Ursolic Acid—A Pentacyclic Triterpenoid with a Wide Spectrum of Pharmacological Activities
Abstract
:1. Introduction
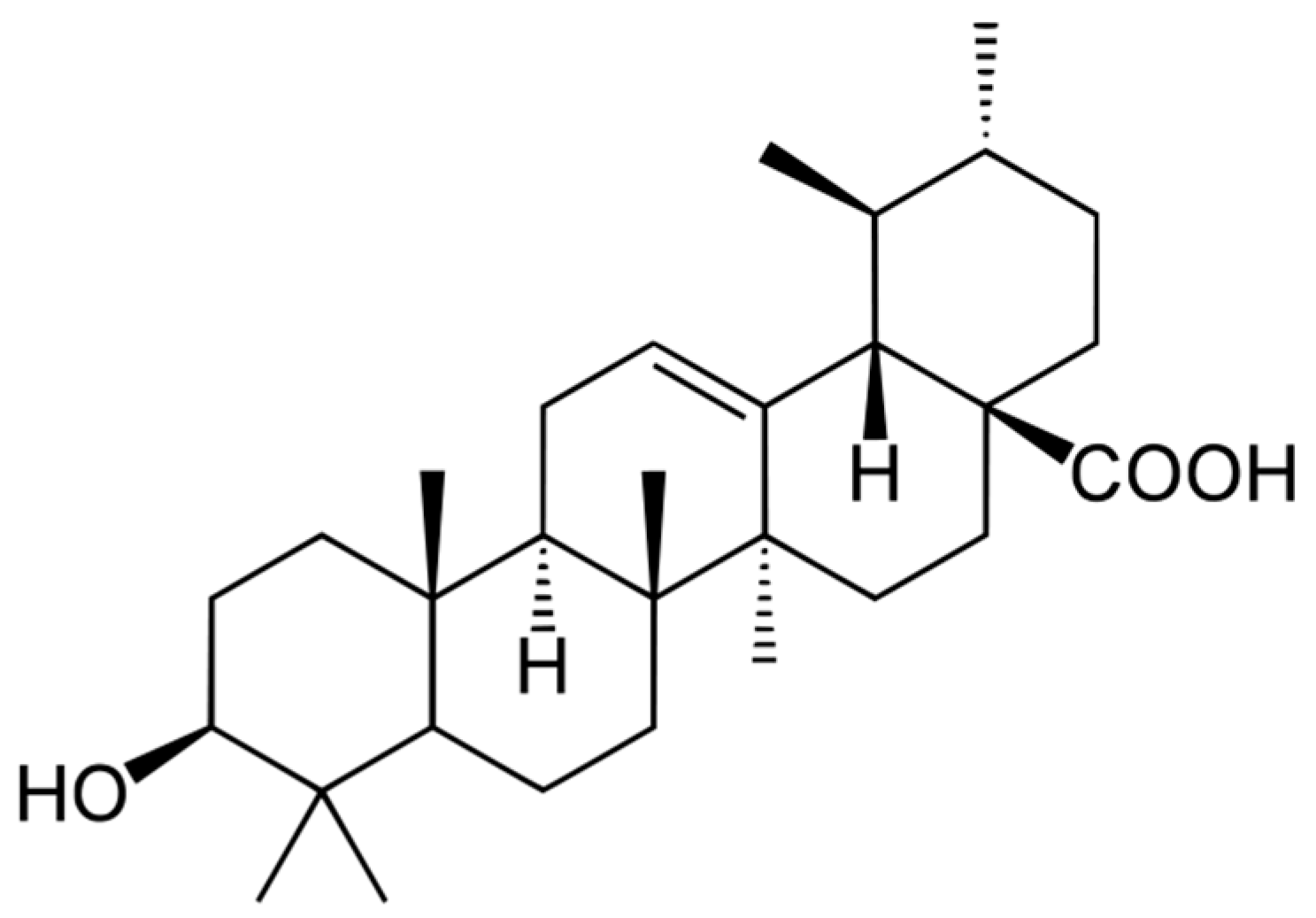
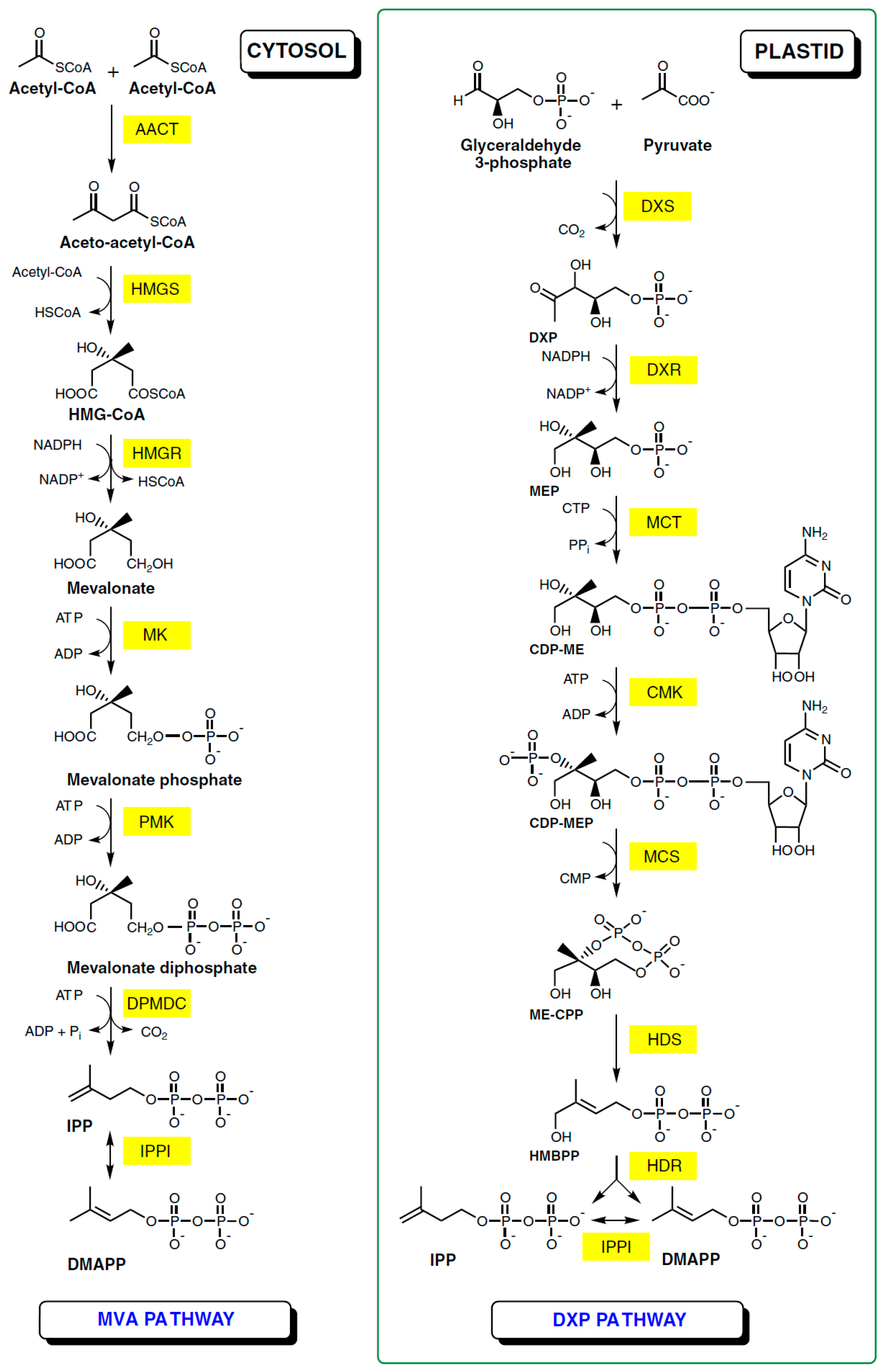
2. Natural Occurrence and Biosynthesis of Ursolic Acid
3. Ursolic Acid as a Tool in Cancer Prevention and Therapy
3.1. General Review of Literature Data on Ursolic Acid Anti-Cancer Activities
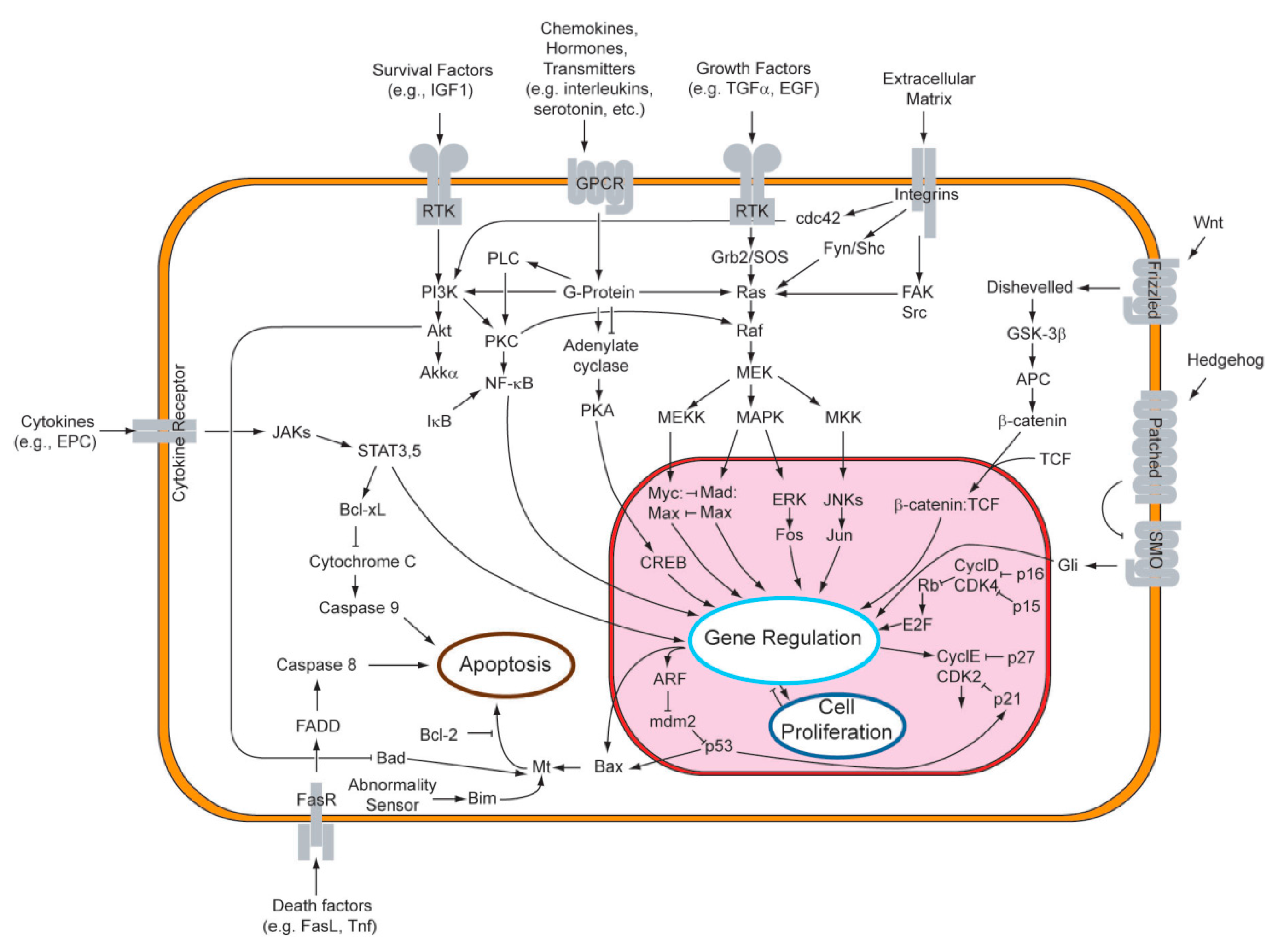
3.2. Cellular Signaling Pathways and Enzyme Inhibition—The Key to the Ursolic Acid Activity against Cancer
| Carcinoma Type | Model Used | Mechanism of Action |
|---|---|---|
| bladder cancer | cell lines (NTUB1 and T24) | |
| breast cancer | rodent model (mice) |
|
| cell lines (MCF-7, MCF-7/ADR and MDA-MB-231) | ||
| cervical cancer | cell lines (HeLa and SiHa) | |
| colorectal cancer | cell lines (Caco-2, CO115, CT26, DLD1, HCT15, HCT116, HT29, SW480 and SW620) | |
| fibrosarcoma | cell lines (HT1080) |
|
| gastric cancer | cell lines (AGS, BGC823, SGC7901 and SNU-484) |
|
| glioma | rodent model (rats) |
|
| cell lines (1321N1, U87 and U251) | ||
| hepatic cancer | rodent model (mice) | |
| cell lines (H22, Hep3B, HepG2 and Huh7) | ||
| melanoma | rodent model (mice) |
|
| cell lines (A375, B16F10 and M4Beu) | ||
| leukemia | cell lines (Jurkat, HL60, HL60/ADR, K562, K562/ADR, THP1 and U937) | |
| lung cancer | cell lines (A549, ASTC-a-1, Calu-6, H640 and H3255) | |
| lymphoma | cell lines (Daudi) |
|
| multiple myeloma | cell lines (U266, RPMI and 8226.MM1.S) |
|
| neuroblastoma | cell lines (IMR32 and SH-SY5Y) | |
| ovarian cancer | cell lines (CAOV and SK-OV-3) |
|
| pancreatic cancer | cell lines (AsPC-1, Capan-1, MIA, Paca-1 and PANC-2) | |
| prostate cancer | rodent model (mice) | |
| cell lines (DU145, LNCaP and PC3) |
| |
| thyroid cancer | cell lines (ARO) |
|
| Preventive Effect | Model Used | Mechanism of Action |
|---|---|---|
| anti-inflammatory | mouse primary splenocytes | inhibition of Th2 cytokines production [76] |
| activated T cells, B cells and macrophages; mice | suppression of NF-κB, AP-1 and NF-AT activity [77] | |
| rat edema tests | unclear, probably connected with glucocorticoids [78] | |
| mice | reductions of Th2 cytokines and ovalbumin-specific IgE production, and eosinophil infiltration via the Th2-GATA-3, STAT6, and IL-17-NF-κB pathways [79] | |
| human intestinal epithelial cells and peritoneal macrophages from mice | inhibition of production of pro-inflammatory cytokines, IκBα phosphorylation/degradation and NF-κB DNA binding activity [80] | |
| rat mast cells | inhibition of histamine release [81] | |
| murine peritoneal macrophages | suppression of NO production and iNOS expression via downregulation of NF-κB activation; attenuation of expression of COX-2 and the secretion of proinflammatory cytokines like TNF-α and IL-6 [82] | |
| PC12 cells | attenuation of H2O2 and MPP-induced release of IL-6 and TNF-α [83] | |
| oedema in mice | attenuation of inflammation [84] | |
| pleurisy in mice | reduction of leukocytes, interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) levels [85] | |
| phagocyte cells | inhibition of histamine release; inhibition of prostaglandins and leukotrienes production [86] | |
| arthritis-induced mice | alteration of Th1/Th2 cytokine production [87] | |
| Th17 cells | suppression of interleukin-17 production by antagonizing function of RORγt protein [88] | |
| biochemical assays | inhibition of cyclooxygenase-2 catalyzed prostaglandin biosynthesis [89] | |
| anti-oxidative | PC12 cells | attenuation of H2O2 and MPP-induced impairment in catalase and superoxide dismutase activity [83] |
| rat liver microsomes | protection against lipid peroxidation [90] | |
| RAW247 cells | inhibition of NO production [91] | |
| isolated rat heart mitochondria | decrease in H2O2 production in the mitochondria [92] | |
| human blood lymphocytes | normalization of antioxidant levels; reduction of lipid peroxidation [93] | |
| Caco-2 cells | protection of DNA against oxidative damage [94] | |
| chemical-induced cancer | mouse skin | inhibition of binding benzo(a)pyrene and 7,12-dimethylbenz(a)anthracene to DNA [95] |
| rats | suppression of preneoplastic lesions formation by 1,2-dimethylhydrazine [96] | |
| rats | inhibition of formation of aberrant crypt foci by azoxymethane [97] | |
| human bronchial epithelial cells and mice | inhibition of tobacco smoke extract-induced cell injury [98] | |
| radiation-induced cancer | mice | enhancement of hematopoietic system recovery [99] |
| ROS-induced cancer | murine T cells | inhibition of cell activation through modulation of NF-κB signaling [100] |
| rats | attenuation of hepatocellular carcinoma induction by diethylnitrosamine-induced reactive oxygen species [101] | |
| keranocite cell line and mice | skin cancer prevention; protection against hydrogen peroxide induced DNA damage [102] | |
| viral-induced cancer | Raji cell line and mice | inhibition of Epstein-Barr virus activation induced by 12-O-tetradecanoylphorbol-13-acetate (TPA) [103,104,105] |
3.2.1. Signaling Pathways
3.2.2. FOXM1 Transcription Factor
3.2.3. Apoptosis Regulating Proteins
3.2.4. Endogenous Reverse Transcriptase
3.2.5. Factors Involved in Metastasis and Angiogenesis
3.2.6. Cyclooxygenase-2 (COX-2)
3.3. Protection against Tumor-Inducing Agents
3.4. Ursolic Acid as a Drug—Clinical Trials
4. Impact of Ursolic Acid on Condition and Functioning of Body Organs
4.1. The Liver
4.2. The Heart
4.3. The Brain
4.4. Skeletal Muscles
4.5. Bones
4.6. Other Organs
5. Anti-Microbial Properties of Ursolic Acid
5.1. Anti-Bacterial Activity
| Species | References |
|---|---|
| Bacteria | |
| Aeromonas caveae | [171] |
| Bacillus cereus | [171,172] |
| Bacillus sphaericus | [173] |
| Bacillus subtilis | [173,176] |
| Enterococcus faecalis | [177] |
| Escherichia coli | [171,176,177] |
| Klebisiella pneumoniae | [171] |
| Listeria monocytogenes | [171,175,178] |
| Mycobacterium tuberculosis | [179,180,181] |
| Pseudomonas aeruginosa | [171,177] |
| Pseudomonas syrinagae | [173] |
| Ralstonia solanacearum | [175] |
| Shigella flexneri | [171] |
| Staphylococcus aureus | [171,176,177,182,183,184] |
| Staphylococcus epidermis | [175] |
| Streptococcus mutans | [185,186,187] |
| Streptococcus pneumoniae | [178,183] |
| Streptococcus sobrinus | [186] |
| Streptomyces scabies | [176] |
| Vibrio cholerae | [171] |
| Viruses | |
| Human immunodefiency virus | [188,189,190,191,192] |
| Hepatitis C virus | [189,193,194] |
| Herpes simplex virus | [195] |
| Protozoa | |
| Leishmania amazonensis | [196] |
| Plasmodium falciparum | [197,198,199,200] |
| Trypanosoma brucei rhodesiense | [197] |
| Trypanosoma cruzi | [201] |
| Fungi | |
| 11 species | [202] |
| Nematodes (Roundworms) | |
| Brugia malayi | [203] |
| Wuchereria bancrofti | [203] |
5.2. Anti-Viral Properties
5.3. Activity against other Microbes and Parasites
6. Conclusions
Conflicts of Interest
References
- Laszczyk, M.N. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 2009, 75, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Jesus, J.A.; Lago, J.H.G.; Laurenti, M.D.; Yamamoto, E.S.; Passero, L.F. Antimicrobial activity of oleanolic and ursolic acid: An update. Evid. Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.H.; Sethi, G.; Bishayee, A. Ursolic acid in cancer prevention and treatment: Molecular targets, pharmacokinetics and clinical studies. Biochem. Pharmacol. 2013, 85, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Ovesná, Z.; Vachálková, A.; Horváthová, K.; Tóthová, D. Pentacyclic triterpenoic acids: New chemoprotective compounds. Minireview. Neoplasma 2004, 51, 327–333. [Google Scholar] [PubMed]
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Urban, M.; Sarek, J. Pharmacological activities if natural triterpenoids and their therapeutic implications. Nat. Prod. Res. 2006, 23, 394–411. [Google Scholar] [CrossRef]
- Liu, J. Oleanolic and ursolic acid: Research perspectives. J. Ethnopharmacol. 2005, 100, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Pharmacology of oleanolic and ursolic acid. J. Ethnopharmacol. 1995, 49, 57–68. [Google Scholar] [CrossRef]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants—Rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [PubMed]
- Szakiel, A.; Pączkowski, C.; Pensec, F.; Bertsch, C. Fruit cuticular waxes as a source of biologically active triterpenoids. Phytochem. Rev. 2012, 11, 263–284. [Google Scholar] [CrossRef] [PubMed]
- Świeżewska, E.; Danikiewicz, W. Polyisoprenoids: Structure, biosynthesis and function. Prog. Lip. Res. 2005, 44, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Hemmerlin, A.; Harwood, J.L.; Bach, T.J. A raison d’être for two distinct pathways in the early steps of plant isoprenoid biosynthesis? Prog. Lip. Res. 2012, 51, 95–148. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, F.; Rahier, A.; Camara, B. Biogenesis, molecular regulation and function of plant isoprenoids. Prog. Lip. Res. 2005, 44, 357–429. [Google Scholar] [CrossRef] [PubMed]
- Thimmappa, R.; Geisler, K.; Louveau, T.; O’Maille, P.; Osbourn, A. Triterpene biosynthesis in plants. Annu. Rev. Plant Biol. 2015, 65, 225–257. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.Y.; Li, P.P.; Jin, F.S.; Yao, C.; Zhang, G.H.; Zang, T.; Ai, X. Ursolic acid induces ER stress response to activate ASK1-JNK signaling and induce apoptosis in human bladder cancer T24 cells. Cell Signal. 2013, 25, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.Y.; Huang, A.M.; Wei, B.L.; Gan, K.H.; Hour, T.C.; Yang, S.C.; Pu, Y.S.; Lin, C.N. Ursolic acid derivatives induce cell cycle arrest and apoptosis in NTUB1 cells associated with reactive oxygen species. Bioorg. Med. Chem. 2009, 17, 7265–7274. [Google Scholar] [CrossRef] [PubMed]
- De Angel, R.E.; Smith, S.M.; Glickman, R.D.; Perkins, S.N.; Hursting, S.D. Antitumor effects of ursolic acid in a mouse model of postmenopausal breast cancer. Nutr. Cancer 2010, 62, 1074–1086. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.Z.; Xuan, Y.Y.; Ruan, S.Q.; Sun, M. Proliferation-inhibiting and apoptosis-inducing effects of ursolic acid and oleanolic acid on multi-drug resistance cancer cells in vitro. Chin. J. Integr. Med. 2011, 17, 607–611. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, R.L. Triterpenoids isolated from apple peels have potent antiproliferative activity and may be partially responsible for apple’s anticancer activity. J. Agric. Food Chem. 2007, 55, 4366–4370. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.T.; Wu, C.H.; Yen, G.C. Ursolic acid, a naturally occurring triterpenoid, suppresses migration and invasion of human breast cancer cells by modulating C-Jun N-terminal kinase, Akt and mammalian target of rapamycin signaling. Mol. Nutr. Food Res. 2010, 54, 1285–1295. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Seo, H.S.; Choi, H.S.; Choi, I.; Shin, Y.C.; Ko, S.G. Induction of apoptotic cell death by ursolic acid through mitochondrial death pathway and extrinsic death receptor pathway in MDA-MB-231 cells. Arch. Pharm. Res. 2011, 34, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Ren, T.N.; Xi, T. Ursolic acid induces apoptosis by suppressing the expression of in MCF-7 human breast cancer cells. Med. Oncol. 2012, 29, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.M.; Cai, S.Q.; Cui, J.R.; Wang, R.Q.; Tu, P.F.; Hattori, M.; Daneshtalab, M. The cytotoxic activity of ursolic acid derivatives. Eur. J. Med. Chem. 2005, 40, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Mallavadhani, U.V.; Mahapatra, A.; Pattnaik, B.; Vanga, N.; Suri, N.; Saxena, A.K. Synthesis and anti-cancer activity of some novel C-17 analogs of ursolic and oleanolic acids. Med. Chem. Res. 2013, 22, 1263–1269. [Google Scholar] [CrossRef]
- Shao, J.W.; Dai, Y.C.; Xue, J.P.; Wang, J.C.; Lin, F.P.; Guo, Y.H. In vitro and in vivo anticancer activity evaluation of ursolic acid derivatives. Eur. J. Med. Chem. 2011, 46, 2652–2661. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, X.; Qi, H.; Li, X.; Xiao, X.; Gao, J. Ursolic acid induces apoptosis trough mitochondrial intrinsic pathway and suppression of ERK1/2 MAPK in HeLa cells. J. Pharmacol. Sci. 2014, 125, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xing, D.; Chen, Q.; Chen, W.R. Enhancement of chemotherapeutic agent-induced apoptosis by inhibition of NF-κB using ursolic acid. Int. J. Cancer 2010, 127, 462–473. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Kasoju, N.; Bora, U.; Chaturvedi, R. Accumulation of betulinic, oleanolic and ursolic acids in In vitro cell cultures of Lantana camara L. and their significant cytotoxic effects on HeLa cell lines. Biotechnol. Bioprocess Eng. 2010, 15, 1038–1046. [Google Scholar] [CrossRef]
- Xavier, C.P.; Lima, C.F.; Preto, A.; Seruca, R.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Luteolin, quercetin and ursolic acid are potent inhibitors of proliferation and inducers of apoptosis in both KRAS and BRAF mutated human colorectal cancer cells. Cancer Lett. 2009, 281, 162–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Kim, Y.H.; Song, G.Y.; Kim, D.E.; Jeong, Y.J.; Liu, K.H.; Chung, Y.H.; Oh, S. Ursolic acid and its natural derivative corosolic acid suppress the proliferation of APC-mutated colon cancer cells through promotion of β-catenin degradation. Food Chem. Toxicol. 2014, 67, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Yadav, V.; Sung, B.; Reuter, S.; Kannappan, R.; Deorukhkar, A.; Diagaradjane, P.; Wei, C.; Baladandayuthapani, V.; Krishnan, S.; et al. Ursolic acid inhibits growth and metastasis of human colorectal cancer in an orthotopic nude mouse model by targeting multiple cell signaling pathways: Chemosensitization with capecitabine. Clin. Cancer Res. 2012, 18, 4942–4953. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.; Yadav, V.R.; Kannappan, R.; Aggarwal, B.B. Ursolic acid, a pentacyclic triterpene, potentiates TRAIL-induced apoptosis through p53-independent upregulation of death receptors: Evidence for the role of reactive oxygen species and JNK. J. Biol. Chem. 2011, 286, 5546–5557. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.; Kim, M.M. Ursolic acid induces apoptosis of SW480 cells via p53 activation. Food Chem. Toxicol. 2013, 62, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Xavier, C.P.; Lima, C.F.; Pedro, D.F.; Wilson, J.M.; Kristiansen, K.; Pereira-Wilson, C. Ursolic acid induces cell death and modulates autophagy through JNK pathway in apoptosis-resistant colorectal cancer cells. J. Nutr. Biochem. 2013, 24, 706–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limami, Y.; Pinon, A.; Leger, D.Y.; Pinault, E.; Delage, C.; Beneytout, J.L.; Simon, A.; Liarge, B. The P2Y2/Src/p38/COX-2 pathway is involved in the resistance to ursolic acid-induced apoptosis in colorectal and prostate cancer cells. Biochimie 2012, 94, 1754–1763. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.J.; Tak, J.K.; Kim, S.T.; Nam, W.S.; Kim, S.Y.; Park, K.M.; Park, J.W. Sensitization of ionizing radiation-induced apoptosis by ursolic acid. Free Radic. Res. 2012, 46, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Bai, K.K.; Yu, Z.; Chen, F.L.; Li, F.; Li, W.Y.; Guo, Y.H. Synthesis and evaluation of ursolic acid derivatives as potent cytotoxic agents. Bioorg. Med. Chem. Lett. 2012, 7, 2488–2493. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.J.; Bae, S.K.; Lee, H.Y.; Lee, O.H.; Sato, H.; Seiki, M.; Park, B.C.; Kim, K.W. Anti-invasive activity of ursolic acid correlates with the reduced expression of matrix metalloproteinase-9 (MMP-9) in HT1080 human fibrosarcoma cells. Cancer Res. 1996, 56, 2281–2284. [Google Scholar] [PubMed]
- Kim, E.S.; Moon, A. Ursolic acid inhibits the invasive phenotype of SNU-484 human gastric cancer cells. Oncol. Lett. 2015, 9, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, F.; Yang, L.; Mei, Y.; Long, H.; Zhang, X.; Zhang, J.; Qimuge-Suyila; Su, X. Ursolic acid inhibits proliferation and induces apoptosis of cancer cells in vitro and in vivo. J. Biomed. Biotechnol. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, X.; Ding, J.; Xu, H.; Dai, X.; Hou, Z.; Zhang, K.; Sun, K.; Sun, W. Delivery of ursolic acid (UA) in polymeric nanoparticles effectively promotes the apoptosis of gastric cancer cells through enhanced inhibition of cyclooxygenase 2 (COX-2). Int. J. Pharm. 2013, 441, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Huang, C.Y.; Lin-Shiau, S.Y.; Lin, J.K. Ursolic acid inhibits IL-2 beta or TF-alpha-induced C6 glioma invasion through suppressing the association ZIP/p62 with PKC-zeta and downregulating the MMP-9 expression. Mol. Carcinog. 2009, 48, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Wang, X.; Jiang, C. Ursolic acid inhibits proliferation and induces apoptosis in human glioblastoma cell lines U251 by suppressing TGFβ-1/miR-21/PDCD4 pathway. Basic Clin. Pharmacol. Toxicol. 2012, 111, 106–112. [Google Scholar] [PubMed]
- Bonaccorsi, I.; Altieri, F.; Sciamanna, I.; Oricchio, E.; Grillo, C.; Contartese, G.; Galati, E.M. Endogenous reverse transcriptase as a mediator of ursolic acid’s anti-proliferative and differentiating effects in human cancer cell lines. Cancer Lett. 2008, 263, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Carvalho, J.; Ibeas, E.; Hernández, M.; Ruiz-Guttierez, V.; Nieto, M.L. Acidic triterpenes compromise growth and survival of astrocytoma cell lines by regulating reactive oxygen species accumulation. Cancer Res. 2007, 67, 3741–3751. [Google Scholar] [CrossRef] [PubMed]
- Shih, W.L.; Yu, F.L.; Chang, C.D.; Liao, M.H.; Wu, H.Y.; Lin, P.Y. Suppression of AMF/PGI-mediated tumorigenic activities by ursolic acid in cultured hepatoma cells and in a mouse model. Mol. Carcinog. 2013, 52, 800–812. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Lin, G.; Zheng, R.X.; Huang, F.; Yang, M.S.; Xiao, P.G. Anti-hepatoma activity and mechanism of ursolic acid and its derivatives isolated from Aralia decaisneana. World J. Gastroenterol. 2006, 12, 874–879. [Google Scholar] [PubMed]
- Liu, L.; Zhang, J.; Li, M.; Zhang, X.; Zhang, J.; Li, Z.; Wang, L.; Wu, J.; Luo, C. Inhibition of HepG2 cell proliferation by ursolic acid and polysaccharides via the downregulation of cyclooxygenase-2. Mol. Med. Rep. 2014, 9, 2505–2511. [Google Scholar] [CrossRef] [PubMed]
- Shyu, M.H.; Kao, T.C.; Yen, G.C. Oleanolic acid and ursolic acid induce apoptosis in HuH7 human hepatocellular carcinoma cells through a mitochondrial-dependent pathway and downregulation of XIAP. J. Agric. Food Chem. 2010, 58, 6110–6118. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Lu, Y.H.; Xie, J.H.; Wang, F.; Zou, J.N.; Yang, J.S.; Xing, Y.Y.; Xi, T. Downregulation of survivin and activation of caspase-3 through the PI3K/Akt pathway in ursolic acid-induced HepG2 cell apoptosis. Anti-Cancer Drugs 2009, 20, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Huang, C.Y.; Mong, M.C.; Chan, C.Y.; Yin, M.C. Antiangiogenic potential of three triterpenic acids in human liver cancer cells. J. Agric. Food Chem. 2011, 59, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Kanjoormana, M.; Kuttan, G. Antiangiogenic activity of ursolic acid. Integr. Cancer Ther. 2010, 9, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Harmand, P.O.; Duval, R.; Delage, C.; Simon, A. Ursolic acid induces apoptosis through mitochondrial intrinsic pathway and caspase-3 activation in M4Beu melanoma cells. J. Cancer 2005, 114, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Duval, R.E.; Harmand, P.O.; Jayat-Vignoles, C.; Cook-Moreau, J.; Pinon, A.; Delage, C.; Simon, A. Differential involvement of mitochondria during ursolic acid-induced apoptotic process in HaCaT and M4Beu cells. Oncol. Rep. 2008, 19, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Manu, K.A.; Kutan, G. Ursolic acid induces apoptosis by activating p53 and caspase-3 gene expressions and suppressing NF-kappaB mediated activation of bcl-2 in B16F-10 melanoma cells. Int. Immunopharmacol. 2008, 8, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhou, T.; Liu, L.; Chen, J.; Zhao, Z.; Peng, Y.; Li, P.; Gao, N. Ezrin dephosphorylation/downregulation contributes to ursolic acid-mediated cell death in human leukemia cells. Blood Cancer J. 2013, 3, e108. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wang, X.; Chi, Z.F.; Hu, R.; Zhang, R.; Yang, W.; Liu, Z.G. Ursolic acid-induced apoptosis in K562 cells involving upregulation of PTEN gene expression and inactivation of the PI3K/Akt pathway. Arch. Pharm. Res. 2012, 35, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Cheng, S.; Budhjara, A.; Gao, Z.; Chen, J.; Liu, E.H.; Huang, C.; Chen, D.; Yang, Z.; Liu, Q.; et al. Ursolic acid induces apoptosis in human leukaemia cells and inhibits anti-leukaemic activity in nude mice through the PKB pathway. Br. J. Pharmacol. 2012, 165, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.H.; Lee, Y.S.; Kang, C.M.; Kim, J.A.; Kwon, K.S.; Son, H.C.; Kim, K.W. Intracellular Ca2+ release mediates ursolic acid-induced apoptosis in human leukemic HL-60 cells. Int. J. Cancer 1997, 73, 725–728. [Google Scholar] [CrossRef]
- Zhang, T.; He, Y.M.; Wang, J.S.; Shen, J.; Xing, Y.Y.; Xi, T. Ursolic acid induces HL60 monocytic differentiation and upregulates C/EBPβ expression by ERK pathway activation. Anticancer Drugs 2011, 22, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Lin, C.Y.; Tsai, C.W.; Yin, M.C. Inhibition of cell proliferation, invasion and migration by ursolic acid in human lung cancer cell lines. Toxicol. Vitro 2011, 25, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Guo, L.; Miao, L.; Bao, W.; Yang, J.; Li, X.; Xi, T.; Zhao, W. Ursolic acid inhibits epithelial-mesenchymal transition by suppressing the expression of astrocyte-elevated gene-1 in human nonsmall cell lung cancer A549 cells. Anticancer Drugs 2013, 24, 494–503. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.Y.; Leung, H.W.C.; Yang, W.H.; Chen, W.H.; Lee, H.Z. Upregulation of matrix metalloproteinase family gene involvement in ursolic acid induced human lung non-small carcinoma cell apoptosis. Anticancer Res. 2007, 27, 145–153. [Google Scholar] [PubMed]
- Lauthier, F.; Taillet, L.; Trouillas, P.; Delage, C.; Simon, A. Ursolic acid triggers calcium-dependent apoptosis in human Daudi cells. Anticancer Drugs 2000, 11, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Pathak, A.K.; Bhutani, M.; Nair, A.S.; Ahn, K.S.; Chakraborty, A.; Kadara, H.; Guha, S.; Sethi, G.; Aggarwal, B.B. Ursolic acid inhibits STAT3 activation pathway leading to suppression of proliferation and chemosensitization of human myeloma cells. Mol. Cancer Res. 2007, 5, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, L.; Wang, B.; Xiang, J. Effects of ursolic acid on the proliferation and apoptosis of human ovarian cancer cells. J. Huazhong Univ. Sci. Technol. Med. Sci. 2009, 29, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Jeong, S.J.; Kwon, H.Y.; Kim, B.; Sim, S.H.; Yoo, D.Y. Ursolic acid from Oldenlandia diffusa induces apoptosis via activation of caspases and phosphorylation of glycogen synthase kinase 3 beta in SK-OV-3 ovarian cancer cells. Biol. Pharm. Bull. 2012, 35, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.S.; Wang, R.; Salvador, J.A.; Jing, Y. Synthesis of novel ursolic acid heterocyclic derivatives with improved abilities of antiproliferation and induction of p53, p21waf1 and NOXA in pancreatic cancer cells. Bioorg. Med. Chem. 2012, 20, 5774–5786. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liang, X.; Yang, X. Ursolic acid inhibits growth and induces apoptosis in gemcitabine-resistant human pancreatic cancer via the JNK and PI3K/Akt/NF-κB pathways. Oncol. Rep. 2012, 28, 501–510. [Google Scholar] [PubMed]
- Shanmugam, M.K.; Manu, K.A.; Ong, T.H.; Ramachandran, L.; Surana, R.; Bist, P.; Lim, L.H.K.; Kumar, A.P.; Hui, K.M.; Sethi, G. Inhibition of CXCR4/CXCL12 signaling axis by ursolic acid leads to suppression of metastasis in transgenic adenocarcinoma of mouse prostate model. Int. J. Cancer 2011, 129, 1552–1563. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Ong, T.H.; Kumar, A.P.; Lun, C.K.; Ho, P.C.; Wong, P.T.; Hui, K.M.; Sethi, G. Ursolic acid inhibits the initiation, progression of prostate cancer and prolongs the survival of TRAMP mice by modulating pro-inflammatory pathways. PLoS ONE 2012, 7, e32476. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Baek, J.H.; Yoo, M.A.; Chung, H.Y.; Kim, N.D.; Kim, K.W. Induction of apoptosis by ursolic acid through activation of caspases and down-regulation of c-IAPs in human prostate epithelial cells. Int. J. Oncol. 2000, 17, 565–636. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.W.; Kim, S.Y.; Park, J.W. Autophagy inhibition enhances ursolic acid-induced apoptosis in PC3 cells. Biochim. Biophys. Acta 2012, 1823, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kong, C.; Zeng, Y.; Wang, L.; Li, Z.; Wang, H.; Xu, C.; Sun, Y. Ursolic acid induces PC-3 cell apoptosis via activation of JNK and inhibition of Akt pathways in vitro. Mol. Carcinogen. 2010, 49, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kong, C.; Wang, L.; Li, J.; Liu, X.; Xu, B.; Xu, C.; Sun, Y. Ursolic acid overcomes Bcl-2-mediated resistance to apoptosis in prostate cancer cells involving activation of JNK-induced Bcl-2 phosphorylation and degradation. J. Cell Biochem. 2010, 109, 764–773. [Google Scholar] [CrossRef] [PubMed]
- Kassi, E.; Papoutsi, Z.; Pratsinis, H.; Aligiannis, N.; Manoussakis, M.; Moutsatsou, P. Ursolic acid, a naturally occurring triterpenoid, demonstrates anticancer activity on human prostate cancer cells. J. Cancer Res. Clin. Oncol. 2007, 133, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Ku, C.M.; Lin, J.Y. Anti-inflammatory effect of 27 selected terpenoid compounds tested through modulating Th1/Th2 cytokine secretion profiles using murine primary splenocytes. Food Chem. 2013, 141, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Checker, R.; Sandur, S.K.; Sharma, D.; Patwardhan, R.S.; Jayakumar, S.; Kohli, V.; Sethi, G.; Aggarwal, B.B.; Sainis, K.B. Potent anti-inflammatory activity of ursolic acid, a triterpenoid antioxidant, is mediated through suppression of NF-κB, AP-1 and NF-AT. PLoS ONE 2012, 7, e31318. [Google Scholar] [CrossRef] [PubMed]
- Recio, M.C.; Giner, R.M.; Máñez, S.; Gueho, J.; Julien, H.R.; Hostettmann, K.; Rios, J.L. Investigations on the steroidal anti-inflammatory activity of triterpenoids from Diospyros leucomelas. Planta Med. 1995, 61, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Hong, J.H.; Lee, J.C. Ursolic acid, a potential PPARγ agonist, suppresses ovalbumin-induced airway inflammation and Penh by down-regulating IL-5, IL-13 and IL-17 in a mouse model of allergic asthma. Eur. J. Pharmacol. 2013, 701, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Lee, C.; Hwang, S.W.; Im, J.P.; Kim, J.S. Ursolic acid inhibits nuclear factor-κB signaling in intestinal epithelial cells and macrophages, and attenuates experimental colitis in mice. Life Sci. 2014, 110, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Tsuruga, T.; Chun, Y.T.; Ebizuka, Y.; Sankawa, U. Biologically active constituents of Melaleuca leucadendron: Inhibitors of induced histamine release from rat mast cells. Chem. Pharm. Bull. 1991, 39, 3276–3278. [Google Scholar] [CrossRef] [PubMed]
- Cha, D.S.; Eun, J.S.; Jeon, H. Anti-inflammatory and antinociceptive properties of the leaves of Eriobotrya japonica. J. Ethnopharmacol. 2011, 134, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.J.; Yin, M.C. Antioxidative and anti-inflammatory protection of oleanolic acid and ursolic acid in PC12 cells. J. Food Sci. 2008, 73, H174–H178. [Google Scholar] [CrossRef] [PubMed]
- Baricevic, D.; Sosa, S.; Della Loggia, R.; Tubaro, A.; Simonovska, B.; Krasna, A.; Zupancic, A. Topical anti-inflammatory activity of Salvia officinalis L. leaves: The relevance of ursolic acid. J. Ethnopharmacol. 2001, 75, 125–132. [Google Scholar] [CrossRef]
- Benincá, J.P.; Dalmarco, J.B.; Pizzolatti, M.G.; Fröde, T.S. Analysis of the anti-inflammatory properties of Rosmarinus officinalis L. in mice. Food Chem. 2011, 124, 468–475. [Google Scholar] [CrossRef]
- Santos Rosa, C.; Garcia Gimenez, M.D.; Saenz Rodriguez, M.T.; de la Puerta Vazquez, R. Antihistaminic and antieicosanoid effects of oleanolic and ursolic acid fraction from Helichrysum picardii. Int. J. Pharm. Sci. 2007, 62, 459–462. [Google Scholar]
- Ahmad, S.F.; Khan, B.; Bani, S.; Suri, K.A.; Satti, N.K.; Qazi, G.N. Amelioration of adjuvant-induced arthritis by ursolic acid through altered Th1/Th2 cytokine production. Pharmacol. Res. 2006, 53, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wang, X.; Zhong, B.; Nurieva, R.I.; Ding, S.; Dong, C. Ursolic acid suppresses interleukin-17 (Il-17) production by selectively antagonizing the function of RORγt protein. J. Biol. Chem. 2011, 286, 22707–22710. [Google Scholar] [CrossRef] [PubMed]
- Ringbom, T.; Segura, L.; Noreen, Y.; Perera, P.; Bohlin, L. Ursolic acid from Plantago major, a selective inhibitor of cyclooxygenase-2 catalyzed prostaglandin biosynthesis. J. Nat. Prod. 1998, 61, 1212–1215. [Google Scholar] [CrossRef] [PubMed]
- Balanehru, S.; Nagarajan, B. Protective effect of oleanolic and ursolic acid against lipid peroxidation. Biochem. Int. 1991, 24, 981–990. [Google Scholar] [PubMed]
- Kwon, T.H.; Lee, B.; Chung, S.H.; Kim, D.H.; Lee, Y.S. Synthesis and NO production inhibitory activities of ursolic acid and oleanolic acid derivatives. Bull. Korean Chem. Soc. 2009, 30, 119–123. [Google Scholar] [CrossRef]
- Liobikas, J.; Majiene, D.; Trumbeckaite, S.; Kursvietiene, L.; Masteikova, R.; Kopustinskien, D.M.; Savickas, A.; Bernatoniene, J. Uncoupling and antioxidant effect of ursolic acid in isolated rat heart mitochondria. J. Nat. Prod. 2011, 74, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, S.; Rajendra Prasad, N.; Pugalendi, K.V.; Menon, V.P. Modulation of UVB-induced oxidative stress by ursolic acid in human blood lymphocytes. Asian J. Biochem. 2008, 3, 11–18. [Google Scholar] [CrossRef]
- Ramos, A.A.; Pereira-Wilson, C.; Collins, A.R. Protective effects of ursolic acid and luteolin against oxidative DNA damage include enhancement of DNA repair in Caco-2 cells. Mutat. Res. 2010, 692, 6–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, M.T.; Ho, C.T.; Wang, Z.Y.; Ferraro, T.; Lou, Y.R.; Stauber, K.; Ma, W.; Georgiadis, C.; Laskin, J.D.; Conney, A.H. Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Res. 1994, 54, 701–708. [Google Scholar] [PubMed]
- Furtado, R.A.; Rodrigues, É.P.; Araújo, F.R.R.; Oliveira, W.L.; Furtado, M.A.; Castro, M.B.; Cunha, W.R.; Tavares, D.C. Ursolic acid and oleanolic acid suppress preneoplastic lesions induced by 1,2-dimethylhydrazine in rat colon. Toxicol. Pathol. 2008, 36, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.; Cheng, Y.; Duan, R.D. Ursolic acid inhibits the formation of aberrant crypt foci and affects colonic sphingomyelin hydrolyzing enzymes in azoxymethane–treated rats. J. Cancer Res. Clin. Oncol. 2008, 134, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tan, X.; Shu, L.; Song, J.; Jin, P.; Yu, S.; Sun, M.; Jia, X. Ursolic acid inhibits cigarette smoke extract-induced human bronchial epithelial cell injury and prevents development of lung cancer. Molecules 2012, 17, 9104–9115. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Yang, J.J.; Lin, C.C. Effects of oleanolic and ursolic acid on inhibiting tumor growth and enhancing the recovery of hematopoietic system postirradiation in mice. Cancer Lett. 1997, 111, 7–13. [Google Scholar] [CrossRef]
- Zeng, G.; Chen, J.; Liang, Q.H.; You, W.H.; Wu, H.J.; Xiong, X.G. Ursolic acid inhibits T-cell activation through modulating nuclear factor-κB signaling. Chin. J. Integr. Med. 2012, 18, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Gayathri, R.; Priya, D.K.; Gunassekaran, G.R.; Sathisekaran, D. Ursolic acid attenuates oxidative stress-mediated hepatocellular carcinoma induction by diethylnitrosamine in male Wistar rats. Asian Pac. J. Cancer Prev. 2009, 10, 933–938. [Google Scholar] [PubMed]
- Kowalczyk, M.C.; Walaszek, Z.; Kowalczyk, P.; Kinjo, T.; Hanausek, M.; Slaga, T.J. Differential effect of several phytochemicals and their derivatives on murine keratinocytes in vitro and in vivo: Implications for skin cancer prevention. Carcinogenesis 2009, 30, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Ohigashi, H.; Takamura, H.; Koshimizu, K.; Tokuda, H.; Ito, Y. Search of possible antitumor promoters by inhibition of 12-O-tetradecanoyphorbol-13-acetate-induced Epstein-Barr virus activation. Ursolic acid and oleanolic acid from an anti-inflammatory Chinese medicinal plant, Glechoma hederaceae L. Cancer Lett. 1986, 30, 143–151. [Google Scholar] [CrossRef]
- Ito, H.; Kobayashi, E.; Li, S.; Hatano, T.; Sugita, D.; Kubo, N.; Shimura, S.; Itoh, Y.; Tokuda, H.; Nishino, H. Antitumor activity of compounds isolated from leaves of Eriobotrya japonica. J. Agric. Food Chem. 2002, 50, 2400–2403. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, H.; Ohigashi, H.; Koshimizu, K.; Ito, Y. Inhibitory effects of ursolic and oleanolic acid on skin tumor promotion by 12-O-tetradecanoyphorbol-13-acetate. Cancer Lett. 1986, 33, 279–285. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Steelman, L.S.; Abrams, S.L.; Lee, J.T.; Chang, F.; Bertrand, F.E.; Navolanic, P.M.; Terrian, D.M.; Franklin, R.A.; D’Assoro, A.B.; et al. Roles of the RAF /MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv. Enzym. Regul. 2006, 46, 249–279. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, T.D. Introduction to NF-κB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef] [PubMed]
- Gemenetzidis, E.; Bose, A.; Riaz, A.M.; Chaplin, T.; Young, B.D.; Ali, M.; Sudgen, D.; Thurlow, J.K.; Cheong, S.C.; Teo, S.H.; et al. FOXM1 upregulation is an early event in human squamous cell carcinoma and it is enhanced by nicotine during malignant transformation. PLoS ONE 2009, 4, e4849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Ahmad, A.; Li, Y.; Benerjee, S.; Kong, D.; Sarkat, F.H. Forkhead box M1 transcription factor: A novel target for cancer therapy. Cancer Ther. Rev. 2010, 36, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Chao, D.T.; Korsmeyer, S.J. BCL-2 family: Regulators of cell death. Annu. Rev. Immunol. 1998, 16, 395–419. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.D.; Orrenius, S.; Zhivotovsky, B. Review: Nuclear events in apoptosis. J. Struct. Biol. 2000, 129, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Nachmias, B.; Ashhab, Y.; Ben-Yehuda, D. The inhibitor of apoptosis protein family (IAPs): An emerging therapeutic target in cancer. Semin. Cancer Biol. 2004, 14, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Sciamanna, I.; Landriscina, M.; Pittoggi, C.; Quirino, M.; Mearelli, C.; Beraldi, R.; Mattei, E.; Serafino, A.; Cassano, A.; Sinibaldi-Vallebona, P.; et al. Inhibition of endogenous reverse transcriptase antagonizes human tumor growth. Oncogene 2005, 24, 3923–3931. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, C.; Quesada, A.R.; Medina, M.A. Effects of ursolic acid on different steps of the angiogenic process. Biochem. Biophys. Res. Commun. 2004, 320, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Vane, J.R.; Bakhle, Y.S.; Botting, R.M. Cyclooxygenases 1 and 2. Annu. Rev. Pharmacol. Toxicol. 1998, 38, 97–120. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Qian, Z.; Yan, Z.; Zhao, C.; Wang, H.; Ying, G. A phase I pharmacokinetic study of ursolic acid nanoliposomes in healthy volunteers and patients with advanced solid tumors. Int. J. Nanomed. 2013, 8, 129–136. [Google Scholar]
- Wang, X.H.; Zhou, S.Y.; Qian, Z.Z.; Zhang, H.L.; Qiu, L.H.; Song, Z.; Zhao, J.; Wang, P.; Hao, X.S.; Wang, H.Q. Evaluation of toxicity and single-dose pharmacokinetics of intravenous ursolic acid liposomes in healthy adult volunteers and patients with advanced solid tumors. Expert Opin. Drug Metab. Toxicol. 2013, 9, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Wang, X.; Song, Z.; Zhang, H.; Zhou, S.; Zhao, J.; Wang, H. A phase I trial to evaluate the multiple-dose safety and antitumor activity of ursolic acid liposomes in subjects with advanced solid tumors. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Saraswat, B.; Visen, P.K.S.; Agarwal, D.P. Ursolic acid isolated from Eucalyptus tereticornis protects against ethanol toxicity in isolated rat hepatocytes. Phytother. Res. 2000, 14, 163–166. [Google Scholar] [CrossRef]
- Saravanan, R.; Pugalendi, V.; Pugalendi, K.V. Protective effect of ursolic acid on ethanol-mediated experimental liver damage in rats. Life Sci. 2006, 78, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Shukla, B.; Visen, P.K.S.; Patnaik, G.K.; Tripathi, S.C.; Srimal, R.C.; Dayal, R.; Dobhal, P.C. Hepatoprotective activity in the rat of ursolic acid isolated from Eucalyptus hybrid. Phytother. Res. 1992, 6, 74–79. [Google Scholar] [CrossRef]
- Martin-Aragón, S.; de las Heras, B.; Sanchez-Reus, M.I.; Benedi, J. Pharmacological modification of endogenous antioxidant enzymes by ursolic acid on tetrachloride-induced liver damage in rats and primary cultures of rat hepatocytes. Exp. Toxicol. Pathol. 2001, 53, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Sundaresan, A.; Radhiga, T.; Pugalendi, K.V. Effect of ursolic acid and rosiglitazone combination on hepatic lipid accumulation in high fat diet-fed C57BL/6J mice. Eur. J. Pharmacol. 2014, 741, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liao, X.; Meng, F.; Wang, Y.; Sun, Z.; Guo, F.; Li, X.; Meng, M.; Li, Y.; Sun, C. Therapeutic role of ursolic acid on ameliorating hepatic steatosis and improving metabolic disorders in high-fat diet-induced non-alcoholic fatty liver disease rats. PLoS ONE 2014, 29, e86724. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ikejima, K.; Kon, K.; Arai, K.; Aoyama, T.; Okumura, K.; Abe, W.; Sato, N.; Watanabe, S. Ursolic acid ameliorates hepatic fibrosis in the rat by specific induction of apoptosis in hepatic stellate cells. J. Hepatol. 2011, 55, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.R.; Jin, J.L.; Li, C.H.; Piao, X.X.; Jin, N.G. Ursolic acid enhances mouse liver regeneration after partial hepatectomy. Pharm. Biol. 2012, 50, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Ness, A.R.; Powles, J.W. Fruit and vegetables, and cardiovascular disease: A review. Int. J. Epidemiol. 1997, 26, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Somova, L.O.; Nadar, A.; Rammanan, P.; Shode, F.O. Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine 2003, 10, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Crespo, F.; Vergara-Galicia, J.; Villalobos-Molina, R.; López-Guerrero, J.J.; Navarette-Vázquez, G.; Estrada-Soto, S. Ursolic acid mediates the vasorelaxant activity of Lepechinia caulescens via NO release in isolated rat thoracic aorta. Life Sci. 2006, 79, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Rios, M.Y.; López-Martínez, S.; López-Vallejo, F.; Medina-Franco, J.L.; Villalobos-Molina, R.; Ibarra-Barajas, M.; Navarette-Vazquez, G.; Hidalgo-Figueroa, S.; Hernández-Abreu, O.; Estrada-Soto, S. Vasorelaxant activity of some structurally related triterpenic acids from Phoradendron reichenbachianum (Viscaceae) mainly by NO production: Ex vivo and in silico studies. Fitoterapia 2012, 83, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Shimada, A.; Inagaki, M. Angiotensin I-converting enzyme (ACE) inhibitory activity of ursolic acid isolated from Thymus vulgaris L. Food Sci. Technol. Res. 2014, 3, 711–714. [Google Scholar] [CrossRef]
- Senthil, S.; Sridevi, M.; Pugalendi, K.V. Protective effect of ursolic acid against myocardial ischemia induced by isoproterenol in rats. Toxicol. Mech. Methods 2007, 17, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Radhiga, T.; Rajamanickam, C.; Senthil, S.; Pugalendi, K.V. Effect of ursolic acid on cardiac marker enzymes, lipid profile and macroscopic enzyme mapping assay in isoproterenol-induced myocardial ischemic rats. Food Chem. Toxicol. 2012, 50, 3971–3977. [Google Scholar] [CrossRef] [PubMed]
- Radhiga, T.; Rajamanickam, C.; Sundaresan, A.; Ezhumalai, M.; Pugalendi, K.V. Effect of ursolic acid treatment on apoptosis and DNA damage in isoproterenol-induced myocardial infarction. Biochimie 2012, 94, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, R.; Pugalendi, V. Impact of ursolic acid on chronic ethanol-induced oxidative stress in the rat heart. Pharmacol. Rep. 2006, 58, 41–47. [Google Scholar] [PubMed]
- Pozo, M.; Castilla, V.; Gutierrez, C.; de Nicolás, R.; Egido, J.; González-Cabrero, J. Ursolic acid inhibits neointima formation in the rat carotid artery injury model. Atherosclerosis 2006, 184, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.Y.; Jin, Y.; Han, G.Z.; Liu, Y.X.; Wu, T.; Liu, P.; Zhou, Q.; Liu, K.X.; Sun, H.J. Ursolic acid suppresses IL-6 induced C-reactive protein expression in HepG2 and protects HUVECs from injury induced by CRP. Eur. J. Pharm. Sci. 2012, 45, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Tannock, L.R. Ursolic acid on atherosclerosis: Apples and apples, or apples and oranges? Atherosclerosis 2011, 219, 397–398. [Google Scholar] [CrossRef] [PubMed]
- Ullevig, S.L.; Zhao, Q.; Zamora, D.; Asmis, R. Ursolic acid protects diabetic mice against monocyte dysfunction and accelerated atherosclerosis. Atherosclerosis 2011, 219, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Messner, B.; Zeller, I.; Ploner, C.; Frotschnig, S.; Ringer, T.; Steinacher-Nigish, A.; Ritsch, A.; Laufer, G.; Huck, C.; Bernhard, D. Ursolic acid causes DNA-damage, P53-mediated, mitochondria- and caspase-dependent human endothelial cell apoptosis, and accelerates atherosclerotic plaque formation in vivo. Atherosclerosis 2011, 219, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Han, C.H.; Lee, M.Y. Enhancement of platelet aggregation by ursolic acid and oleanolic acid. Biomol. Ther. 2014, 22, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Bondy, S.C.; LeBel, C.P. The relationship between excitotoxicity and oxidative stress in the central nervous system. Free Radic. Biol. Med. 1993, 14, 633–642. [Google Scholar] [CrossRef]
- Shih, Y.H.; Chein, Y.C.; Wang, J.Y.; Fu, Y.S. Ursolic acid protects hippocampal neurons against kainate-induced excitotoxicity in rats. Neurosci. Lett. 2004, 362, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zheng, Y.L.; Wu, D.M.; Luo, L.; Sun, D.X.; Shan, Q. Ursolic acid ameliorates cognition deficits and attenuates oxidative damage in the brain of senescent mice induced by d-galactose. Biochem. Pharmacol. 2007, 74, 1078–1090. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wu, D.M.; Zheng, Y.L.; Hu, B.; Zhang, Z.F.; Ye, Q.; Liu, C.M.; Shan, Q.; Wang, Y.L. Ursolic acid attenuates D-galactose-induced inflammatory response in mouse prefrontal cortex through inhibiting AGEs/RAGE/NF-κB pathway activation. Cereb. Cortex 2010, 20, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Lu, J.; Wu, D.M.; Zheng, Z.H.; Zheng, Y.L.; Wang, X.H.; Ruan, J.; Sun, X.; Shan, Q.; Zhang, Z.F. Ursolic acid attenuates lipopolysaccharide-induced cognitive deficits in mouse brain through suppressing p38/NF-κB mediated inflammatory pathways. Neurobiol. Learn. Mem. 2011, 96, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, X.; Cui, L.; Wang, L.; Liu, H.; Ji, H.; Du, Y. Ursolic acid promotes the neuroprotection by activating Nrf2 pathway after cerebral ischemia in mice. Brain Res. 2013, 1497, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.M.; Lu, J.; Zhang, Y.Q.; Zheng, Y.L.; Hu, B.; Cheng, W.; Zhang, Z.F.; Li, M.Q. Ursolic acid improves domoic acid-induced cognitive deficits in mice. Toxicol. Appl. Pharmacol. 2013, 271, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.G.; Neis, V.B.; Balen, G.O.; Colla, A.; Cunha, M.P.; Dalmarco, J.B.; Pizzolatti, M.G.; Prediger, R.D.; Rodrigues, A.L. Antidepressant-like effect of ursolic acid isolated from Rosmarinus officinalis L. in mice: Evidence of involvement of the dopaminergic system. Pharmacol. Biochem. Behav. 2012, 103, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Colla, A.R.; Oliveira, A.; Pazini, F.L.; Rosa, J.M.; Manosso, L.M.; Cunha, M.P.; Rodrigues, A.L. Serotonergic and noradrenergic systems are implicated in the antidepressant-like effect of ursolic acid in mice. Pharmacol. Biochem. Behav. 2014, 124, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Colla, A.R.; Rosa, J.M.; Cunha, M.P.; Rodrigues, A.L. Anxiolytic-like effects of ursolic acid in mice. Eur. J. Pharmacol. 2015, 758, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, K.; Yamaguchi, A. Novel intriguing strategies attenuating to sarcopenia. J. Aging Res. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, S.D.; Suneja, M.; Ebert, S.M.; Bongers, K.S.; Fox, D.K.; Malmberg, S.E.; Alipour, F.; Shields, R.K.; Adams, C.M. mRNA expression signatures of human skeletal muscle atrophy identify a natural compound that increases muscle mass. Cell Metab. 2011, 13, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, S.D.; Elmore, C.J.; Bongers, K.S.; Ebert, S.M.; Fox, D.K.; Dyle, M.C.; Bullard, S.A.; Adams, C.M. Ursolic acid increases skeletal muscle and brown fat and decreases diet-induced obesity, glucose intolerance and fatty liver disease. PLoS ONE 2012, 7, e39332. [Google Scholar] [CrossRef] [PubMed]
- Bakhtiari, N.; Hosseinkhani, S.; Tashakor, A.; Hemmati, R. Ursolic acid ameliorates aging-metabolic phenotype through promoting of skeletal muscle rejuvenation. Med. Hypotheses 2015, 85, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Bang, H.S.; Seo, D.Y.; Chung, Y.M.; Oh, K.M.; Park, J.J.; Arturo, F.; Jeong, H.S.; Kim, N.; Han, J. Ursolic acid-induced elevation of serum irisin augments muscle strength during resistance training in men. Korean J. Physiol. Pharmacol. 2014, 18, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Raggatt, L.J.; Partridge, N.C. Cellular and molecular mechanisms of bone remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 2000, 21, 115–137. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.U.; Park, S.J.; Kwak, H.B.; Oh, J.; Min, Y.K.; Kim, S.H. Anabolic activity of ursolic acid in bone: Stimulating osteoblast differentiation in vitro and inducing new bone formation in vivo. Pharmacol. Res. 2008, 58, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Furuta, S.; Nagata, T.; Ohnuki, K.; Akasaka, T.; Shirouchi, B.; Sato, M.; Kondo, R.; Shimizu, K. Inhibitory effect of the leaves of loquat (Eriobotrya japonica) on bone mineral density loss in ovariectomized mice and osteoclast differentiation. J. Agric. Food Chem. 2014, 62, 836–841. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Ashour, A.; Katakura, Y.; Shimizu, K. A structure-activity relationship study on antiosteoclastogenesis effect of triterpenoids from leaves of loquat (Eriobotrya japonica). Phytomedicine 2015, 22, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Xiao, F.; Gu, X.; Zhai, Z.; Liu, X.; Wang, W.; Tang, T.; Wang, Y.; Zhu, Z.; Dai, K.; et al. Inhibitory effects of ursolic acid on osteoclastogenesis and titanium particle-induced osteolysis are mediated primarily via suppression of NF-κB signaling. Biochimie 2015, 111, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.G.; Zhang, C.J.; Xu, X.E.; Sun, J.H.; Zhang, L.; Yu, P.F. Ursolic acid derivative ameliorates streptozotocin-induced diabestic bone deleterious effect in mice. Int. J. Clin. Exp. Pathol. 2015, 8, 3681–3690. [Google Scholar] [PubMed]
- Fu, H.J.; Zhou, Y.R.; Bao, B.H.; Jia, M.X.; Zhao, Y.; Zhang, L.; Li, J.X.; He, H.L.; Zhou, X.M. Tryptophan hydroxylase 1 (Tph-1)-targeted bone anabolic agents for osteoporosis. J. Med. Chem. 2014, 57, 4692–4709. [Google Scholar] [CrossRef] [PubMed]
- Both, D.M.; Goodtzova, K.; Yarosh, D.B.; Brown, D.A. Liposome-encapsulated ursolic acid increases ceramides and collagen in human skin cells. Arch. Derm. Res. 2002, 293, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Wójciak-Kosior, M.; Paduch, R.; Matysik-Woźniak, A.; Niedziela, P.; Donica, H. The effect of ursolic and oleanolic acids on human skin fibroblast cells. Folia Hystochem. Cytobiol. 2011, 49, 664–669. [Google Scholar] [CrossRef]
- Ding, Y.J.; Sun, C.Y.; Wen, C.C.; Chen, Y.H. Nephroprotective role of resveratrol and ursolic acid in aristolochic acid intoxicated zebrafish. Toxins 2015, 7, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Pai, P.G.; Chamari Nawarathna, S.; Kulkarni, A.; Habeeba, U.; Reddy, S.C.; Teerthanath, S.; Shenoy, J.P. Nephroprotective effect of ursolic acid in a murine model of gentamicin-induced renal damage. ISRN Pharmacol. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wan, Y.; Zhou, T.; Li, J.; Wei, Y. Ursolic acid attenuates lipopolysaccharide-induced acute lung injury in a mouse model. Immunotherapy 2013, 5, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, P.G.; Lemos, T.L.; Bizerra, A.M.; Arriaga, Â.M.; Ferreira, D.A.; Santiago, G.M.; Braz-Filho, R.; Costa, J.G. Antibacterial and antioxidant activities of ursolic acid and derivatives. Molecules 2014, 19, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Cunha, W.R.; de Matos, G.X.; Souza, M.G.; Tozatti, M.G.; Andrade e Silva, M.L.; Martins, C.H.; da Silva, R.; da Silva Filho, A.A. Evaluation of the antibacterial activity of the methylene chloride extract of Miconia ligustroides, isolated triterpene acids, and ursolic acid derivatives. Pharm. Biol. 2010, 48, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Mallavadhani, U.V.; Mahapatra, A.; Jamil, K.; Reddy, P.S. Antimicrobial activity of some pentacyclic triterpenes and their synthesized 3-O-lipophilic chains. Biol. Pharm. Bull. 2004, 27, 1576–1579. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Gu, Z.; Sun, M.; Zhang, K.; Gao, P.; Yang, Q.; Yuan, Y. Ursolic acid improves survival and attenuates lung injury in septic rats induced by cecal ligation and puncture. J. Surg. Res. 2015, 194, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Kurek, A.; Grudniak, A.M.; Szwed, M.; Klicka, A.; Samluk, L.; Wolska, K.I.; Janiszowska, W.; Popowska, M. Oleanolic acid and ursolic acid effect peptidoglycan metabolism in Listeria monocytogenes. Antonie Van Leeuwenhoek 2010, 97, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Luo, H.; Li, Q.; Wang, D.; Zhang, J.; Hao, X.; Yang, X. Pentacyclic triterpene derivatives possessing polyhydroxyl ring A inhibit Gram-positive bacteria growth by regulating metabolism and virulence genes expression. Eur. J. Med. Chem. 2015, 95, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Fontanay, S.; Grare, M.; Mayer, J.; Finance, C.; Duval, R.E. Ursolic, oleanolic and betulinic acids: Antibacterial spectra and selectivity indexes. J. Ethnopharmacol. 2008, 120, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Kurek, A.; Nadkowska, P.; Pliszka, S.; Wolska, K.I. Modulation of antibiotic resistance in bacterial pathogens by oleanolic acid and ursolic acid. Phytomedicine 2012, 19, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Woldemichael, G.M.; Franzblau, S.G.; Zhang, F.; Wang, Y.; Timmermann, B.N. Inhibitory effect of sterols from Ruprechtia triflora and diterpenes from Calceolaria pinnifolia on the growth of Mycobacterium tuberculosis. Planta Med. 2003, 69, 628–631. [Google Scholar] [PubMed]
- Jiménez, A.; Meckes, M.; Alvarez, V.; Torres, J.; Parra, R. Secondary metabolites from Chamaedora tepejilote (Palmae) are active against Mycobacterium tuberculosis. Phytother. Res. 2005, 19, 320–322. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Arellanes, A.; Luna-Herrera, J.; Cornejo-Garrido, J.; López-Garcia, S.; Castro-Mussot, M.E.; Meckes-Fisher, M.; Mata-Espinosa, D.; Marquina, B.; Torres, J.; Hernández-Pando, R. Ursolic and oleanolic acids as antimicrobial and immunomodulatory compounds for tuberculosis treatment. BMC Complement. Altern. Med. 2013, 13, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Kim, M.J.; Jin, D.; Park, S.N.; Cho, E.; Freire, M.O.; Jang, S.J.; Park, Y.J.; Kook, J.K. Antimicrobial effect of ursolic acid and oleanolic acid against methicillin-resistant Staphylococcus aureus. Korean J. Microbiol. 2012, 48, 212–215. [Google Scholar] [CrossRef]
- Horiuchi, K.; Shiota, S.; Hatano, T.; Yoshida, T.; Kuroda, T.; Tsuchiya, T. Antimicrobial activity of oleanolic acid from Salvia officinalis and related compounds on vancomycin-resistant enterococci (VRE). Biol. Pharm. Bull. 2007, 30, 1147–1149. [Google Scholar] [CrossRef] [PubMed]
- Chung, P.Y.; Navaratnam, P.; Chung, L.Y. Synergistic antimicrobial activity between pentacyclic triterpenoids and antibiotics against Staphylococcus aureus strains. Ann. Clin. Microbiol. Antimicrob. 2011, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Kozai, K.; Miyake, Y.; Kohda, H.; Kametaka, S.; Yamasaki, K.; Suginaka, H.; Nagasaka, N. Inhibition of glucosyltransferase form Streptococcus mutans by oleanolic acid and ursolic acid. Caries Res. 1987, 21, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, C.S.; Park, J.Y.; Lim, Y.K.; Park, S.N.; Ahn, S.J.; Jin, D.C.; Kim, T.H.; Kook, J.K. Antimicrobial effects of ursolic acid against mutans Streptococci isolated from Koreans. Int. J. Oral Biol. 2011, 36, 7–11. [Google Scholar]
- Kim, S.; Song, M.; Roh, B.D.; Park, S.H.; Park, J.W. Inhibition of Streptococcus mutans biofilm formation on composite resins containing ursolic acid. Restor. Dent. Endod. 2013, 38, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Filho, J.R.; de Sousa Falcão, H.; Batista, L.M.; Filho, J.M.; Piuvezam, M.R. Effects of plant extract on HIV-1 protease. Curr. HIV Res. 2010, 8, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Hattori, M.; Ma, C.M.; Wei, Y.; Salah El Dine, R.; Sato, N. Survey of Anti-HIV and Anti-HCV compounds from natural sources. Can. Chem. Tran. 2013, 1, 116–140. [Google Scholar]
- Kashiwada, Y.; Wang, H.K.; Nagao, T.; Kitanaka, S.; Yasuda, I.; Fujioka, T.; Yamagishi, T.; Cosentino, L.M.; Kozuka, M.; Okabe, H.; et al. Anti-AIDS agents. 30. Anti-HIV activity of oleanolic acid, pomolic acid, and structurally related triterpenoids. J. Nat. Prod. 1998, 61, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.M.; Nakamura, N.; Miyashiro, H.; Hattori, M.; Shimotohno, K. Inhibitory effect of constituents from Cynomorium songaricum and related triterpene derivatives on HIV-1 protease. Chem. Pharm. Bull. 1999, 47, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Quéré, L.; Wenger, T.; Schramm, H.J. Triterpenes as potential dimerization inhibitors of HIV-1 protease. Biochem. Biophys. Res. Commun. 1996, 227, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Li, S.; Liao, Q.; Zhang, Y.; Sun, R.; Zhu, X.; Zhang, Q.; Wang, J.; Wu, X.; Fang, X.; et al. Oleanolic acid and ursolic acid: Novel hepatitis C virus antivirals that inhibit NS5B activity. Antivir. Res. 2013, 98, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Risco, M.R.; Vázquez, E.; Sheldon, J.; Steinmann, E.; Riebesehl, N.; Fornari, T.; Reglero, G. Supercritical fluid extraction of heather (Calluna vulgaris) and evaluation of anti-hepatitis C virus activity of the extracts. Virus Res. 2015, 198, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Bag, P.; Chattopadhyay, D.; Mukherjee, H.; Ojha, D.; Mandal, N.; Sarkar, M.C.; Chatterjee, T.; Das, G.; Chakraborti, S. Anti-herpes virus activities of bioactive fraction and isolated pure constituents of Mallotus peltatus an ethnomedicine from Andaman Islands. Virol. J. 2012, 9, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, E.S.; Campos, B.L.S.; Laurenti, M.D.; Lago, J.H.G.; dos Santos Grecco, S.; Corbett, C.E.P.; Passero, L.F.D. Treatment with triterpenic fraction purified from Baccharis uncinella leaves inhibits Leishmania (Leishmania) amazonensis spreading and improves Th1 immune response in infected mice. Parasitol. Res. 2014, 113, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Van Baren, C.; Anao, I.; Leo Di Lira, P.; Debenedetti, S.; Houghton, P.; Croft, S.; Martino, V. Triterpenic acids and flavonoids from Satureja parvifolia. Evaluation of their antiprotozoal activity. Z. Naturforschung C. 2006, 61, 189–192. [Google Scholar] [CrossRef]
- Innocente, A.; Silva, G.N.; Cruz, L.N.; Moraes, M.S.; Nakabashi, M.; Sonnet, P.; Gosmann, G.; Garcia, C.R.; Gnoatto, S.C. Synthesis and antiplasmodial activity of betulinic and ursolic analogues. Molecules 2012, 17, 12003–12014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Della-Vechia, L.; Dassonville-Klimpt, A.; Grellier, P.; Sonnet, P.; Gosmann, G.; Gnoatto, S.C.B. The Beckmann rearrangement applied to ursolic acid with antimalarial activity in medicinal chemistry studies. Lett. Org. Chem. 2012, 9, 92–95. [Google Scholar] [CrossRef]
- Cimanga, R.K.; Tona, G.L.; Mesia, G.K.; Kambu, O.K.; Bakana, D.P.; Kalenda, P.D.T.; Penge, A.O.; Muyembe, J.J.T.; Totté, J.; Pieters, L.; et al. Bioassay-guided isolation of antimalarial triterpenoid acids from the leaves of Morinda lucida. Pharm. Biol. 2006, 44, 677–681. [Google Scholar] [CrossRef]
- Da Silva Ferreira, D.; Esperandim, V.R.; Toldo, M.P.; Kuehn, C.C.; do Prado Júnior, J.C.; Cunha, W.R.; e Silva, M.L.; de Albuquerque, S. In vivo activity of ursolic and oleanolic acid during the acute phase of Trypanosoma cruzi infection. Exp. Parasitol. 2013, 134, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Innocente, A.; Casanova, B.B.; Klein, F.; Lana, A.D.; Pereira, D.; Muniz, M.N.; Sonnet, P.; Gosmann, G.; Fuentefria, A.M.; Gnoatto, S.C.B. Synthesis of isosteric triterpenoid derivatives and antifungal activity. Chem. Biol. Drug Des. 2014, 83, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Saini, P.; Gayen, P.; Kumar, D.; Nayak, A.; Mukherjee, N.; Mukherjee, S.; Pal, B.C.; Sinha Babu, S.P. Antifilarial effect of ursolic acid from Nyctanthes arbortristis: Molecular and biochemical evidences. Parasitol. Int. 2014, 63, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Wu, C.; Zhou, X.; Wu, F.; Li, J.; Li, T.; Yin, Y. Disturbance of the intestinal microbial community by ursolic acid contributes to its function as a regulator of fat deposition. J. Funct. Foods 2015, 14, 456–468. [Google Scholar] [CrossRef]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woźniak, Ł.; Skąpska, S.; Marszałek, K. Ursolic Acid—A Pentacyclic Triterpenoid with a Wide Spectrum of Pharmacological Activities. Molecules 2015, 20, 20614-20641. https://doi.org/10.3390/molecules201119721
Woźniak Ł, Skąpska S, Marszałek K. Ursolic Acid—A Pentacyclic Triterpenoid with a Wide Spectrum of Pharmacological Activities. Molecules. 2015; 20(11):20614-20641. https://doi.org/10.3390/molecules201119721
Chicago/Turabian StyleWoźniak, Łukasz, Sylwia Skąpska, and Krystian Marszałek. 2015. "Ursolic Acid—A Pentacyclic Triterpenoid with a Wide Spectrum of Pharmacological Activities" Molecules 20, no. 11: 20614-20641. https://doi.org/10.3390/molecules201119721
APA StyleWoźniak, Ł., Skąpska, S., & Marszałek, K. (2015). Ursolic Acid—A Pentacyclic Triterpenoid with a Wide Spectrum of Pharmacological Activities. Molecules, 20(11), 20614-20641. https://doi.org/10.3390/molecules201119721







