Effects of Flavonoids from Food and Dietary Supplements on Glial and Glioblastoma Multiforme Cells
Abstract
:1. Introduction
2. Flavonoids
| Chromane Ring |  | |||||
|---|---|---|---|---|---|---|
| General Structural Formula of Flavonoids (2-Phenylchromane) |  | |||||
| Class | Group | C2-C3 Double Bond | 3-OH | 4-keto | Structural Formula | Example |
| Anthoxanthins | Flavones | Yes | No | Yes |  | Apigenin, luteolin. |
| Flavonols | Yes | Yes | Yes |  | Quercetin, kaempferol | |
| Flavanones | / | No | No | Yes |  | Naringenin |
| Flavanonols | / | No | Yes | Yes |  | Taxifolin |
| Flavans | Flavan-3-ols | No | Yes | No |  | Catechin and its derivatives. |
| Flavan-4-ols | No | No (4-OH instead) | No |  | Apiforol | |
| Flavan-3,4-diols | No | Yes (also 4-OH) | No |  | Leucocyanidin | |
| Proanthocyanidins | No | Depending on monomers. | No | Figure 3 | Dimers and oligomers of flavanols | |
| Anthocyanidins | / | No (aromatic ring) | Yes | No |  | Cyanidin |
| Signaling Pathways (Red = Pathway Inhibition, Green = Pathway Activation) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Flavonoid | Ras/MAPK | EGF/PI3K/Akt | NF-κB | Wnt/β-catenin | TNFα/NADPH-oxidase | JAK/STAT | Notch | ER | AHR | Nrf2 | Ig-E | IRF-1 |
| Flavonols | Quercetin | ||||||||||||
| Kaempferol | |||||||||||||
| 3′-HF | |||||||||||||
| Flavan-3-ols | Catechin/CG | ||||||||||||
| Epicatechin | |||||||||||||
| Epicatechin metabolites | |||||||||||||
| ECG | |||||||||||||
| EGCG | |||||||||||||
| PACs | Dimeric procyanidins | ||||||||||||
| Hexameric procyanidins | |||||||||||||
| Flavones | Apigenin | ||||||||||||
| Luteolin | |||||||||||||
| Tangeretin | |||||||||||||
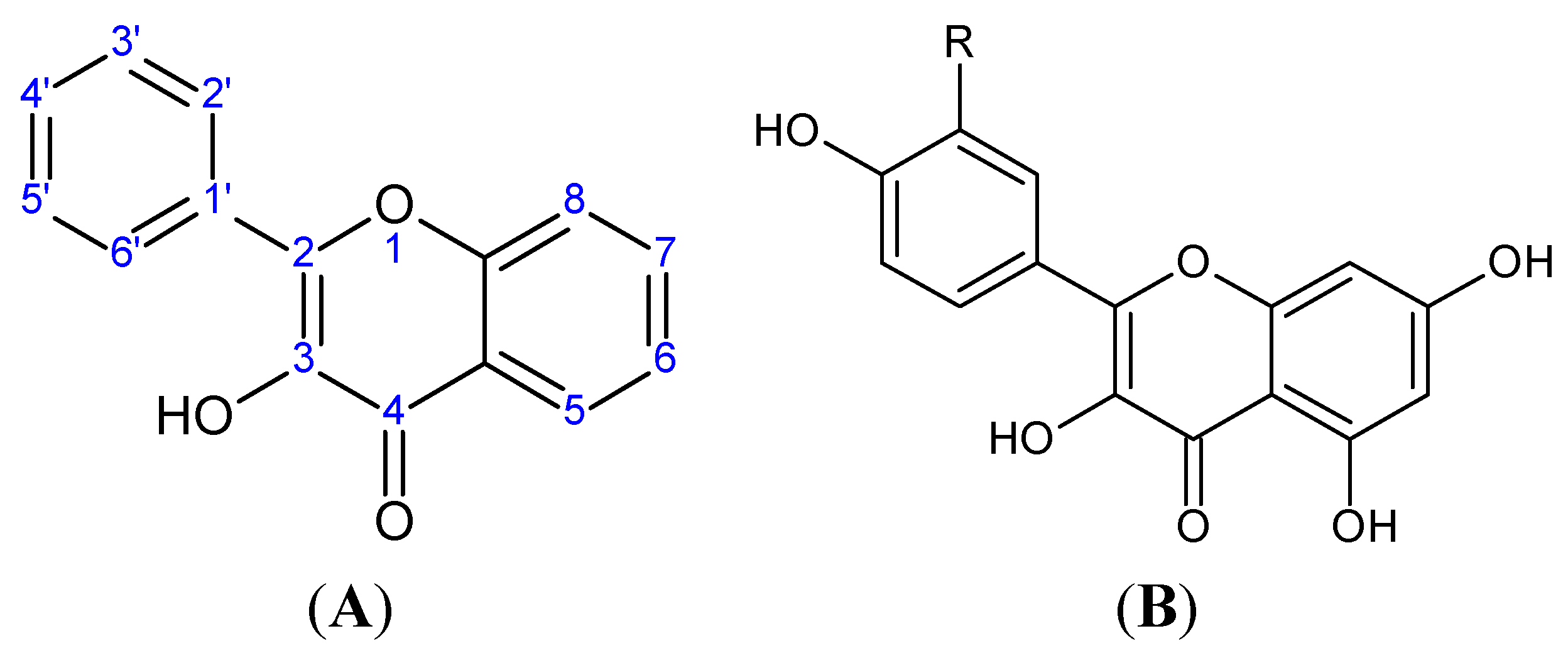
3. Quercetin
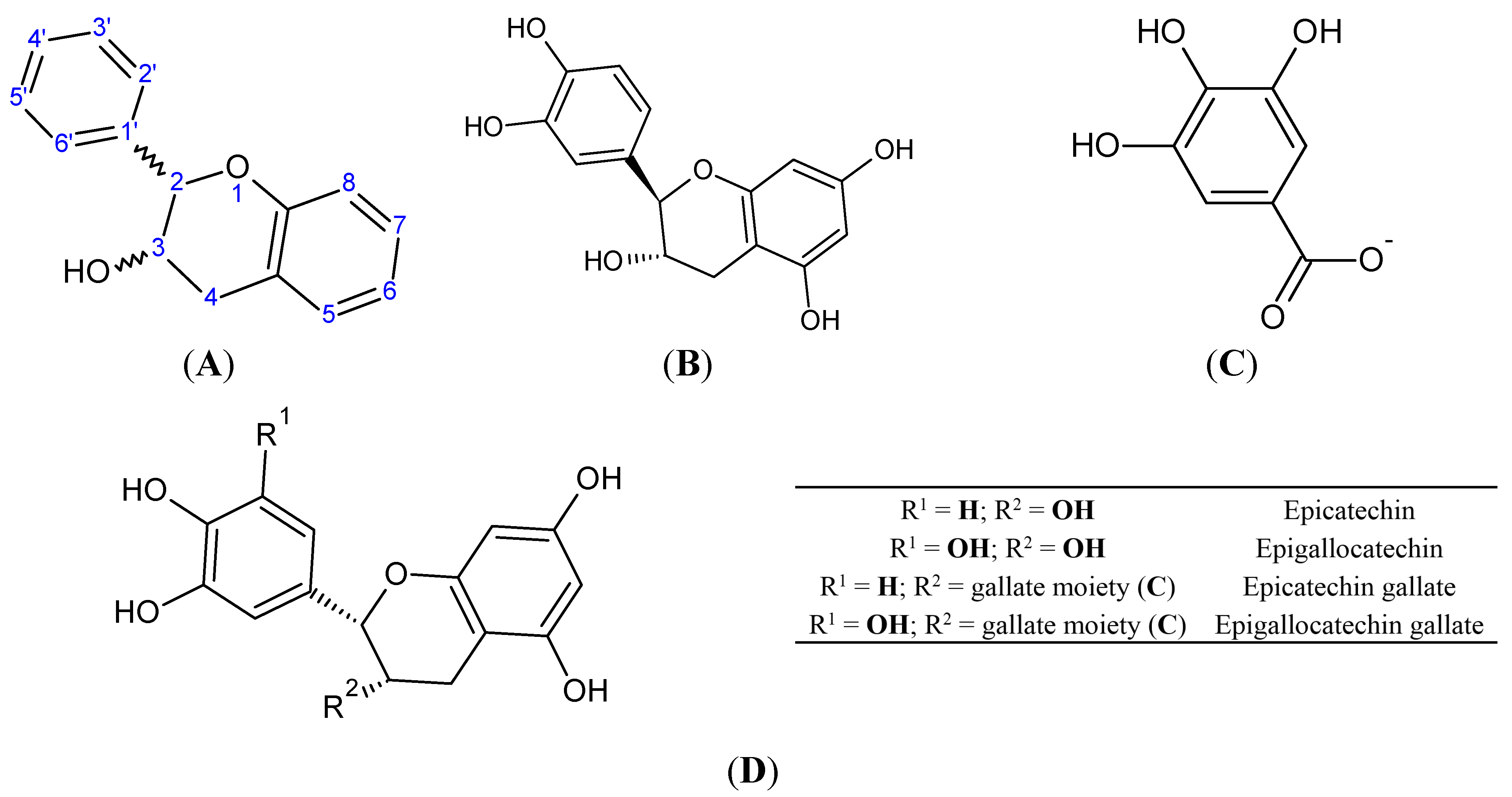
4. Catechins
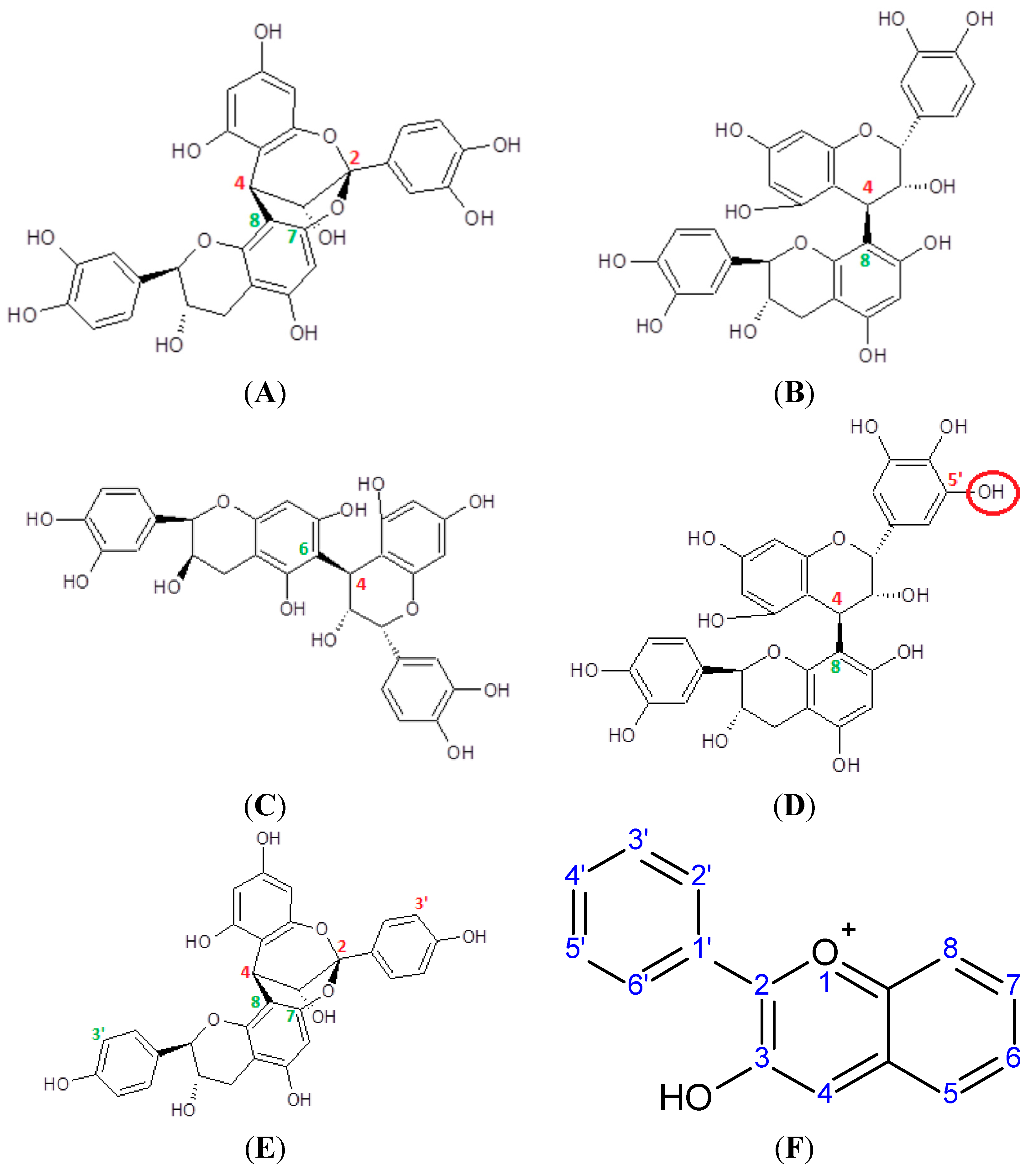
5. Proanthocyanidins
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 who classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.R.; O’Neill, B.P. Glioblastoma survival in the united states before and during the temozolomide era. J. Neurooncol. 2012, 107, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Eyler, C.E.; Rich, J.N. Survival of the fittest: Cancer stem cells in therapeutic resistance and angiogenesis. J. Clin. Oncol. 2008, 26, 2839–2845. [Google Scholar] [CrossRef] [PubMed]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Eramo, A.; Ricci-Vitiani, L.; Zeuner, A.; Pallini, R.; Lotti, F.; Sette, G.; Pilozzi, E.; Larocca, L.M.; Peschle, C.; de Maria, R. Chemotherapy resistance of glioblastoma stem cells. Cell Death Differ. 2006, 13, 1238–1241. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.J.; Woodard, C.A. Glioblastoma cells do not intravasate into blood vessels. Neurosurgery 1995, 36, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Silbergeld, D.L.; Rostomily, R.C.; Alvord, E.C. The cause of death in patients with glioblastoma is multifactorial: Clinical factors and autopsy findings in 117 cases of supratentorial glioblastoma in adults. J. Neurooncol. 1991, 10, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Rajaraman, P.; Hutchinson, A.; Rothman, N.; Black, P.M.; Fine, H.A.; Loeffler, J.S.; Selker, R.G.; Shapiro, W.R.; Linet, M.S.; Inskip, P.D. Oxidative response gene polymorphisms and risk of adult brain tumors. Neuro Oncol. 2008, 10, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Cotelle, N. Role of flavonoids in oxidative stress. Curr. Top. Med. Chem. 2001, 1, 569–590. [Google Scholar] [CrossRef] [PubMed]
- Chun, O.K.; Chung, S.J.; Song, W.O. Estimated dietary flavonoid intake and major food sources of U.S. adults. J. Nutr. 2007, 137, 1244–1252. [Google Scholar] [PubMed]
- Treutter, D. Significance of flavonoids in plant resistance and enhancement of their biosynthesis. Plant Biol. 2005, 7, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Das, D.K. Naturally occurring flavonoids: Structure, chemistry, and high-performance liquid chromatography methods for separation and characterization. Methods Enzymol. 1994, 234, 410–420. [Google Scholar] [PubMed]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [PubMed]
- Wolffram, S.; Blöck, M.; Ader, P. Quercetin-3-glucoside is transported by the glucose carrier SGLT1 across the brush border membrane of rat small intestine. J. Nutr. 2002, 132, 630–635. [Google Scholar] [PubMed]
- Hollman, P.C.; Katan, M.B. Absorption, metabolism and health effects of dietary flavonoids in man. Biomed. Pharmacother. 1997, 51, 305–310. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, M. Absorption and metabolism of flavonoids in the Caco-2 cell culture model and a perused rat intestinal model. Drug Metab. Dispos. 2002, 30, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Shukitt-Hale, B.; Joseph, J.A. Flavonoids and the brain: Interactions at the blood-brain barrier and their physiological effects on the central nervous system. Free Radic. Biol. Med. 2004, 37, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Youdim, K.A.; Dobbie, M.S.; Kuhnle, G.; Proteggente, A.R.; Abbott, N.J.; Rice-Evans, C. Interaction between flavonoids and the blood-brain barrier: In vitro studies. J. Neurochem. 2003, 85, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Begley, D. Efflux mechanisms in the central nervous system: A powerful influence on drug distribution within the brain. In Blood-Spinal Cord and Brain Barriers in Health and Disease; Sharma, S., Westman, J., Eds.; Elsevier Science: Amsterdam, The Netherlands; New York, NY, USA, 2004; pp. 83–97. [Google Scholar]
- Youdim, K.A.; Qaiser, M.Z.; Begley, D.J.; Rice-Evans, C.A.; Abbott, N.J. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radic. Biol. Med. 2004, 36, 592–604. [Google Scholar] [CrossRef] [PubMed]
- Borst, P.; Elferink, R.O. Mammalian abc transporters in health and disease. Annu. Rev. Biochem. 2002, 71, 537–592. [Google Scholar] [CrossRef] [PubMed]
- Ishige, K.; Schubert, D.; Sagara, Y. Flavonoids protect neuronal cells from oxidative stress by three distinct mechanisms. Free Radic. Biol. Med. 2001, 30, 433–446. [Google Scholar] [CrossRef]
- Mandel, S.; Youdim, M.B. Catechin polyphenols: Neurodegeneration and neuroprotection in neurodegenerative diseases. Free Radic. Biol. Med. 2004, 37, 304–317. [Google Scholar] [CrossRef] [PubMed]
- Raso, G.M.; Meli, R.; di Carlo, G.; Pacilio, M.; di Carlo, R. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 expression by flavonoids in macrophage J774A.1. Life Sci. 2001, 68, 921–931. [Google Scholar] [CrossRef]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Fruehauf, J.P.; Meyskens, F.L. Reactive oxygen species: A breath of life or death? Clin. Cancer Res. 2007, 13, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Leslie, E.M.; Mao, Q.; Oleschuk, C.J.; Deeley, R.G.; Cole, S.P. Modulation of multidrug resistance protein 1 (MRP1/ABCC1) transport and atpase activities by interaction with dietary flavonoids. Mol. Pharmacol. 2001, 59, 1171–1180. [Google Scholar] [PubMed]
- López-Lázaro, M.; Martín-Cordero, C.; Toro, M.V.; Ayuso, M.J. Flavonoids as DNA topoisomerase I poisons. J. Enzyme Inhib. Med. Chem. 2002, 17, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Yang, E.B.; Zhang, K.; Mack, P. Quercetin-induced apoptosis in the monoblastoid cell line U937 in vitro and the regulation of heat shock proteins expression. Anticancer Res. 2000, 20, 4339–4345. [Google Scholar] [PubMed]
- Kobuchi, H.; Roy, S.; Sen, C.K.; Nguyen, H.G.; Packer, L. Quercetin inhibits inducible ICAM-1 expression in human endothelial cells through the JNK pathway. Am. J. Physiol. 1999, 277, C403–C411. [Google Scholar] [PubMed]
- Ngomuo, A.J.; Jones, R.S. Cytotoxicity studies of quercetin, shikimate, cyclohexanecarboxylate and ptaquiloside. Vet. Hum. Toxicol. 1996, 38, 14–18. [Google Scholar] [PubMed]
- Yamakoshi, J.; Saito, M.; Kataoka, S.; Kikuchi, M. Safety evaluation of proanthocyanidin-rich extract from grape seeds. Food Chem. Toxicol. 2002, 40, 599–607. [Google Scholar] [CrossRef]
- Hodek, P.; Trefil, P.; Stiborová, M. Flavonoids-potent and versatile biologically active compounds interacting with cytochromes P450. Chem. Biol. Interact. 2002, 139, 1–21. [Google Scholar] [CrossRef]
- Ross, J.A. Maternal diet and infant leukemia: A role for DNA topoisomerase II inhibitors? Int. J. Cancer Suppl. 1998, 11, 26–28. [Google Scholar] [CrossRef]
- Pérez-Coll, C.S.; Herkovits, J. Lethal and teratogenic effects of naringenin evaluated by means of an amphibian embryo toxicity test (AMPHITOX). Food Chem. Toxicol. 2004, 42, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Schönthal, A.H. Adverse effects of concentrated green tea extracts. Mol. Nutr. Food Res. 2011, 55, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Choi, Y.J.; Shin, S.Y.; Li, J.; Kang, S.W.; Bae, J.Y.; Kim, D.S.; Ji, G.E.; Kang, J.S.; Kang, Y.H. Dietary flavonoids differentially reduce oxidized LDL-induced apoptosis in human endothelial cells: Role of MAPK- and JAK/STAT-signaling. J. Nutr. 2008, 138, 983–990. [Google Scholar] [PubMed]
- Parker-Athill, E.; Luo, D.; Bailey, A.; Giunta, B.; Tian, J.; Shytle, R.D.; Murphy, T.; Legradi, G.; Tan, J. Flavonoids, a prenatal prophylaxis via targeting JAK2/STAT3 signaling to oppose IL-6/MIA associated autism. J. Neuroimmunol. 2009, 217, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Virgili, F.; Acconcia, F.; Ambra, R.; Rinna, A.; Totta, P.; Marino, M. Nutritional flavonoids modulate estrogen receptor alpha signaling. IUBMB Life 2004, 56, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.R.; Kang, Y.J.; Kim, J.H.; Lee, H.T.; Cho, S.G. Modulation of apoptosis in HaCaT keratinocytes via differential regulation of ERK signaling pathway by flavonoids. J. Biol. Chem. 2005, 280, 31498–31507. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Oteiza, P.I. Dietary flavonoids: Role of (−)-epicatechin and related procyanidins in cell signaling. Free Radic. Biol. Med. 2011, 51, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, P.A.; Haller, D. Functional diversity of flavonoids in the inhibition of the proinflammatory NF-κB, IRF, and Akt signaling pathways in murine intestinal epithelial cells. J. Nutr. 2006, 136, 664–671. [Google Scholar] [PubMed]
- Schroeter, H.; Boyd, C.; Spencer, J.P.; Williams, R.J.; Cadenas, E.; Rice-Evans, C. MAPK signaling in neurodegeneration: Influences of flavonoids and of nitric oxide. Neurobiol. Aging 2002, 23, 861–880. [Google Scholar] [CrossRef]
- Amado, N.G.; Fonseca, B.F.; Cerqueira, D.M.; Neto, V.M.; Abreu, J.G. Flavonoids: Potential Wnt/beta-catenin signaling modulators in cancer. Life Sci. 2011, 89, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Muthian, G.; Bright, J.J. Quercetin, a flavonoid phytoestrogen, ameliorates experimental allergic encephalomyelitis by blocking IL-12 signaling through JAK-STAT pathway in T lymphocyte. J. Clin. Immunol. 2004, 24, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Krol, W.; Czuba, Z.; Scheller, S.; Paradowski, Z.; Shani, J. Structure-activity relationship in the ability of flavonols to inhibit chemiluminescence. J. Ethnopharmacol. 1994, 41, 121–126. [Google Scholar] [CrossRef]
- Sampson, L.; Rimm, E.; Hollman, P.C.; de Vries, J.H.; Katan, M.B. Flavonol and flavone intakes in us health professionals. J. Am. Diet Assoc. 2002, 102, 1414–1420. [Google Scholar] [CrossRef]
- Mennen, L.I.; Walker, R.; Bennetau-Pelissero, C.; Scalbert, A. Risks and safety of polyphenol consumption. Am. J. Clin. Nutr. 2005, 81, 326S–329S. [Google Scholar] [PubMed]
- Sak, K. Site-specific anticancer effects of dietary flavonoid quercetin. Nutr. Cancer 2014, 66, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Aherne, S.A.; O’Brien, N.M. Dietary flavonols: Chemistry, food content, and metabolism. Nutrition 2002, 18, 75–81. [Google Scholar] [CrossRef]
- Dajas, F. Life or death: Neuroprotective and anticancer effects of quercetin. J. Ethnopharmacol. 2012, 143, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Egert, S.; Wolffram, S.; Bosy-Westphal, A.; Boesch-Saadatmandi, C.; Wagner, A.E.; Frank, J.; Rimbach, G.; Mueller, M.J. Daily quercetin supplementation dose-dependently increases plasma quercetin concentrations in healthy humans. J. Nutr. 2008, 138, 1615–1621. [Google Scholar] [PubMed]
- Shimoi, K.; Yoshizumi, K.; Kido, T.; Usui, Y.; Yumoto, T. Absorption and urinary excretion of quercetin, rutin, and alphag-rutin, a water soluble flavonoid, in rats. J. Agric. Food Chem. 2003, 51, 2785–2789. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Kavuru, P.; Wojtas, L.; Zaworotko, M.J.; Shytle, R.D. Cocrystals of quercetin with improved solubility and oral bioavailability. Mol. Pharm. 2011, 8, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; de Vries, J.H.; van Leeuwen, S.D.; Mengelers, M.J.; Katan, M.B. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am. J. Clin. Nutr. 1995, 62, 1276–1282. [Google Scholar] [PubMed]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Arts, M.; Dallinga, J.; Voss, H.; Haenen, G.; Bast, A. A new approach to assess the total antioxidant capacity using the teac assay. Food Chem. 2004, 88, 567–570. [Google Scholar] [CrossRef]
- Hirvonen, T.; Virtamo, J.; Korhonen, P.; Albanes, D.; Pietinen, P. Flavonol and flavone intake and the risk of cancer in male smokers (Finland). Cancer Causes Control 2001, 12, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Knekt, P.; Kumpulainen, J.; Järvinen, R.; Rissanen, H.; Heliövaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [PubMed]
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The zutphen elderly study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef]
- MacNee, W. Oxidative stress and lung inflammation in airways disease. Eur. J. Pharmacol. 2001, 429, 195–207. [Google Scholar] [CrossRef]
- Rahman, I.; Gilmour, P.S.; Jimenez, L.A.; MacNee, W. Oxidative stress and TNF-α induce histone acetylation and NF-κB/AP-1 activation in alveolar epithelial cells: Potential mechanism in gene transcription in lung inflammation. Mol. Cell Biochem. 2002, 37, 239–248. [Google Scholar] [CrossRef]
- Geraets, L.; Moonen, H.J.; Brauers, K.; Wouters, E.F.; Bast, A.; Hageman, G.J. Dietary flavones and flavonoles are inhibitors of poly(ADP-ribose)polymerase-1 in pulmonary epithelial cells. J. Nutr. 2007, 137, 2190–2195. [Google Scholar] [PubMed]
- Bureau, G.; Longpré, F.; Martinoli, M.G. Resveratrol and quercetin, two natural polyphenols, reduce apoptotic neuronal cell death induced by neuroinflammation. J. Neurosci. Res. 2008, 86, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B. Quercetin inhibits LPS-induced nitric oxide and tumor necrosis factor-α production in murine macrophages. Int. J. Immunopharmacol. 1999, 21, 435–443. [Google Scholar]
- Lee, E.S.; Lee, H.E.; Shin, J.Y.; Yoon, S.; Moon, J.O. The flavonoid quercetin inhibits dimethylnitrosamine-induced liver damage in rats. J. Pharm. Pharmacol. 2003, 55, 1169–1174. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Bucki, R.; Pastore, J.J.; Giraud, F.; Sulpice, J.C.; Janmey, P.A. Flavonoid inhibition of platelet procoagulant activity and phosphoinositide synthesis. J. Thromb. Haemost. 2003, 1, 1820–1828. [Google Scholar] [CrossRef] [PubMed]
- De Whalley, C.V.; Rankin, S.M.; Hoult, J.R.; Jessup, W.; Leake, D.S. Flavonoids inhibit the oxidative modification of low density lipoproteins by macrophages. Biochem. Pharmacol. 1990, 39, 1743–1750. [Google Scholar] [CrossRef]
- Duarte, J.; Pérez-Palencia, R.; Vargas, F.; Ocete, M.A.; Pérez-Vizcaino, F.; Zarzuelo, A.; Tamargo, J. Antihypertensive effects of the flavonoid quercetin in spontaneously hypertensive rats. Br. J. Pharmacol. 2001, 133, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.M. Antiproliferative potency of structurally distinct dietary flavonoids on human colon cancer cells. Cancer Lett. 1996, 110, 41–48. [Google Scholar] [CrossRef]
- Gulati, N.; Laudet, B.; Zohrabian, V.M.; Murali, R.; Jhanwar-Uniyal, M. The antiproliferative effect of quercetin in cancer cells is mediated via inhibition of the PI3K-Akt/PKB pathway. Anticancer Res. 2006, 26, 1177–1181. [Google Scholar] [PubMed]
- Gitika, B.; Sai Ram, M.; Sharma, S.K.; Ilavazhagan, G.; Banerjee, P.K. Quercetin protects C6 glial cells from oxidative stress induced by tertiary-butylhydroperoxide. Free Radic. Res. 2006, 40, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.J.; Jeng, J.Y.; Lin, C.W.; Wu, C.Y.; Chen, Y.C. Quercetin inhibition of ROS-dependent and -independent apoptosis in rat glioma C6 cells. Toxicology 2006, 223, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.Y.; Yu, A.C. Quercetin inhibits c-fos, heat shock protein, and glial fibrillary acidic protein expression in injured astrocytes. J. Neurosci. Res. 2000, 62, 730–736. [Google Scholar] [CrossRef]
- Panickar, K.S.; Anderson, R.A. Mechanisms underlying the protective effects of myricetin and quercetin following oxygen-glucose deprivation-induced cell swelling and the reduction in glutamate uptake in glial cells. Neuroscience 2011, 183, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Volk, C.; Kempski, B.; Kempski, O.S. Inhibition of lactate export by quercetin acidifies rat glial cells in vitro. Neurosci. Lett. 1997, 223, 121–124. [Google Scholar] [CrossRef]
- Jakubowicz-Gil, J.; Langner, E.; Bądziul, D.; Wertel, I.; Rzeski, W. Apoptosis induction in human glioblastoma multiforme T98G cells upon temozolomide and quercetin treatment. Tumour Biol. 2013, 34, 2367–2378. [Google Scholar] [CrossRef] [PubMed]
- Braganhol, E.; Zamin, L.L.; Canedo, A.D.; Horn, F.; Tamajusuku, A.S.; Wink, M.R.; Salbego, C.; Battastini, A.M. Antiproliferative effect of quercetin in the human U138MG glioma cell line. Anticancer Drugs 2006, 17, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Choi, C.H.; Park, J.Y.; Kang, S.K.; Kim, Y.K. Underlying mechanism of quercetin-induced cell death in human glioma cells. Neurochem. Res. 2008, 33, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Siegelin, M.D.; Reuss, D.E.; Habel, A.; Rami, A.; von Deimling, A. Quercetin promotes degradation of survivin and thereby enhances death-receptor-mediated apoptosis in glioma cells. Neuro Oncol. 2009, 11, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Moon, J.Y.; Ahn, K.S.; Cho, S.K. Quercetin induces mitochondrial mediated apoptosis and protective autophagy in human glioblastoma U373MG cells. Oxid. Med. Cell. Longev. 2013, 2013, 596496. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz-Gil, J.; Langner, E.; Wertel, I.; Piersiak, T.; Rzeski, W. Temozolomide, quercetin and cell death in the moggccm astrocytoma cell line. Chem. Biol. Interact. 2010, 188, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Michaud-Levesque, J.; Bousquet-Gagnon, N.; Béliveau, R. Quercetin abrogates IL-6/STAT3 signaling and inhibits glioblastoma cell line growth and migration. Exp. Cell Res. 2012, 318, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Amado, N.G.; Cerqueira, D.M.; Menezes, F.S.; da Silva, J.F.; Neto, V.M.; Abreu, J.G. Isoquercitrin isolated from hyptis fasciculata reduces glioblastoma cell proliferation and changes beta-catenin cellular localization. Anticancer Drugs 2009, 20, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Siegelin, M.D.; Reuss, D.E.; Habel, A.; Herold-Mende, C.; von Deimling, A. The flavonoid kaempferol sensitizes human glioma cells to trail-mediated apoptosis by proteasomal degradation of survivin. Mol. Cancer Ther. 2008, 7, 3566–3574. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Shen, S.C.; Chien, C.C.; Yang, L.Y.; Shia, L.T.; Chen, Y.C. 12-O-tetradecanoylphorbol-13-acetate-induced invasion/migration of glioblastoma cells through activating PKCα/ERK/NF-κB-dependent MMP-9 expression. J. Cell. Physiol. 2010, 225, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Banik, N.L.; Ray, S.K. Flavonoids activated caspases for apoptosis in human glioblastoma T98G and U87MG cells but not in human normal astrocytes. Cancer 2010, 116, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Hung, C.M.; Tsai, J.C.; Lee, J.C.; Chen, Y.L.; Wei, C.W.; Kao, J.Y.; Way, T.D. Hispidulin potently inhibits human glioblastoma multiforme cells through activation of AMP-activated protein kinase (AMPK). J. Agric. Food Chem. 2010, 58, 9511–9517. [Google Scholar] [CrossRef] [PubMed]
- Poncet-Legrand, C.; Edelmann, A.; Putaux, J.; Cartalade, D.; Sarni-Manchado, P.; Vernhet, A. Poly(l-proline) interactions with flavan-3-ols units: Influence of the molecular structure and the polyphenol/protein ratio. Food Hydrocolloids 2006, 20, 687–697. [Google Scholar] [CrossRef]
- Hashimoto, F.; Ono, M.; Masuoka, C.; Ito, Y.; Sakata, Y.; Shimizu, K.; Nonaka, G.; Nishioka, I.; Nohara, T. Evaluation of the anti-oxidative effect (in vitro) of tea polyphenols. Biosci. Biotechnol. Biochem. 2003, 67, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.H.; Zhang, Y.J.; Xu, M.; Yang, C.R. Puerins A and B, two new 8-C substituted flavan-3-ols from pu-er tea. J. Agric. Food Chem. 2005, 53, 8614–8617. [Google Scholar] [CrossRef] [PubMed]
- Graham, H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Arts, I.C.; Hollman, P.C.; Kromhout, D. Chocolate as a source of tea flavonoids. Lancet 1999, 354, 488. [Google Scholar] [CrossRef]
- Auger, C.; Al-Awwadi, N.; Bornet, A.; Rouanet, J.; Gasc, F.; Cros, G.; Teissedre, P. Catechins and procyanidins in mediterranean diets. Food Res. Int. 2004, 37, 233–245. [Google Scholar] [CrossRef]
- De Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Quantitative analysis of flavan-3-ols in Spanish foodstuffs and beverages. J. Agric. Food Chem. 2000, 48, 5331–5337. [Google Scholar] [CrossRef] [PubMed]
- Carando, S.; Teissedre, P.L. Catechin and procyanidin levels in french wines: Contribution to dietary intake. Basic Life Sci. 1999, 66, 725–737. [Google Scholar] [PubMed]
- Seeram, N.P.; Henning, S.M.; Niu, Y.; Lee, R.; Scheuller, H.S.; Heber, D. Catechin and caffeine content of green tea dietary supplements and correlation with antioxidant capacity. J. Agric. Food Chem. 2006, 54, 1599–1603. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Chen, Y.; Li, R.C. Oral absorption and bioavailability of tea catechins. Planta Med. 2000, 66, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Warden, B.A.; Smith, L.S.; Beecher, G.R.; Balentine, D.A.; Clevidence, B.A. Catechins are bioavailable in men and women drinking black tea throughout the day. J. Nutr. 2001, 131, 1731–1737. [Google Scholar] [PubMed]
- Baba, S.; Osakabe, N.; Natsume, M.; Muto, Y.; Takizawa, T.; Terao, J. In vivo comparison of the bioavailability of (+)-catechin, (−)-epicatechin and their mixture in orally administered rats. J. Nutr. 2001, 131, 2885–2891. [Google Scholar] [PubMed]
- Nakagawa, K.; Okuda, S.; Miyazawa, T. Dose-dependent incorporation of tea catechins, (−)-epigallocatechin-3-gallate and (−)-epigallocatechin, into human plasma. Biosci. Biotechnol. Biochem. 1997, 61, 1981–1985. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, J.B.; Walle, T. Cellular uptake and efflux of the tea flavonoid (−)-epicatechin-3-gallate in the human intestinal cell line Caco-2. J. Pharmacol. Exp. Ther. 2003, 307, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Faria, A.; Pestana, D.; Teixeira, D.; Couraud, P.O.; Romero, I.; Weksler, B.; de Freitas, V.; Mateus, N.; Calhau, C. Insights into the putative catechin and epicatechin transport across blood-brain barrier. Food Funct. 2011, 2, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhang, Q.L.; Zhang, X.Y.; Lv, C.; Li, J.; Yuan, Y.; Yin, F.X. Pharmacokinetics and blood-brain barrier penetration of (+)-catechin and (−)-epicatechin in rats by microdialysis sampling coupled to high-performance liquid chromatography with chemiluminescence detection. J. Agric. Food Chem. 2012, 60, 9377–9383. [Google Scholar] [CrossRef] [PubMed]
- Abd El Mohsen, M.M.; Kuhnle, G.; Rechner, A.R.; Schroeter, H.; Rose, S.; Jenner, P.; Rice-Evans, C.A. Uptake and metabolism of epicatechin and its access to the brain after oral ingestion. Free Radic. Biol. Med. 2002, 33, 1693–1702. [Google Scholar] [CrossRef]
- Yokozawa, T.; Nakagawa, T.; Kitani, K. Antioxidative activity of green tea polyphenol in cholesterol-fed rats. J. Agric. Food Chem. 2002, 50, 3549–3552. [Google Scholar] [CrossRef] [PubMed]
- Skrzydlewska, E.; Ostrowska, J.; Farbiszewski, R.; Michalak, K. Protective effect of green tea against lipid peroxidation in the rat liver, blood serum and the brain. Phytomedicine 2002, 9, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lee, M.J.; Li, H.; Yang, C.S. Absorption, distribution, elimination of tea polyphenols in rats. Drug Metab. Dispos. 1997, 25, 1045–1050. [Google Scholar] [PubMed]
- Crespy, V.; Williamson, G. A review of the health effects of green tea catechins in in vivo animal models. J. Nutr. 2004, 134, 3431S–3440S. [Google Scholar] [PubMed]
- Jia, X.D.; Han, C. Chemoprevention of tea on colorectal cancer induced by dimethylhydrazine in wistar rats. World J. Gastroenterol. 2000, 6, 699–703. [Google Scholar] [PubMed]
- Jia, X.; Han, C. Effects of green tea on colonic aberrant crypt foci and proliferative indexes in rats. Nutr. Cancer 2001, 39, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Yamane, T.; Inagake, M.; Nakatani, H.; Iwata, Y.; Takahashi, T.; Nishimura, H.; Nishino, H.; Nakagawa, K.; Miyazawa, T. Inhibition of mucosal lipid hyperoxidation by green tea extract in 1,2-dimethylhydrazine-induced rat colonic carcinogenesis. Cancer Lett. 1996, 104, 205–209. [Google Scholar] [CrossRef]
- Li, N.; Han, C.; Chen, J. Tea preparations protect against DMBA-induced oral carcinogenesis in hamsters. Nutr. Cancer 1999, 35, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Xu, Y.; Chen, J.; Klaunig, J.E. Chemopreventive effects of green and black tea on pulmonary and hepatic carcinogenesis. Fundam. Appl. Toxicol. 1996, 29, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Hirose, M.; Hasegawa, R.; Kimura, J.; Akagi, K.; Yoshida, Y.; Tanaka, H.; Miki, T.; Satoh, T.; Wakabayashi, K.; Ito, N. Inhibitory effects of 1-O-hexyl-2,3,5-trimethylhydroquinone (HTHQ), green tea catechins and other antioxidants on 2-amino-6-methyldipyrido[1,2-a: 3′,2′-d]imidazole (Glu-P-1)-induced rat hepatocarcinogenesis and dose-dependent inhibition by HTHQ of lesion induction by Glu-P-1 or 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx). Carcinogenesis 1995, 16, 3049–3055. [Google Scholar] [PubMed]
- Tanaka, H.; Hirose, M.; Kawabe, M.; Sano, M.; Takesada, Y.; Hagiwara, A.; Shirai, T. Post-initiation inhibitory effects of green tea catechins on 7,12-dimethylbenz[a]anthracene-induced mammary gland carcinogenesis in female Sprague-Dawley rats. Cancer Lett. 1997, 116, 47–52. [Google Scholar] [CrossRef]
- Kavanagh, K.T.; Hafer, L.J.; Kim, D.W.; Mann, K.K.; Sherr, D.H.; Rogers, A.E.; Sonenshein, G.E. Green tea extracts decrease carcinogen-induced mammary tumor burden in rats and rate of breast cancer cell proliferation in culture. J. Cell. Biochem. 2001, 82, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Hastak, K.; Ahmad, N.; Lewin, J.S.; Mukhtar, H. Inhibition of prostate carcinogenesis in tramp mice by oral infusion of green tea polyphenols. Proc. Natl. Acad. Sci. USA 2001, 98, 10350–10355. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ho, C.T.; Amin, S.G.; Han, C.; Chung, F.L. Inhibition of tobacco-specific nitrosamine-induced lung tumorigenesis in A/J mice by green tea and its major polyphenol as antioxidants. Cancer Res. 1992, 52, 3875–3879. [Google Scholar] [PubMed]
- Sazuka, M.; Murakami, S.; Isemura, M.; Satoh, K.; Nukiwa, T. Inhibitory effects of green tea infusion on in vitro invasion and in vivo metastasis of mouse lung carcinoma cells. Cancer Lett. 1995, 98, 27–31. [Google Scholar] [CrossRef]
- Zhu, M.; Gong, Y.; Yang, Z.; Ge, G.; Han, C.; Chen, J. Green tea and its major components ameliorate immune dysfunction in mice bearing lewis lung carcinoma and treated with the carcinogen nnk. Nutr. Cancer 1999, 35, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Miura, Y.; Yagasaki, K. Effects of dietary powdered green tea and theanine on tumor growth and endogenous hyperlipidemia in hepatoma-bearing rats. Biosci. Biotechnol. Biochem. 2002, 66, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Sai, K.; Kai, S.; Umemura, T.; Tanimura, A.; Hasegawa, R.; Inoue, T.; Kurokawa, Y. Protective effects of green tea on hepatotoxicity, oxidative dna damage and cell proliferation in the rat liver induced by repeated oral administration of 2-nitropropane. Food Chem. Toxicol. 1998, 36, 1043–1051. [Google Scholar] [PubMed]
- Hasegawa, R.; Chujo, T.; Sai-Kato, K.; Umemura, T.; Tanimura, A.; Kurokawa, Y. Preventive effects of green tea against liver oxidative dna damage and hepatotoxicity in rats treated with 2-nitropropane. Food Chem. Toxicol. 1995, 33, 961–970. [Google Scholar] [PubMed]
- Rhee, S.J.; Kim, M.J.; Kwag, O.G. Effects of green tea catechin on prostaglandin synthesis of renal glomerular and renal dysfunction in streptozotocin-induced diabetic rats. Asia Pac. J. Clin. Nutr. 2002, 11, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.J.; Choi, J.H.; Park, M.R. Green tea catechin improves microsomal phospholipase A2 activity and the arachidonic acid cascade system in the kidney of diabetic rats. Asia Pac. J. Clin. Nutr. 2002, 11, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.A.; Choi, J.H.; Rhee, S.J. Effects of green tea catechin on phospholipase A2 activity and antithrombus in streptozotocin diabetic rats. J. Nutr. Sci. Vitaminol. 1999, 45, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P. Flavonoids and brain health: Multiple effects underpinned by common mechanisms. Genes Nutr. 2009, 4, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P. The interactions of flavonoids within neuronal signalling pathways. Genes Nutr. 2007, 2, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wu, L.J.; Tashiro, S.; Gao, H.Y.; Onodera, S.; Ikejima, T. (+)-catechin, an ingredient of green tea, protects murine microglia from oxidative stress-induced dna damage and cell cycle arrest. J. Pharmacol. Sci. 2005, 98, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Huang, Y.G.; Fang, D.; Le, W.D. (−)-epigallocatechin gallate inhibits lipopolysaccharide-induced microglial activation and protects against inflammation-mediated dopaminergic neuronal injury. J. Neurosci. Res. 2004, 78, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Park, I.J.; Lee, Y.K.; Hwang, J.T.; Kwon, D.Y.; Ha, J.; Park, O.J. Green tea catechin controls apoptosis in colon cancer cells by attenuation of H2O2-stimulated COX-2 expression via the ampk signaling pathway at low-dose H2O2. Ann. N. Y. Acad. Sci. 2009, 1171, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Noreen, Y.; Serrano, G.; Perera, P.; Bohlin, L. Flavan-3-ols isolated from some medicinal plants inhibiting COX-1 and COX-2 catalysed prostaglandin biosynthesis. Planta Med. 1998, 64, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Mazzio, E.A.; Harris, N.; Soliman, K.F. Food constituents attenuate monoamine oxidase activity and peroxide levels in C6 astrocyte cells. Planta Med. 1998, 64, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Soliman, K.F.; Mazzio, E.A. In vitro attenuation of nitric oxide production in C6 astrocyte cell culture by various dietary compounds. Proc. Soc. Exp. Biol. Med. 1998, 218, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Panickar, K.S.; Polansky, M.M.; Anderson, R.A. Green tea polyphenols attenuate glial swelling and mitochondrial dysfunction following oxygen-glucose deprivation in cultures. Nutr. Neurosci. 2009, 12, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Abib, R.T.; Quincozes-Santos, A.; Nardin, P.; Wofchuk, S.T.; Perry, M.L.; Gonçalves, C.A.; Gottfried, C. Epicatechin gallate increases glutamate uptake and S100B secretion in C6 cell lineage. Mol. Cell. Biochem. 2008, 310, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Shervington, A.; Pawar, V.; Menon, S.; Thakkar, D.; Patel, R. The sensitization of glioma cells to cisplatin and tamoxifen by the use of catechin. Mol. Biol. Rep. 2009, 36, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Wang, W.; Golden, E.B.; Thomas, S.; Sivakumar, W.; Hofman, F.M.; Louie, S.G.; Schönthal, A.H. Green tea epigallocatechin gallate enhances therapeutic efficacy of temozolomide in orthotopic mouse glioblastoma models. Cancer Lett. 2011, 302, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Z.; Xu, Y.M.; Wu, Y.; Yu, K.K.; Zhang, C.; Ji, Y.H.; Ding, G.; Chen, F.X. Epigallocatechin-3-gallate induces apoptosis, inhibits proliferation and decreases invasion of glioma cell. Neurosci. Bull. 2014, 30, 67–73. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, N.; Annabi, B.; Bouzeghrane, M.; Temme, A.; Bahary, J.P.; Moumdjian, R.; Béliveau, R. The survivin-mediated radioresistant phenotype of glioblastomas is regulated by RhoA and inhibited by the green tea polyphenol (−)-epigallocatechin-3-gallate. Brain Res. 2006, 1071, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Sharma, V.; Tewari, R.; Koul, N.; Joseph, C.; Sen, E. Epigallocatechin-3-gallate exhibits anti-tumor effect by perturbing redox homeostasis, modulating the release of pro-inflammatory mediators and decreasing the invasiveness of glioblastoma cells. Mol. Med. Rep. 2008, 1, 511–515. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, N.; Annabi, B.; Lachambre, M.P.; Kim, K.S.; Bahary, J.P.; Moumdjian, R.; Béliveau, R. Combined low dose ionizing radiation and green tea-derived epigallocatechin-3-gallate treatment induces human brain endothelial cells death. J. Neurooncol. 2006, 80, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Porter, L. Flavans and proanthocyanidins. In The Flavonoids—Advances in Research Since 1986; Harborne, J., Ed.; Chapman and Hall: London, UK, 1994; pp. 23–55. [Google Scholar]
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and tannin-like compounds—Nature, occurrence, dietary intake and effects on nutrition and health. J. Sci. Food Agric. 2000, 80, 1094–1117. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Gebhardt, S.; Prior, R.L. Concentrations of proanthocyanidins in common foods and estimations of normal consumption. J. Nutr. 2004, 134, 613–617. [Google Scholar] [PubMed]
- Rasmussen, S.E.; Frederiksen, H.; Struntze Krogholm, K.; Poulsen, L. Dietary proanthocyanidins: Occurrence, dietary intake, bioavailability, and protection against cardiovascular disease. Mol. Nutr. Food Res. 2005, 49, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Wilmouth, R.C.; Turnbull, J.J.; Welford, R.W.; Clifton, I.J.; Prescott, A.G.; Schofield, C.J. Structure and mechanism of anthocyanidin synthase from arabidopsis thaliana. Structure 2002, 10, 93–103. [Google Scholar] [CrossRef]
- Gu, L.; Kelm, M.A.; Hammerstone, J.F.; Beecher, G.; Holden, J.; Haytowitz, D.; Prior, R.L. Screening of foods containing proanthocyanidins and their structural characterization using LC-MS/MS and thiolytic degradation. J. Agric. Food Chem. 2003, 51, 7513–7521. [Google Scholar] [CrossRef] [PubMed]
- Oliff, H. Scientific and Clinical Monograph for Pycnogenol; American Botanical Council: Austin, TX, USA, 2007; p. 24. [Google Scholar]
- Rohdewald, P. A review of the French maritime pine bark extract (Pycnogenol), a herbal medication with a diverse clinical pharmacology. Int. J. Clin. Pharmacol. Ther. 2002, 40, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Shrikhande, A. Wine by-products with health benefits. Food Res. Int. 2000, 33, 469–474. [Google Scholar] [CrossRef]
- Rios, L.Y.; Bennett, R.N.; Lazarus, S.A.; Rémésy, C.; Scalbert, A.; Williamson, G. Cocoa procyanidins are stable during gastric transit in humans. Am. J. Clin. Nutr. 2002, 76, 1106–1110. [Google Scholar] [PubMed]
- Déprez, S.; Brezillon, C.; Rabot, S.; Philippe, C.; Mila, I.; Lapierre, C.; Scalbert, A. Polymeric proanthocyanidins are catabolized by human colonic microflora into low-molecular-weight phenolic acids. J. Nutr. 2000, 130, 2733–2738. [Google Scholar] [PubMed]
- Gonthier, M.P.; Donovan, J.L.; Texier, O.; Felgines, C.; Remesy, C.; Scalbert, A. Metabolism of dietary procyanidins in rats. Free Radic. Biol. Med. 2003, 35, 837–844. [Google Scholar] [CrossRef]
- Rios, L.Y.; Gonthier, M.P.; Rémésy, C.; Mila, I.; Lapierre, C.; Lazarus, S.A.; Williamson, G.; Scalbert, A. Chocolate intake increases urinary excretion of polyphenol-derived phenolic acids in healthy human subjects. Am. J. Clin. Nutr. 2003, 77, 912–918. [Google Scholar] [PubMed]
- Düweler, K.G.; Rohdewald, P. Urinary metabolites of french maritime pine bark extract in humans. Pharmazie 2000, 55, 364–368. [Google Scholar] [PubMed]
- Deprez, S.; Mila, I.; Huneau, J.F.; Tome, D.; Scalbert, A. Transport of proanthocyanidin dimer, trimer, and polymer across monolayers of human intestinal epithelial Caco-2 cells. Antioxid. Redox Signal. 2001, 3, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.; Schroeter, H.; Shenoy, B.; Srai, S.K.; Debnam, E.S.; Rice-Evans, C. Epicatechin is the primary bioavailable form of the procyanidin dimers B2 and B5 after transfer across the small intestine. Biochem. Biophys. Res. Commun. 2001, 285, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.; Osakabe, N.; Natsume, M.; Terao, J. Absorption and urinary excretion of procyanidin B2 [epicatechin-(4β-8)-epicatechin] in rats. Free Radic. Biol. Med. 2002, 33, 142–148. [Google Scholar] [CrossRef]
- Donovan, J.; Manach, C.; Rios, L.; Morand, C.; Scalbert, A.; Remesy, C. Procyanidins are not bioavailable in rats fed a single meal containing a grapeseed extract or the procyanidin dimer B3. Br. J. Nutr. 2002, 87, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Cahn, J.; Borzeix, M.G. Administration of procyanidolic oligomers in rats. Observed effects on changes in the permeability of the blood-brain barrier. Sem. Hop. 1983, 59, 2031–2034. [Google Scholar] [PubMed]
- Robert, A.M.; Tixier, J.M.; Robert, L.; Legeais, J.M.; Renard, G. Effect of procyanidolic oligomers on the permeability of the blood-brain barrier. Pathol. Biol. 2001, 49, 298–304. [Google Scholar] [CrossRef]
- Prasain, J.K.; Peng, N.; Dai, Y.; Moore, R.; Arabshahi, A.; Wilson, L.; Barnes, S.; Michael Wyss, J.; Kim, H.; Watts, R.L. Liquid chromatography tandem mass spectrometry identification of proanthocyanidins in rat plasma after oral administration of grape seed extract. Phytomedicine 2009, 16, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Gu, L. Occurrence and biological significance of proanthocyanidins in the american diet. Phytochemistry 2005, 66, 2264–2280. [Google Scholar] [CrossRef] [PubMed]
- Natella, F.; Belelli, F.; Gentili, V.; Ursini, F.; Scaccini, C. Grape seed proanthocyanidins prevent plasma postprandial oxidative stress in humans. J. Agric. Food Chem. 2002, 50, 7720–7725. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.J.; Chronopoulos, A.K.; Singh, I.; Francis, M.A.; Moriarty, H.; Pike, M.J.; Turner, A.H.; Mann, N.J.; Sinclair, A.J. Dietary flavanols and procyanidin oligomers from cocoa (Theobroma cacao) inhibit platelet function. Am. J. Clin. Nutr. 2003, 77, 1466–1473. [Google Scholar] [PubMed]
- Wan, Y.; Vinson, J.A.; Etherton, T.D.; Proch, J.; Lazarus, S.A.; Kris-Etherton, P.M. Effects of cocoa powder and dark chocolate on LDL oxidative susceptibility and prostaglandin concentrations in humans. Am. J. Clin. Nutr. 2001, 74, 596–602. [Google Scholar] [PubMed]
- Facino, R.M.; Carini, M.; Aldini, G.; Berti, F.; Rossoni, G.; Bombardelli, E.; Morazzoni, P. Diet enriched with procyanidins enhances antioxidant activity and reduces myocardial post-ischaemic damage in rats. Life Sci. 1999, 64, 627–642. [Google Scholar] [CrossRef]
- Pataki, T.; Bak, I.; Kovacs, P.; Bagchi, D.; Das, D.K.; Tosaki, A. Grape seed proanthocyanidins improved cardiac recovery during reperfusion after ischemia in isolated rat hearts. Am. J. Clin. Nutr. 2002, 75, 894–899. [Google Scholar] [PubMed]
- Sato, M.; Bagchi, D.; Tosaki, A.; Das, D.K. Grape seed proanthocyanidin reduces cardiomyocyte apoptosis by inhibiting ischemia/reperfusion-induced activation of JNK-1 and C-JUN. Free Radic. Biol. Med. 2001, 31, 729–737. [Google Scholar] [CrossRef]
- Ramirez, R.O.; Roa, C.C. The gastroprotective effect of tannins extracted from duhat (Syzygium cumini Skeels) bark on HCl/ethanol induced gastric mucosal injury in Sprague-Dawley rats. Clin. Hemorheol. Microcirc. 2003, 29, 253–261. [Google Scholar] [PubMed]
- Foo, L.Y.; Lu, Y.; Howell, A.B.; Vorsa, N. The structure of cranberry proanthocyanidins which inhibit adherence of uropathogenic P-fimbriated escherichia coli in vitro. Phytochemistry 2000, 54, 173–181. [Google Scholar] [CrossRef]
- Foo, L.Y.; Lu, Y.; Howell, A.B.; Vorsa, N. A-type proanthocyanidin trimers from cranberry that inhibit adherence of uropathogenic P-fimbriated escherichia coli. J. Nat. Prod. 2000, 63, 1225–1228. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Safdar, M.; Ali Khan, M.M.; Khattak, K.N.; Anderson, R.A. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care 2003, 26, 3215–3218. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, N.; Yamagishi, M.; Natsume, M.; Yasuda, A.; Osawa, T. Ingestion of proanthocyanidins derived from cacao inhibits diabetes-induced cataract formation in rats. Exp. Biol. Med. 2004, 229, 33–39. [Google Scholar]
- Kamitani, Y.; Maki, K.; Tofani, I.; Nishikawa, Y.; Tsukamoto, K.; Kimura, M. Effects of grape seed proanthocyanidins extract on mandibles in developing rats. Oral Dis. 2004, 10, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Deshane, J.; Chaves, L.; Sarikonda, K.V.; Isbell, S.; Wilson, L.; Kirk, M.; Grubbs, C.; Barnes, S.; Meleth, S.; Kim, H. Proteomics analysis of rat brain protein modulations by grape seed extract. J. Agric. Food. Chem. 2004, 52, 7872–7883. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, J.X.; Kong, L.D.; Yang, C.; Cheng, C.H.; Zhang, X. Administration of procyanidins from grape seeds reduces serum uric acid levels and decreases hepatic xanthine dehydrogenase/oxidase activities in oxonate-treated mice. Basic Clin. Pharmacol. Toxicol. 2004, 94, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Elmets, C.A.; Katiyar, S.K. Dietary feeding of proanthocyanidins from grape seeds prevents photocarcinogenesis in SKH-1 hairless mice: Relationship to decreased fat and lipid peroxidation. Carcinogenesis 2003, 24, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Singletary, K.W.; Meline, B. Effect of grape seed proanthocyanidins on colon aberrant crypts and breast tumors in a rat dual-organ tumor model. Nutr. Cancer 2001, 39, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Gali-Muhtasib, H.U.; Younes, I.H.; Karchesy, J.J.; El-Sabban, M.E. Plant tannins inhibit the induction of aberrant crypt foci and colonic tumors by 1,2-dimethylhydrazine in mice. Nutr. Cancer 2001, 39, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, M.; Natsume, M.; Osakabe, N.; Okazaki, K.; Furukawa, F.; Imazawa, T.; Nishikawa, A.; Hirose, M. Chemoprevention of lung carcinogenesis by cacao liquor proanthocyanidins in a male rat multi-organ carcinogenesis model. Cancer Lett. 2003, 191, 49–57. [Google Scholar] [CrossRef]
- Panickar, K.S.; Polansky, M.M.; Graves, D.J.; Urban, J.F.; Anderson, R.A. A procyanidin type a trimer from cinnamon extract attenuates glial cell swelling and the reduction in glutamate uptake following ischemia-like injury in vitro. Neuroscience 2012, 202, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, S.; Wolf, G.; Keilhoff, G.; Bagchi, D.; Horn, T. Protection of primary glial cells by grape seed proanthocyanidin extract against nitrosative/oxidative stress. Nitric Oxide 2001, 5, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Fujishita, K.; Ozawa, T.; Shibata, K.; Tanabe, S.; Sato, Y.; Hisamoto, M.; Okuda, T.; Koizumi, S. Grape seed extract acting on astrocytes reveals neuronal protection against oxidative stress via interleukin-6-mediated mechanisms. Cell. Mol. Neurobiol. 2009, 29, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, P.J.; Kurowska, E.M.; Freeman, D.J.; Chambers, A.F.; Koropatnick, J. In vivo inhibition of growth of human tumor lines by flavonoid fractions from cranberry extract. Nutr. Cancer 2006, 56, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.J.; Yang, J.Y.; Mou, Y.H.; Sun, B.S.; Ping, Y.F.; Wang, J.M.; Bian, X.W.; Wu, C.F. Inhibition of U-87 human glioblastoma cell proliferation and formyl peptide receptor function by oligomer procyanidins (F2) isolated from grape seeds. Chem. Biol. Interact. 2009, 179, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Yang, J.; Mou, Y.; Sun, B.; Wang, J.; Wang, F.; Wu, C. Oligomeric procyanidins induce generation of reactive oxygen species and collapse of mitochondrial membrane potential in glioblastoma cell lines. Chin. Herb. Med. 2009, 1, 45–52. [Google Scholar]
- Lotito, S.B.; Actis-Goretta, L.; Renart, M.L.; Caligiuri, M.; Rein, D.; Schmitz, H.H.; Steinberg, F.M.; Keen, C.L.; Fraga, C.G. Influence of oligomer chain length on the antioxidant activity of procyanidins. Biochem. Biophys. Res. Commun. 2000, 276, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Verstraeten, S.V.; Keen, C.L.; Schmitz, H.H.; Fraga, C.G.; Oteiza, P.I. Flavan-3-ols and procyanidins protect liposomes against lipid oxidation and disruption of the bilayer structure. Free Radic. Biol. Med. 2003, 34, 84–92. [Google Scholar] [CrossRef]
- Szegedi, A.; Kohnen, R.; Dienel, A.; Kieser, M. Acute treatment of moderate to severe depression with hypericum extract WS 5570 (St John’s wort): Randomised controlled double blind non-inferiority trial versus paroxetine. BMJ 2005, 330, 503. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.; Ferreres, F.; Malva, O.; Dias, A. Phytochemical and antioxidant characterization of Hypericum perforatum alcoholic extracts. Food Chem. 2005, 90, 157–167. [Google Scholar] [CrossRef]
- Fox, E.; Murphy, R.F.; McCully, C.L.; Adamson, P.C. Plasma pharmacokinetics and cerebrospinal fluid penetration of hypericin in nonhuman primates. Cancer Chemother. Pharmacol. 2001, 47, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.H.; Karas, M.; Müller, W.E.; Volmer, D.A.; Eckert, G.P.; Tawab, M.A.; Blume, H.H.; Dingermann, T.; Schubert-Zsilavecz, M. Determination of hyperforin in mouse brain by high-performance liquid chromatography/tandem mass spectrometry. Anal. Chem. 2003, 75, 6084–6088. [Google Scholar] [CrossRef] [PubMed]
- Paulke, A.; Schubert-Zsilavecz, M.; Wurglics, M. Determination of St. John’s wort flavonoid-metabolites in rat brain through high performance liquid chromatography coupled with fluorescence detection. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2006, 832, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Sovak, M.; Seligson, A.L.; Konas, M.; Hajduch, M.; Dolezal, M.; Machala, M.; Nagourney, R. Herbal composition PC-SPES for management of prostate cancer: Identification of active principles. J. Natl. Cancer Inst. 2002, 94, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Small, E.J.; Frohlich, M.W.; Bok, R.; Shinohara, K.; Grossfeld, G.; Rozenblat, Z.; Kelly, W.K.; Corry, M.; Reese, D.M. Prospective trial of the herbal supplement PC-SPES in patients with progressive prostate cancer. J. Clin. Oncol. 2000, 18, 3595–3603. [Google Scholar] [PubMed]
- Ikezoe, T.; Chen, S.S.; Heber, D.; Taguchi, H.; Koeffler, H.P. Baicalin is a major component of PC-SPES which inhibits the proliferation of human cancer cells via apoptosis and cell cycle arrest. Prostate 2001, 49, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Rafi, M.M.; Rosen, R.T.; Vassil, A.; Ho, C.T.; Zhang, H.; Ghai, G.; Lambert, G.; DiPaola, R.S. Modulation of bcl-2 and cytotoxicity by licochalcone-A, a novel estrogenic flavonoid. Anticancer Res. 2000, 20, 2653–2658. [Google Scholar] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidak, M.; Rozman, D.; Komel, R. Effects of Flavonoids from Food and Dietary Supplements on Glial and Glioblastoma Multiforme Cells. Molecules 2015, 20, 19406-19432. https://doi.org/10.3390/molecules201019406
Vidak M, Rozman D, Komel R. Effects of Flavonoids from Food and Dietary Supplements on Glial and Glioblastoma Multiforme Cells. Molecules. 2015; 20(10):19406-19432. https://doi.org/10.3390/molecules201019406
Chicago/Turabian StyleVidak, Marko, Damjana Rozman, and Radovan Komel. 2015. "Effects of Flavonoids from Food and Dietary Supplements on Glial and Glioblastoma Multiforme Cells" Molecules 20, no. 10: 19406-19432. https://doi.org/10.3390/molecules201019406
APA StyleVidak, M., Rozman, D., & Komel, R. (2015). Effects of Flavonoids from Food and Dietary Supplements on Glial and Glioblastoma Multiforme Cells. Molecules, 20(10), 19406-19432. https://doi.org/10.3390/molecules201019406





