] [CrossRef]
- Woodford, J.N. Density Functional Theory and Atoms-in-Molecules Investigation of Intramolecular Hydrogen Bonding in Derivatives of Malonaldehyde and Implications for Resonance-Assisted Hydrogen Bonding. J. Phys. Chem. A 2007, 111, 8519–8530. [Google Scholar] [CrossRef] [PubMed]
- Rozas, I.; Alkorta, I.; Elguero, J. Intramolecular Hydrogen Bonds in Ortho-Substituted Hydroxybenzenes and in 8-Susbtituted 1-Hydroxynaphthalenes: Can a Methyl Group Be an Acceptor of Hydrogen Bonds? J. Phys. Chem. A 2001, 105, 10462–10467. [Google Scholar] [CrossRef]
- Jabłoński, M.; Monaco, G. Different Zeroes of Interaction Energies As the Cause of Opposite Results on the Stabilizing Nature of C–H···O Intramolecular Interactions. J. Chem. Inf. Model. 2013, 53, 1661–1675. [Google Scholar] [CrossRef] [PubMed]
- Jabłoński, M.; Kaczmarek, A.; Sadlej, A.J. Estimates of the Energy of Intramolecular Hydrogen Bonds. J. Phys. Chem. A 2006, 110, 10890–10898. [Google Scholar] [CrossRef] [PubMed]
- Fliegl, H.; Lehtonen, O.; Sundholm, D.; Kaila, V.R.I. Hydrogen-Bond Strengths by Magnetically Induced Currents. Phys. Chem. Chem. Phys. 2011, 13, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Weinhold, F.; Schleyer, P.v.R.; McKee, W.C. Bay-Type H···H “Bonding” in cis-2-Butene and Related Species: QTAIM Versus NBO Description. J. Comput. Chem. 2014, 35, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Alkorta, I.; Sanchez-Sanz, G.; Elguero, J. Pnicogen Bonds between X=PH3 (X = O, S, NH, CH2) and Phosphorus and Nitrogen Bases. J. Phys. Chem. A 2014, 118, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Monaco, M.; Palusiak, M. The Halogen···Oxygen Interaction in 3-Halogenopropenal Revisited—The Dimer Model vs. QTAIM Indications. Chem. Phys. 2013, 415, 207–213. [Google Scholar]
- Scheiner, S. Interpretation of Spectroscopic Markers of Hydrogen Bonds. ChemPhysChem 2016, 17, 2263–2271. [Google Scholar] [CrossRef] [PubMed]
- Jabłoński, M.; Solà, M. Influence of Confinement on Hydrogen Bond Energy. The Case of the FH···NCH Dimer. J. Phys. Chem. A 2010, 114, 10253–10260. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; Oxford University Press: New York, NY, USA, 1999; p. 507. [Google Scholar]
- Jabłoński, M.; Sadlej, A.J. Blue-Shifting Intramolecular C−H···O Interactions. J. Phys. Chem. A 2007, 111, 3423–3431. [Google Scholar] [CrossRef] [PubMed]
- Gould, I.R.; Cornell, W.D.; Hillier, I.H. A Quantum Mechanical Investigation of the Conformational Energetics of the Alanine and Glycine Dipeptides in the Gas Phase and in Aqueous Solution. J. Am. Chem. Soc. 1994, 116, 9250–9256. [Google Scholar] [CrossRef]
- Stern, H.A.; Kaminski, G.A.; Banks, J.L.; Zhou, R.; Berne, B.J.; Friesner, R.A. Fluctuating Charge, Polarizable Dipole, and Combined Models: Parameterization from Ab Initio Quantum Chemistry. J. Phys. Chem. B 1999, 103, 4730–4737. [Google Scholar] [CrossRef]
- Vargas, R.; Garza, J.; Hay, B.P.; Dixon, D.A. Conformational Study of the Alanine Dipeptide at the MP2 and DFT Levels. J. Phys. Chem. A 2002, 106, 3213–3218. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Duan, Y. Solvation Effects on Alanine Dipeptide: A MP2/Cc-Pvtz//MP2/6–31g** Study of (F,Y) Energy Maps and Conformers in the Gas Phase, Ether, and Water. J. Comput. Chem. 2004, 25, 1699–1716. [Google Scholar] [CrossRef] [PubMed]
- Scarsdale, J.N.; Alsenoy, C.V.; Klimkowski, V.J.; Schaefer, L.; Momany, F.A. Ab Initio Studies of Molecular Geometries. 27. Optimized Molecular Structures and Conformational Analysis of N.Alpha.-Acetyl-N-Methylalaninamide and Comparison with Peptide Crystal Data and Empirical Calculations. J. Am. Chem. Soc. 1983, 105, 3438–3445. [Google Scholar] [CrossRef]
- Head-Gordon, T.; Head-Gordon, M.; Frisch, M.J.; Brooks, C.L.; Pople, J.A. Theoretical Study of Blocked Glycine and Alanine Peptide Analogs. J. Am. Chem. Soc. 1991, 113, 5989–5997. [Google Scholar] [CrossRef]
- Frey, R.F.; Coffin, J.; Newton, S.Q.; Ramek, M.; Cheng, V.K.W.; Momany, F.A.; Schaefer, L. Importance of Correlation-Gradient Geometry Optimization for Molecular Conformational Analyses. J. Am. Chem. Soc. 1992, 114, 5369–5377. [Google Scholar] [CrossRef]
- Beachy, M.D.; Chasman, D.; Murphy, R.B.; Halgren, T.A.; Friesner, R.A. Accurate Ab Initio Quantum Chemical Determination of the Relative Energetics of Peptide Conformations and Assessment of Empirical Force Fields. J. Am. Chem. Soc. 1997, 119, 5908–5920. [Google Scholar] [CrossRef]
- Scheiner, S. Contributions of NH··O and CH··O H-Bonds to the Stability of β-Sheets in Proteins. J. Phys. Chem. B 2006, 110, 18670–18679. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathi, R.; Raman, S.S.; Subramanian, V.; Ramasami, T. Bader’s Electron Density Analysis of Hydrogen Bonding in Secondary Structural Elements of Proteins. J. Phys. Chem. A 2007, 111, 7141–7148. [Google Scholar] [CrossRef] [PubMed]
- Vener, M.V.; Egorova, A.N.; Fomin, D.P.; Tsirelson, V.G. QTAIM Study of the Closed-Shell Interactions in Peptide Secondary Structures: A Cluster Treatment of Oligo- and Polyalanines. Chem. Phys. Lett. 2007, 440, 279–285. [Google Scholar] [CrossRef]
- Vener, M.V.; Egorova, A.N.; Fomin, D.P.; Tsirel'son, V.G. A Quantum-Topological Analysis of Noncovalent Interactions in Secondary Polyalanine Structures. Russ. J. Phys. Chem. B. 2009, 3, 541–547. [Google Scholar] [CrossRef]
- Hudson, K.L.; Bartlett, G.J.; Diehl, R.C.; Agirre, J.; Gallagher, T.; Kiessling, L.L.; Woolfson, D.N. Carbohydrate–Aromatic Interactions in Proteins. J. Am. Chem. Soc. 2015, 137, 15152–15160. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, M.; Nikpour, M.; Bauzá, A.; Frontera, A. On the Importance of C-H/π and C-H···H-C Interactions in the Solid State Structure of 15-Lipoxygenase Inhibitors Based on Eugenol Derivatives. ChemPhysChem 2015, 16, 2260–2266. [Google Scholar] [CrossRef] [PubMed]
- Aliev, A.E.; Arendorf, J.R.T.; Pavlakos, I.; Moreno, R.B.; Porter, M.J.; Rzepa, H.S.; Motherwell, W.B. Surfing π Clouds for Noncovalent Interactions: Arenes versus Alkenes. Angew. Chem. Int. Ed. 2015, 54, 551–555. [Google Scholar] [CrossRef]
- Banerjee, P.; Chakraborty, T. Correlation of νOH Spectral Shifts of Phenol–Benzene O–H···π Hydrogen-Bonded Complexes with Donor’s Acidity: A Combined Matrix Isolation, Infrared Spectroscopy, and Quantum Chemistry Study. J. Phys. Chem. A 2014, 118, 7074–7084. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M.; Umezawa, Y.; Fantini, J.; Weiss, M.S.; Chakrabarti, P. CH–π Hydrogen Bonds in Biological Macromolecules. Phys. Chem. Chem. Phys. 2014, 16, 12648–12683. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, O.; Kohno, Y.; Saito, K. Molecular Orbital Calculations of the Substituent Effect on Intermolecular CH/π Interaction in C2H3X--C6H6 Complexes (X=H, F, Cl, Br, and OH). Chem. Phys. Lett. 2013, 378, 509–515. [Google Scholar] [CrossRef]
- Ganguly, H.K.; Majumder, B.; Chattopadhyay, S.; Chakrabarti, P.; Basu, G. Direct Evidence for CH···π Interaction Mediated Stabilization of Pro-Cispro Bond in Peptides with Pro-Pro-Aromatic Motifs. J. Am. Chem. Soc. 2012, 134, 4661–4669. [Google Scholar] [CrossRef] [PubMed]
- Saggu, M.; Levinson, N.M.; Boxer, S.G. Experimental Quantification of Electrostatics in X–H···π Hydrogen Bonds. J. Am. Chem. Soc. 2012, 134, 18986–18997. [Google Scholar] [CrossRef] [PubMed]
- Weinhold, F.; Klein, R.A. Anti-Electrostatic Hydrogen Bonds. Angew. Chem. Int. Ed. 2014, 53, 11214–11217. [Google Scholar] [CrossRef] [PubMed]
- Alkorta, I.; Mata, I.; Molins, E.; Espinosa, E. Charged Versus Neutral Hydrogen-Bonded Complexes: Is There a Difference in the Nature of the Hydrogen Bonds? Chem. Eur. J. 2016, 22, 9226–9234. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Keith, T.A. Aimall; TK Gristmill Software: Overland Park, KS, USA, 2013. [Google Scholar]
- Jones, C.R.; Baruah, P.K.; Thompson, A.L.; Scheiner, S.; Smith, M.D. Can a C-H···O Interaction Be a Determinant of Conformation. J. Am. Chem. Soc. 2012, 134, 12064–12071. [Google Scholar] [CrossRef] [PubMed]
- Dian, B.C.; Longarte, A.; Mercier, S.; Evans, D.A.; Wale, D.J.; Zwier, T.S. The Infrared and Ultraviolet Spectra of Single Conformations of Methyl-Capped Dipeptides: N-Acetyl Tryptophan Amide and N-Acetyl Tryptophan Methyl Amide. J. Chem. Phys. 2002, 117, 10688. [Google Scholar] [CrossRef]
- Chin, W.; Dognon, J.-P.; Canuel, C.; Piuzzi, F.; Dimicoli, I.; Mons, M.; Compagnon, I.; Helden, G.V.; Meijer, G. Secondary Structures of Short Peptide Chains in the Gas Phase: Double Resonance Spectroscopy of Protected Dipeptides. J. Chem. Phys. 2005, 122, 054317. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Relative Strengths of NH··O and CH··O Hydrogen Bonds between Polypeptide Chain Segments. J. Phys. Chem. B 2005, 109, 16132–16141. [Google Scholar] [CrossRef] [PubMed]
- Lehninger, A.L.; Nelson, D.L.; Cox, M.M. Principles of Biochemistry, 2nd ed.; Worth: New York, NY, USA, 1993; p. 1013. [Google Scholar]
- Pauling, L.; Corey, R.B. Configurations of Polypeptide Chains with Favored Orientations around Single Bonds: Two New Pleated Sheets. Proc. Natl. Acad. Sci. USA 1951, 37, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, T.W.; Martyna, G.J.; Allison, S.; Bates, S.P.; Crain, J. Liquid NMA: A Surprisingly Realistic Model for Hydrogen Bonding Motifs in Proteins. Chem. Phys. Lett. 2005, 414, 210–214. [Google Scholar] [CrossRef]
- Whitfield, T.W.; Martyna, G.J.; Allison, S.; Bates, S.P.; Vass, H.; Crain, J. Structure and Hydrogen Bonding in Neat N-Methylacetamide: Classical Molecular Dynamics and Raman Spectroscopy Studies of a Liquid of Peptidic Fragments. J. Phys. Chem. B 2006, 110, 3624–3637. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Gorin, A.; Guo, H. A Peptide-Linkage Deletion Procedure for Estimate of Energetic Contributions of Individual Peptide Groups in a Complex Environment: Application to Parallel β-Sheets. Interdiscip. Sci. Comput. Life Sci. 2009, 1, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Pohl, G.; Plumley, J.A.; Dannenberg, J.J. The Interactions of Phenylalanines in β-Sheet-Like Structures from Molecular Orbital Calculations Using Density Functional Theory (DFT), MP2, and CCSD(T) Methods. J. Chem. Phys. 2013, 138, 245102. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S.; Kar, T.; Pattanayak, J. Comparison of Various Types of Hydrogen Bonds Involving Aromatic Amino Acids. J. Am. Chem. Soc. 2002, 124, 13257–13264. [Google Scholar] [CrossRef] [PubMed]
- Bankiewicz, B.; Matczak, P.; Palusiak, M. Electron Density Characteristics in Bond Critical Point (QTAIM) Versus Interaction Energy Components (SAPT): The Case of Charge-Assisted Hydrogen Bonding. J. Phys. Chem. A 2012, 116, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Hunt, P.A.; Ashworth, C.R.; Matthews, R.P. Hydrogen Bonding in Ionic Liquids. Chem. Soc. Rev. 2015, 44, 1257–1288. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, Z.; Zhang, Z.; Yang, Z.; Liu, Y.; Wang, J.; Cai, T.; Li, S.; Chen, K.; Shi, J.; et al. Like-Charge Guanidinium Pairing between Ligand and Receptor: An Unusual Interaction for Drug Discovery and Design? J. Phys. Chem. B 2015, 119, 11988–11997. [Google Scholar] [CrossRef] [PubMed]
- Mata, I.; Molins, E.; Alkorta, I.; Espinosa, E. The Paradox of Hydrogen-Bonded Anion–Anion Aggregates in Oxoanions: A Fundamental Electrostatic Problem Explained in Terms of Electrophilic···Nucleophilic Interactions. J. Phys. Chem. A 2014, 119, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, J.; Seok, C. What Stabilizes Close Arginine Pairing in Proteins? Phys. Chem. Chem. Phys. 2013, 15, 5844–5853. [Google Scholar] [CrossRef] [PubMed]
- Vondrášek, J.; Mason, P.E.; Heyda, J.; Collins, K.D.; Jungwirth, P. The Molecular Origin of Like-Charge Arginine−Arginine Pairing in Water. J. Phys. Chem. B 2009, 113, 9041–9045. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
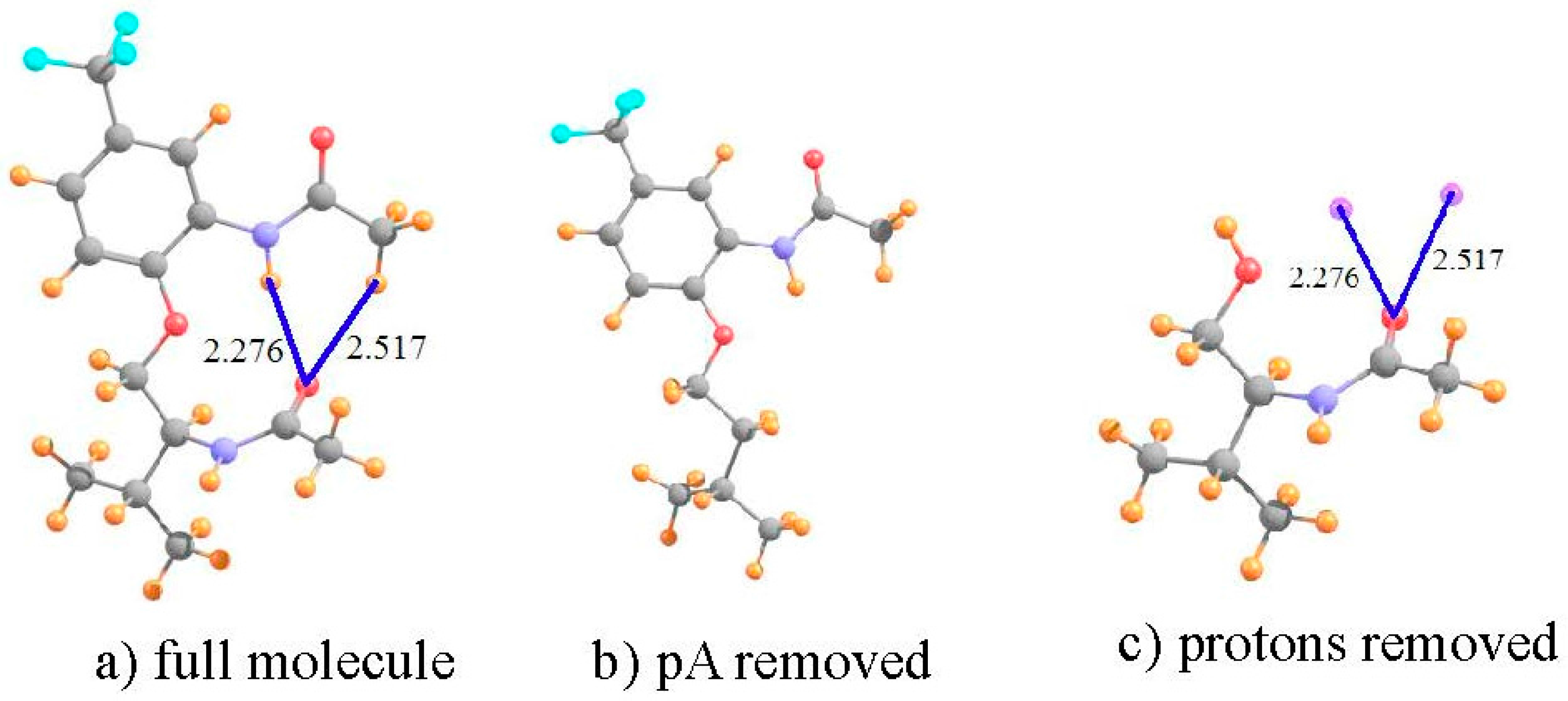
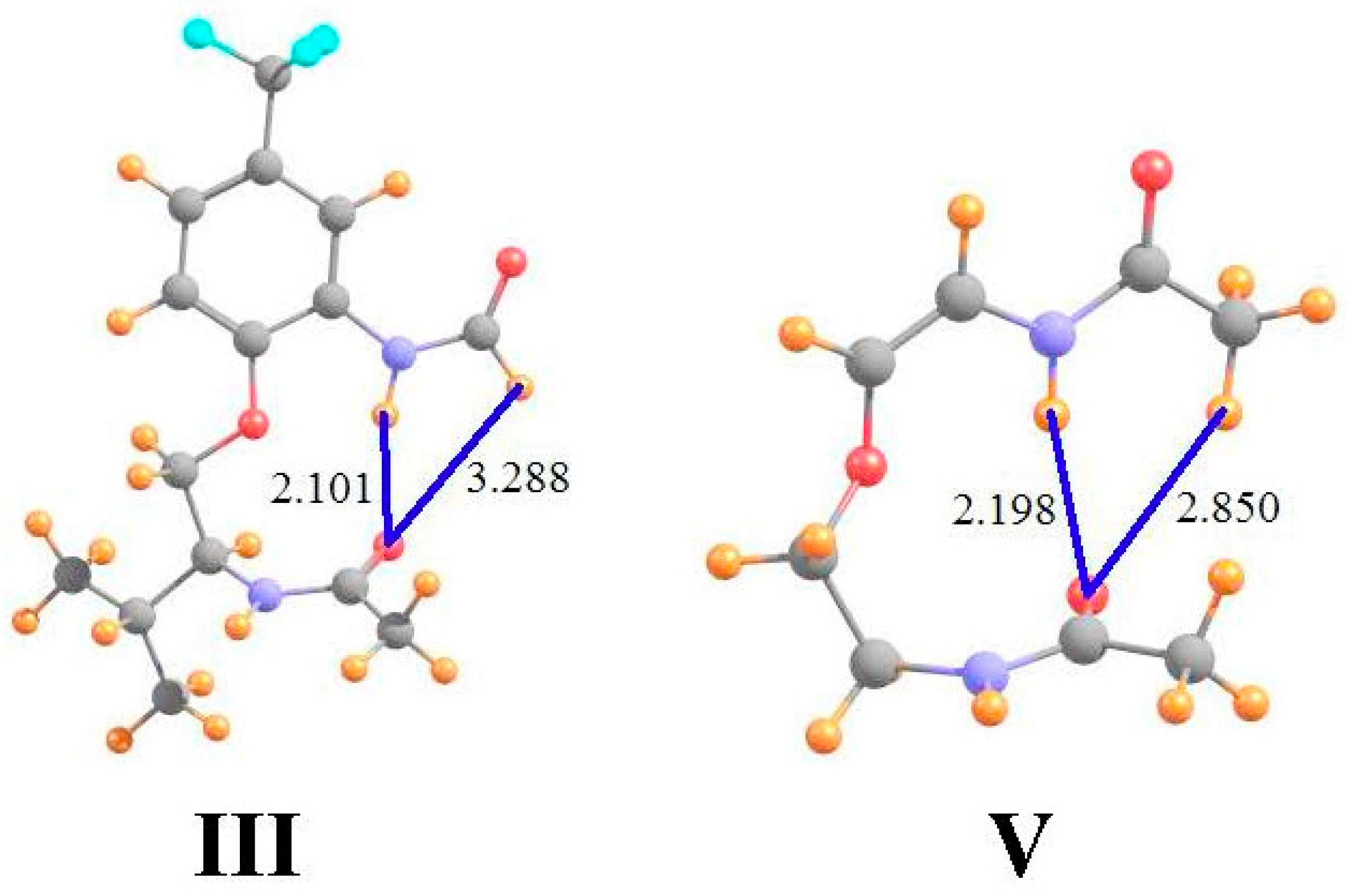


| I a | III b | V b | ||||
|---|---|---|---|---|---|---|
| CH | NH | CH | NH | CH | NH | |
| full molecule | 28.912 | 23.078 | 22.882 | 22.809 | 29.592 | 23.438 |
| pA removed | 30.163 | 23.682 | 23.091 | 23.930 | 30.349 | 24.350 |
| shift | −1.251 | −0.603 | −0.209 | −1.121 | −0.756 | −0.912 |
| protons removed | −0.166 | +0.588 | −0.048 | +0.640 | −0.101 | +0.588 |
| shift due to HB | −1.085 | −1.191 | −0.161 | −1.761 | −0.656 | −1.500 |
| ρBCP | 8 | 12 | - | 17 | 5 | 14 |
| C5 | |
|---|---|
| full molecule | 25.450 |
| pA removed | 27.312 |
| shift | −1.862 |
| proton removed | −0.011 |
| shift due to HB | −1.752 |
| ρBCP | 20.0 |
| NH | CHa | CHb | |
|---|---|---|---|
| full molecule | 23.947 | 27.297 | 28.071 |
| pA removed | 26.748 | 28.166 | 28.067 |
| shift | −2.801 | −0.869 | +0.004 |
| protons removed | −0.468 | −0.269 | −0.066 |
| shift due to HB | −2.333 | −0.600 | +0.070 |
| ρBCP | 20.6 | 9.1 | - |
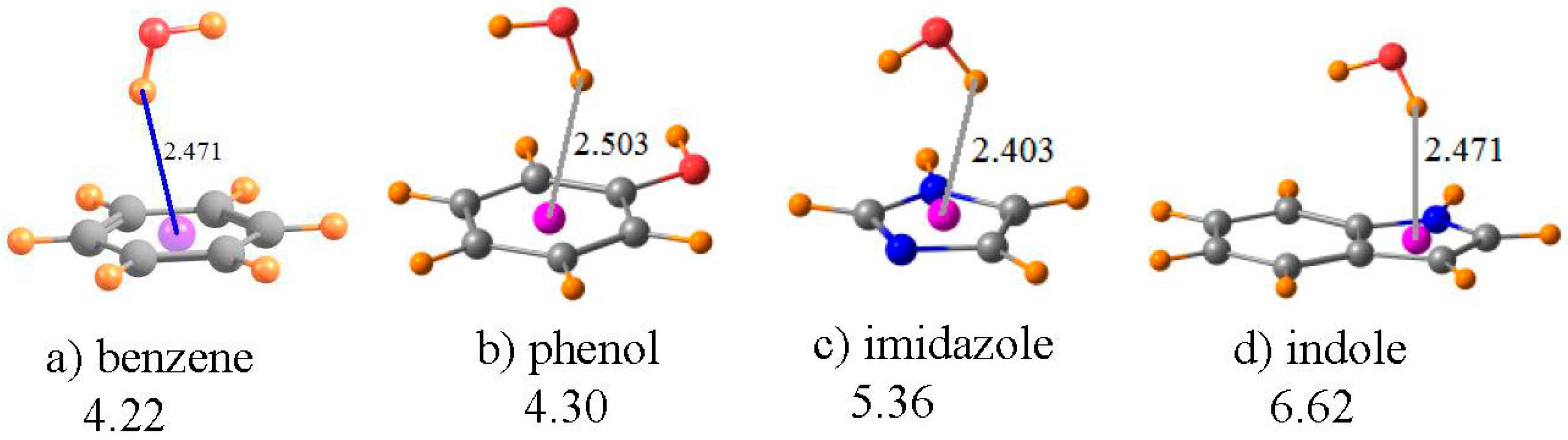
| Benzene | Phenol | Imidazole | Indole | |
|---|---|---|---|---|
| full dimer | 33.071 | 32.688 | 32.308 | 33.055 |
| pA removed | 30.546 | 30.743 | 30.788 | 30.741 |
| shift | 2.524 | 1.945 | 1.520 | 2.314 |
| HOH removed | 3.189 | 2.818 | 2.600 | 3.448 |
| shift due to HB | −0.665 | −0.873 | −1.081 | −1.134 |
| ρBCP | 8.2 | 8.4 | 9.5 | 8.8 |
| −∆E (kcal/mol) | 4.22 | 4.30 | 5.36 | 6.62 |
| HCO3− + F− | (NH3COOH+)2 | (COOH+)2 Ring a | |
|---|---|---|---|
| full dimer | 21.101 | 20.948 | 21.645 |
| pA removed | 27.125 | 23.113 | 24.036 |
| shift | −6.025 | −2.165 | −2.391 |
| pD removed | +0.862 | −0.129 | +0.101 |
| shift due to HB | −6.887 | −2.036 | −2.491 |
| ρBCP | 32.7 | 20.9 | 21.0 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scheiner, S. Assessment of the Presence and Strength of H-Bonds by Means of Corrected NMR. Molecules 2016, 21, 1426. https://doi.org/10.3390/molecules21111426
Scheiner S. Assessment of the Presence and Strength of H-Bonds by Means of Corrected NMR. Molecules. 2016; 21(11):1426. https://doi.org/10.3390/molecules21111426
Chicago/Turabian StyleScheiner, Steve. 2016. "Assessment of the Presence and Strength of H-Bonds by Means of Corrected NMR" Molecules 21, no. 11: 1426. https://doi.org/10.3390/molecules21111426