Comparison of the Separation Performances of Cinchona Alkaloid-Based Zwitterionic Stationary Phases in the Enantioseparation of β2- and β3-Amino Acids
Abstract
:1. Introduction
2. Results and Discussion
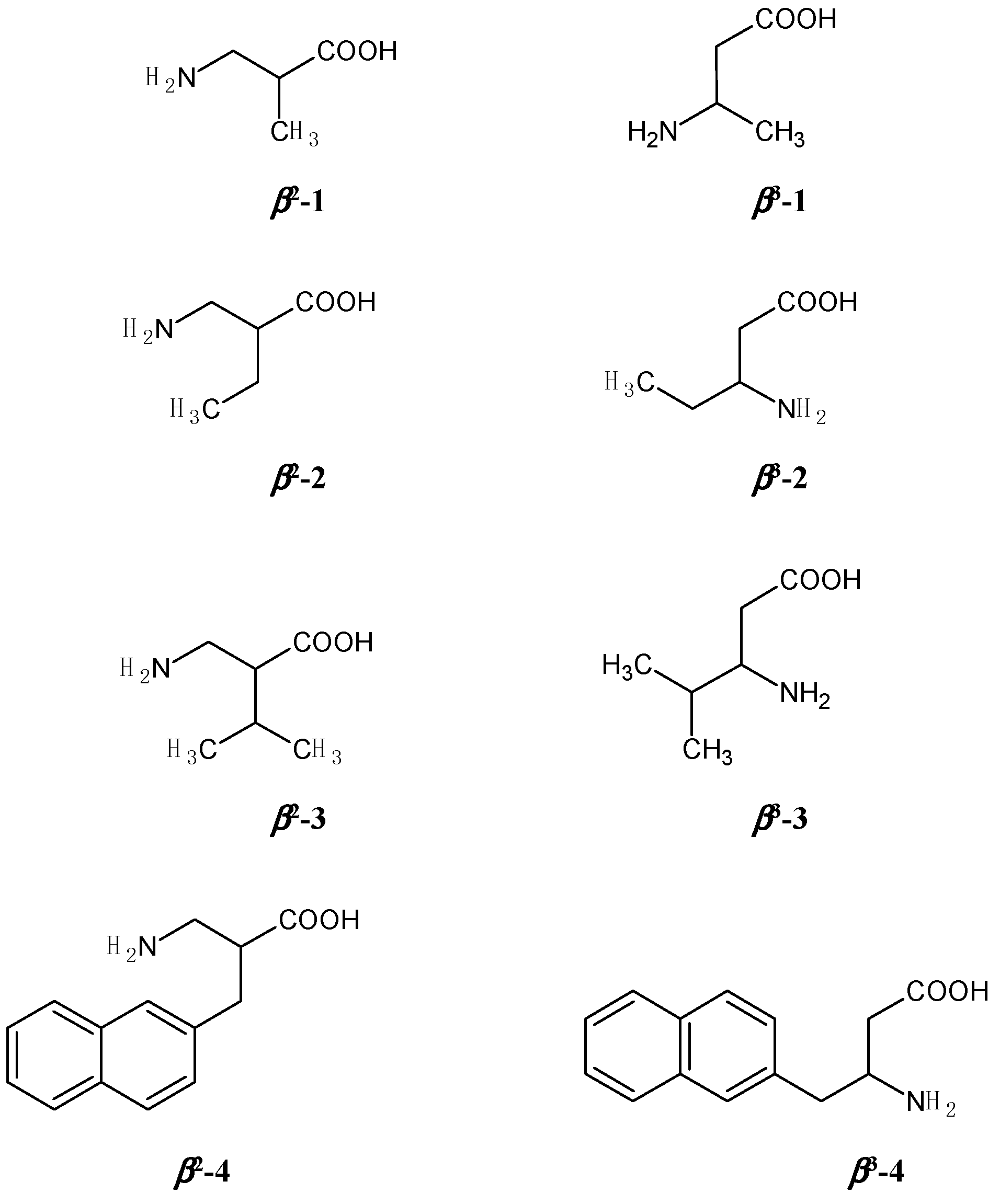


2.1. Effect of the Mobile Phase Composition
 : for β2-1,
: for β2-1,  : for β3-1,
: for β3-1,  : for β2-4 and
: for β2-4 and  : for β3-4; α:
: for β3-4; α:  : for β2-1,
: for β2-1,  : β3-1,
: β3-1,  : for β2-4 and
: for β2-4 and  : for β3-4; RS:
: for β3-4; RS:  : for β2-1,
: for β2-1,  : β3-1,
: β3-1,  : for β2-4 and
: for β2-4 and  : for β3-4.
: for β3-4.
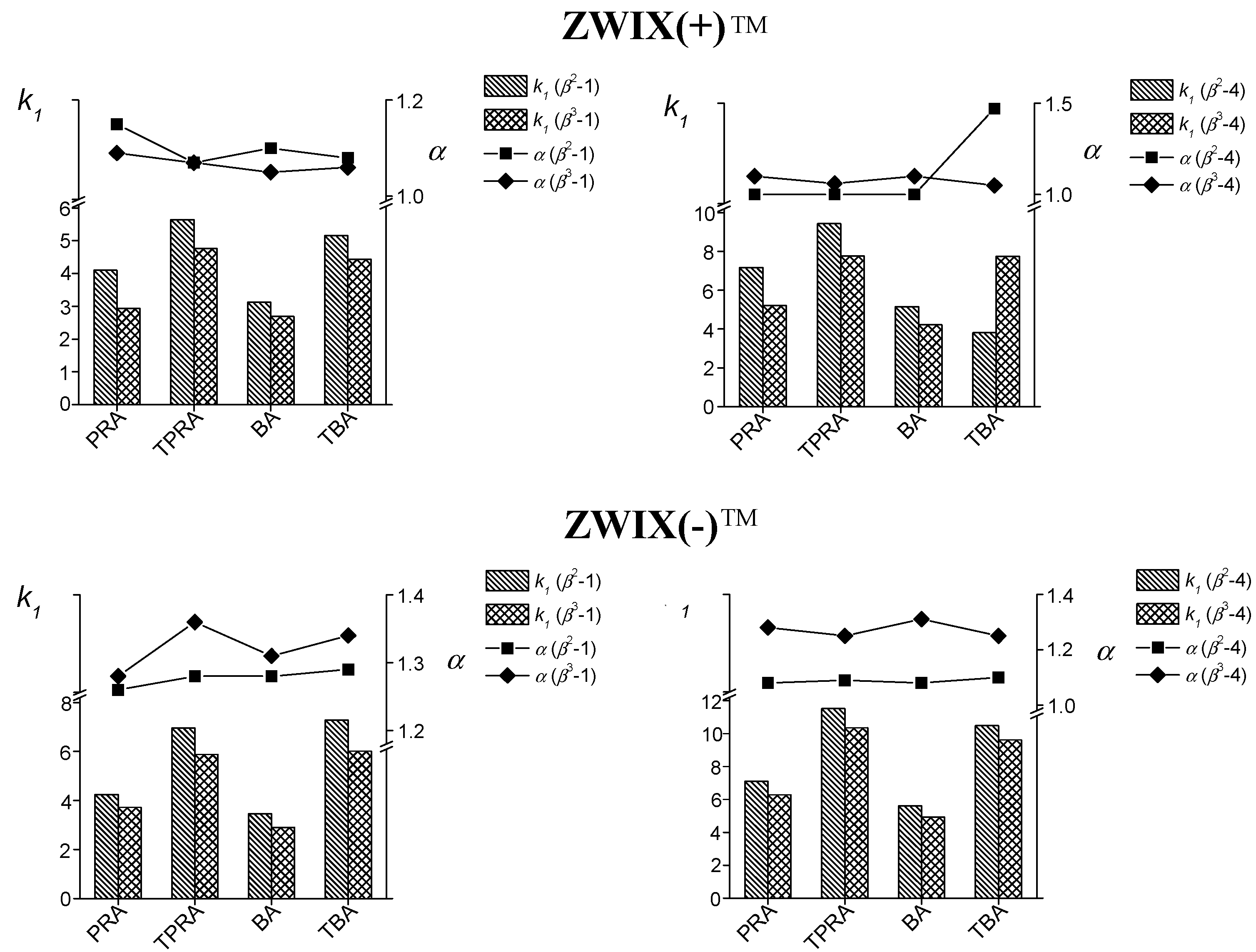
 : for β2-1,
: for β2-1,  : for β3-1,
: for β3-1,  : for β2-4 and
: for β2-4 and  : for β3-4; α:
: for β3-4; α:  : for β2-1,
: for β2-1,  : β3-1,
: β3-1,  : for β2-4 and
: for β2-4 and  : for β3-4; RS:
: for β3-4; RS:  : for β2-1,
: for β2-1,  : β3-1,
: β3-1,  : for β2-4 and
: for β2-4 and  : for β3-4.
: for β3-4.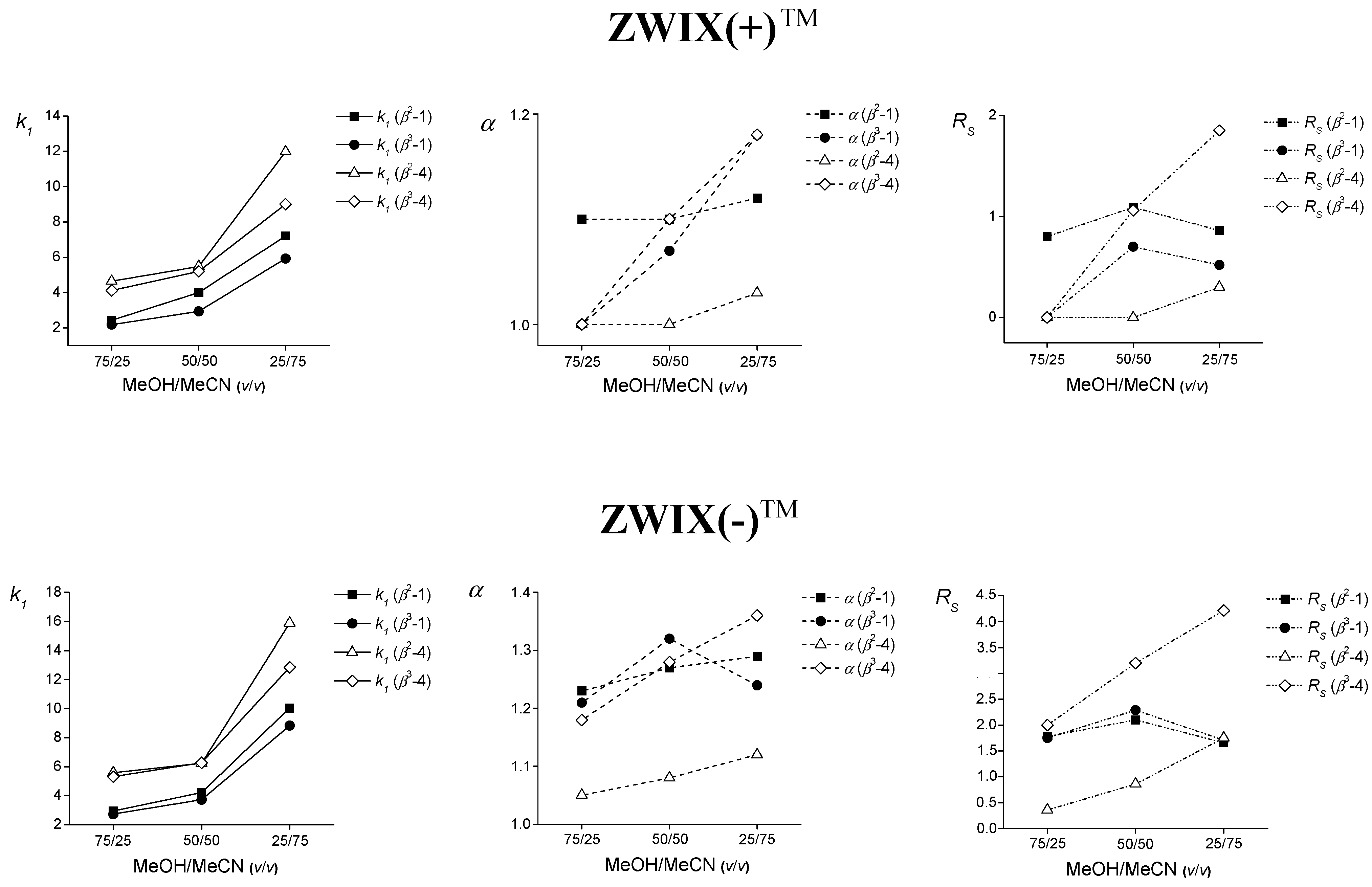
| Compound | Eluent | k1 | α | RS | Elution sequence |
|---|---|---|---|---|---|
| β2-1 | g | 3.12 | 1.10 | 0.63 | - |
| h | 5.15 | 1.08 | 0.32 | - | |
| β3-1 | g | 2.69 | 1.05 | 0.43 | R < S |
| h | 4.44 | 1.06 | 0.32 | R < S | |
| β2-2 | b | 3.45 | 1.29 | 2.80 | - |
| e | 5.35 | 1.28 | 1.96 | - | |
| g | 3.06 | 1.28 | 2.62 | - | |
| h | 5.39 | 1.25 | 1.98 | - | |
| β3-2 | b | 2.85 | 1.13 | 1.37 | - |
| e | 4.24 | 1.13 | 1.50 | - | |
| g | 2.52 | 1.13 | 1.19 | - | |
| h | 4.17 | 1.13 | 1.04 | - | |
| β2-3 | b | 3.10 | 1.57 | 4.56 | R < S |
| e | 5.09 | 1.45 | 4.00 | R < S | |
| g | 2.78 | 1.53 | 4.25 | R < S | |
| h | 5.02 | 1.46 | 3.48 | R < S | |
| β3-3 | b | 2.48 | 1.18 | 1.88 | - |
| e | 3.62 | 1.19 | 2.23 | - | |
| g | 2.21 | 1.18 | 1.60 | - | |
| h | 3.57 | 1.19 | 1.27 | - | |
| β2-4 | g | 5.16 | 1.00 | 0.00 | - |
| h | 3.81 | 1.47 | 3.27 | - | |
| β3-4 | g | 4.22 | 1.10 | 0.83 | R < S |
| h | 7.75 | 1.05 | 0.36 | R < S | |
| β2-5 | b | 4.43 | 1.03 | 0.26 | - |
| e | 6.87 | 1.05 | 0.42 | - | |
| g | 3.92 | 1.03 | <0.20 | - | |
| h | 7.02 | 1.04 | 0.31 | - | |
| β3-5 | b | 3.67 | 1.10 | 1.05 | R < S |
| e | 5.60 | 1.07 | 0.79 | R < S | |
| g | 3.26 | 1.10 | 1.05 | R < S | |
| h | 5.63 | 1.07 | 0.80 | R < S | |
| β2-6 | b | 5.08 | 1.02 | <0.20 | - |
| e | 8.26 | 1.06 | 0.60 | - | |
| g | 4.51 | 1.00 | 0.00 | - | |
| h | 8.39 | 1.05 | 0.36 | - | |
| β3-6 | b | 4.35 | 1.15 | 1.67 | R < S |
| e | 6.80 | 1.12 | 1.31 | R < S | |
| g | 3.82 | 1.14 | 1.47 | R < S | |
| h | 6.79 | 1.13 | 1.41 | R < S |
| Compound | Eluent | k1 | α | RS | Elution sequence |
|---|---|---|---|---|---|
| β2-1 | g | 3.46 | 1.28 | 3.39 | - |
| h | 7.29 | 1.29 | 3.08 | - | |
| β3-1 | g | 2.91 | 1.31 | 3.34 | S < R |
| h | 6.02 | 1.34 | 4.32 | S < R | |
| β2-2 | b | 2.15 | 1.38 | 2.36 | - |
| e | 6.94 | 1.35 | 3.85 | - | |
| g | 3.19 | 1.40 | 3.88 | - | |
| h | 7.11 | 1.35 | 3.67 | - | |
| β3-2 | b | 2.96 | 1.36 | 3.71 | - |
| e | 5.55 | 1.42 | 5.35 | - | |
| g | 2.71 | 1.35 | 3.29 | - | |
| h | 5.67 | 1.58 | 5.29 | - | |
| β2-3 | b | 3.87 | 1.62 | 5.89 | S < R |
| e | 6.04 | 1.68 | 6.90 | S < R | |
| g | 2.97 | 1.64 | 6.19 | S < R | |
| h | 6.79 | 1.53 | 6.81 | S < R | |
| β3-3 | b | 2.75 | 1.62 | 3.25 | - |
| e | 4.74 | 1.50 | 3.92 | - | |
| g | 2.44 | 1.46 | 4.03 | - | |
| h | 4.81 | 1.49 | 4.19 | - | |
| β2-4 | g | 5.60 | 1.08 | 0.98 | - |
| h | 10.47 | 1.10 | 1.07 | - | |
| β3-4 | g | 4.92 | 1.31 | 3.73 | S < R |
| h | 9.62 | 1.25 | 3.38 | S < R | |
| β2-5 | b | 4.57 | 1.12 | 1.59 | - |
| e | 7.70 | 1.14 | 2.19 | - | |
| g | 4.27 | 1.12 | 1.59 | - | |
| h | 8.01 | 1.16 | 1.84 | - | |
| β3-5 | b | 3.91 | 1.35 | 3.64 | S < R |
| e | 6.39 | 1.33 | 2.80 | S < R | |
| g | 3.59 | 1.36 | 3.43 | S < R | |
| h | 6.64 | 1.34 | 3.28 | S < R | |
| β2-6 | b | 6.25 | 1.13 | 1.59 | - |
| e | 9.29 | 1.16 | 2.24 | - | |
| g | 4.92 | 1.12 | 1.56 | - | |
| h | 9.76 | 1.16 | 1.92 | - | |
| β3-6 | b | 5.82 | 1.35 | 3.56 | S < R |
| e | 8.25 | 1.30 | 3.13 | S < R | |
| g | 4.39 | 1.35 | 3.72 | S < R | |
| h | 8.66 | 1.29 | 3.00 | S < R |
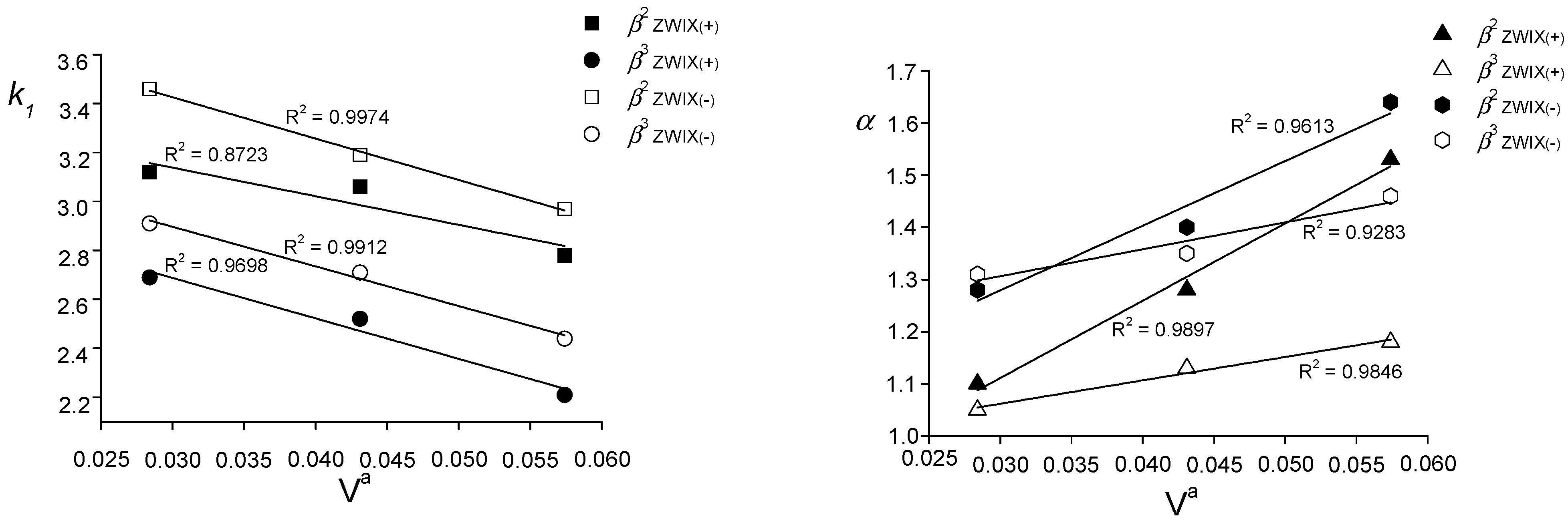
2.2. Structure-Retention (Selectivity) Relationship
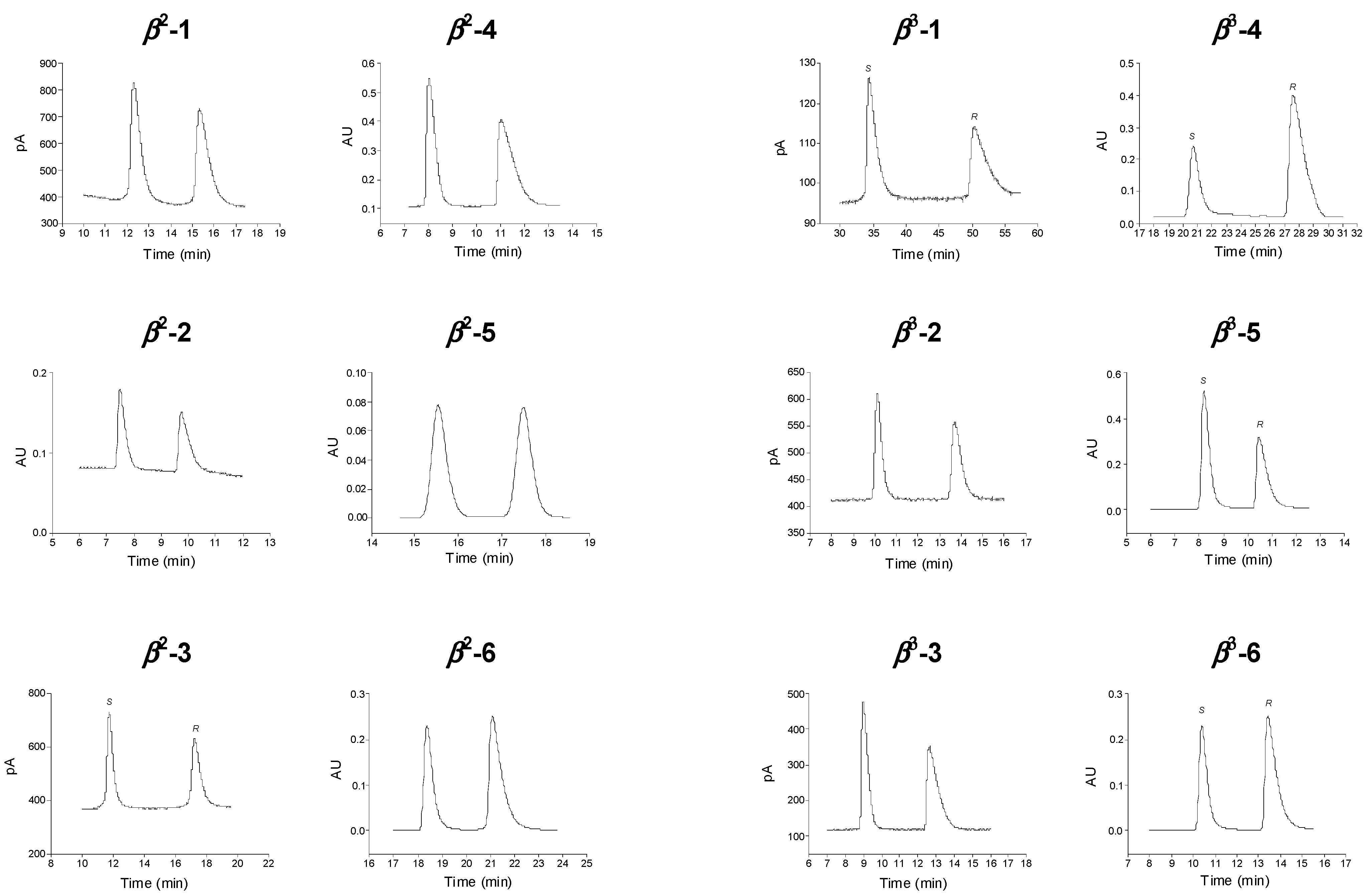
2.3. Effects of Temperature and Thermodynamic Parameters
| Analyte | Column | −∆(∆H°) (kJ·mol−1) | −∆(∆S°) (J·mol−1·K−1) | Corr. coeff. (R2) | −Tx∆(∆S°)298K (kJ·mol−1) | −∆(∆G°)298K (kJ·mol−1) |
|---|---|---|---|---|---|---|
| β2-1 | ZWIX(+)™ | 0.4 | 0.6 | 0.9956 | 0.2 | 0.2 |
| ZWIX(−)™ | 0.9 | 1.0 | 0.9914 | 0.3 | 0.6 | |
| β3-1 | ZWIX(+)™ | 0.8 | 2.3 | 0.9932 | 0.7 | 0.1 |
| ZWIX(−)™ | 2.9 | 7.3 | 0.9926 | 2.2 | 0.7 | |
| β2-3 | ZWIX(+)™ | 3.8 | 9.0 | 0.9945 | 2.7 | 1.1 |
| ZWIX(−)™ | 3.3 | 7.1 | 0.9965 | 2.1 | 1.2 | |
| β3-3 | ZWIX(+)™ * | 3.9 * | 9.7 * | 0.9935 | 2.9 * | 1.0 * |
| ZWIX(+)™ ** | 9.7 ** | 29.1 ** | 0.9996 | 8.7 ** | 1.0 ** | |
| ZWIX(−)™ | 7.7 | 21.7 | 0.9953 | 6.5 | 1.2 | |
| β2-4 | ZWIX(+)™ | - | - | - | - | - |
| ZWIX(−)™ | −0.3 | −1.7 | 0.9918 | −0.5 | 0.2 | |
| β3-4 | ZWIX(+)™ | 0.4 | 0.5 | 0.9945 | 0.2 | 0.2 |
| ZWIX(−)™ | 1.8 | 4.0 | 0.9908 | 1.2 | 0.6 | |
| β2-6 | ZWIX(+)™ * | 0.6 * | 1.9 * | 0.9998 | 0.6 * | 0.0 * |
| ZWIX(−)™ | 0.3 | 0.1 | 0.9972 | 0.04 | 0.3 | |
| β3-6 | ZWIX(+)™ | 0.5 | 0.5 | 0.9936 | 0.1 | 0.4 |
| ZWIX(−)™ | 1.9 | 3.8 | 0.9962 | 1.1 | 0.8 |
3. Experimental
3.1. Chemicals and Syntheses
3.2. Apparatus and Chromatography
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ma, J.S. Unnatural amino acids in drug discovery. Chim. Oggi 2003, 21, 65–68. [Google Scholar]
- Ziora, Z.; Skwarczynski, M.; Kiso, Y. Medicinal chemistry of α-hydroxy-β-amino acids. In Amino Acids, Peptides and Proteins in Organic Chemistry, Volume 4, Protection Reactions, Medicinal Chemistry, Combinatorial Synthesis; Hughes, A.B., Ed.; Wiley-VCH: Weinheim, Germany, 2011; pp. 189–245. [Google Scholar]
- Cheng, R.P.; Gellman, S.; DeGrado, W.F. β-Peptides: From structure to function. Chem. Rev. 2001, 101, 3219–3232. [Google Scholar] [CrossRef] [PubMed]
- Fülöp, F.; Martinek, T.A. Peptidic foldamers: Ramping up diversity. Chem. Soc. Rev. 2012, 41, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Sleebs, B.E.; van Nguyen, T.T.; Hughes, A.B. Recent advances in stereoselective synthesis and application of β-amino acids. Org. Prep. Proc. Int. 2009, 41, 429–478. [Google Scholar] [CrossRef]
- Seebach, D.; Beck, A.K.; Capone, S.; Gildas, D.; Grošelj, U.; Zass, E. Enantioselective preparation of β2-amino acid derivatives for β-peptide synthesis. Synthesis 2009, 1–32. [Google Scholar]
- Bandala, Y.; Juaristi, E. Recent developments in the synthesis of β-amino acids. In Amino Acids, Peptides and Proteins in Organic Chemistry, Volume 1, Origins and Synthesis of Amino Acids; Hughes, A.B., Ed.; Wiley-VCH: Weinheim, Germany, 2009; pp. 291–365. [Google Scholar]
- Kiss, L.; Fülöp, F. Synthesis of carbocyclic and heterocyclic β-aminocarboxylic acids. Chem. Rev. 2014, 114, 1116–1169. [Google Scholar] [CrossRef]
- Bojarski, J.; Aboul-enein, H.Y.; Ghanem, A. What’s new in chromatographic enantioseparations. Curr. Anal. Chem. 2005, 1, 59–77. [Google Scholar] [CrossRef]
- Ilisz, I.; Aranyi, A.; Pataj, Z.; Péter, A. Recent advances in the direct and indirect liquid chromatographic enantioseparation of amino acids and related compounds: A review. J. Pharm. Biomed. Anal. 2012, 69, 28–41. [Google Scholar] [PubMed]
- Lämmerhofer, M. Chiral recognition by enantioselective liquid chromatography: Mechanisms and modern chiral stationary phases. J. Chromatogr. A 2010, 1217, 814–856. [Google Scholar] [CrossRef] [PubMed]
- D’Acquarica, I.; Gasparrini, F.; Misiti, D.; Zappia, G.; Cimarelli, C.; Palmieri, G.; Carotti, A.; Cellamare, S.; Villani, C. Application of a new chiral stationary phase containing the glycopeptide antibiotic A-40,926 in the direct chromatographic resolution of ß-amino acids. Tetrahedron: Asymmetry 2000, 11, 2375–2385. [Google Scholar] [CrossRef]
- Ilisz, I.; Berkecz, R.; Péter, A. HPLC separation of amino acid enantiomers and small peptides on macrocyclic antibiotic-based chiral stationary phases: A review. J. Sep. Sci. 2006, 29, 1305–1321. [Google Scholar] [CrossRef] [PubMed]
- Ilisz, I.; Berkecz, R.; Péter, A. Retention mechanism of high-performance liquid chromatographic enantioseparation on macrocyclic glycopeptide-based chiral stationary phases. J. Chromatogr. A 2009, 1216, 1845–1860. [Google Scholar] [CrossRef]
- Kyba, E.P.; Siegel, M.G.; Sousa, L.R.; Sogah, G.D.Y.; Cram, D.J. Chiral, hinged, and functionalized multiheteromacrocycles. J. Am. Chem. Soc. 1973, 95, 2691–2692. [Google Scholar] [CrossRef]
- Hyun, M.H.; Cho, Y.J.; Jin, J.S. Liquid chromatographic direct resolution of ß-amino acids on a chiral crown ether stationary phase. J. Sep. Sci. 2002, 25, 648–652. [Google Scholar]
- Hyun, M.H.; Han, S.C.; Whangbo, S.H. New ligand exchange chiral stationary phase for the liquid chromatographic resolution of α- and ß-amino acids. J. Chromatogr. A 2003, 992, 47–56. [Google Scholar] [CrossRef]
- Hyun, M.H.; Han, S.C.; Whangbo, S.H. Liquid chromatographic separation of the enantiomers of ß-amino acids on a ligand exchange chiral stationary phase. Biomed. Chromatogr. 2003, 17, 292–296. [Google Scholar] [CrossRef]
- Hyun, M.H.; Kim, D.H. Spacer length effect of a chiral stationary phase based on (+)-(18-crown-6)-2,3,11, 12-tetracarboxylic acid. Chirality 2004, 16, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Hyun, M.H.; Choi, H.J.; Kang, B.S.; Tan, G.; Cho, Y.J. Resolution of β-amino acids on a chiral stationary phase based on (+)-(18-crown-6)-2,3,11,12-tetracarboxilic acid without extra free aminopropyl groups on silica surface: The effect of ammonium ion mobile phase modifier on the resolution behaviors. Bull. Korean Chem. Soc. 2006, 27, 1775–1779. [Google Scholar] [CrossRef]
- Hyun, M.H.; Song, Y.; Cho, Y.J.; Choi, H.J. Resolution of ß-amino acids on a high performance liquid chromatographic doubly tethered chiral stationary phase containing N-CH3 amide linkage based on (+)-(18-crown-6)-2,3,11,12-tetracarboxylic acid. J. Sep. Sci. 2007, 30, 2539–2543. [Google Scholar] [CrossRef] [PubMed]
- Berkecz, R.; Ilisz, I.; Fülöp, F.; Pataj, Z.; Hyun, M.H.; Péter, A. High-performance liquid chromatographic enantioseparation of ß-3-homo-amino acid stereoisomers on a (+)-(18-crown-6)-2,3,11,12-tetracarboxylic acid-based chiral stationary phase. J. Chromatogr. A 2008, 1189, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Berkecz, R.; Ilisz, I.; Misicka, A.; Tymecka, D.; Fülöp, F.; Choi, H.J.; Hyun, M.H.; Peter, A. HPLC enantioseparation of ß-2-homoamino acids using crown ether-based chiral stationary phase. J. Sep. Sci. 2009, 32, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Gecse, Z.; Ilisz, I.; Nonn, M.; Grecsó, N.; Fülöp, F.; Agneeswari, R.; Hyun, M.H.; Péter, A. High-performance liquid chromatographic enantioseparation of isoxazoline-fused 2-aminocyclopentanecarboxylic acids on a chiral ligand-exchange stationary phase. J. Sep. Sci. 2013, 36, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Pataj, Z.; Ilisz, I.; Gecse, Z.; Szakonyi, Z.; Fülöp, F.; Lindner, W.; Péter, A. Effect of mobile phase composition on the liquid chromatographic enantioseparation of bulky monoterpene-based ß-amino acids by applying chiral stationary phases based on Cinchona alkaloid. J. Sep. Sci. 2014, 37, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Ilisz, I.; Pataj, Z.; Gecse, Z.; Szakonyi, Z.; Fülöp, F.; Lindner, W.; Péter, A. Unusual temperature-induced retention behavior of constrained ß-amino acid enantiomers on the zwitterionic chiral stationary phases ZWIX(+) and ZWIX(−). Chirality 2014, 26, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Ilisz, I.; Grecsó, N.; Palkó, M.; Fülöp, F.; Lindner, W.; Péter, A. Structural and temperature effects on enantiomer separations of bicyclo[2.2.2]octane-based 3-amino-2-carboxylic acids on Cinchona alkaloid-based zwitterionic chiral stationary phases. J. Pharm. Biomed. Anal. 2014, 98, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Schurig, V.; Ossig, J.; Link, R. Evidence for a temperature-dependent reversal of the enantioselectivity in complexation gas-chromatography on chiral phases. Angew. Chem. Int. Ed. Engl. 1989, 28, 194–196. [Google Scholar] [CrossRef]
- Gotmar, G.; Fornstedt, T.; Guiochon, G. Retention mechanism of β-blockers on an immobilized cellulase. Relative importance of the hydrophobic and ionic contributions to their enantioselective and nonselective interactions. Anal. Chem. 2000, 72, 3908–3915. [Google Scholar] [CrossRef] [PubMed]
- Fornstedt, T.; Götmar, G.; Andersson, M.; Guichon, G. Dependence on the mobile-phase ph of the adsorption behavior of propranolol enantiomers on a cellulase protein used as the chiral selector. J. Am. Chem. Soc. 1999, 121, 1164–1174. [Google Scholar] [CrossRef]
- Péter, A.; Török, G.; Armstrong, D.W.; Tóth, G.; Tourwé, D. Effect of temperature on retention of enantiomers of β-methyl amino acids on a teicoplanin chiral stationary phase. J. Chromatogr. A 1998, 828, 177–190. [Google Scholar] [CrossRef]
- Morin, N.; Guillaume, Y.C.; Peyrin, E.; Rouland, J.C. Retention mechanism study of imidazole derivatives on a β-cyclodextrin-bonded stationary phase. Thermal analysis contributions. Anal. Chem. 1998, 70, 2819–2826. [Google Scholar] [CrossRef] [PubMed]
- Cavazzini, A.; Nadalini, G.; Dondi, F.; Gasparrini, F.; Ciogli, A.; Villani, C. Study of mechanisms of chiral discrimination of amino acids and their derivatives on a teicoplanin-based chiral stationary phase. J. Chromatogr. A 2004, 1031, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Chester, T.L.; Coym, J.W. Effect of phase ratio on van’t Hoff analysis in reversed-phase liquid chromatography, and phase-ratio-independent estimation of transfer enthalpy. J. Chromatogr. A 2003, 1003, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Fornstedt, T.; Sajonz, P.; Guiochon, G. Thermodynamic study of an unusual chiral separation. Propranolol enantiomers on an immobilized cellulase. J. Am. Chem. Soc. 1997, 119, 1254–1264. [Google Scholar] [CrossRef]
- Samuelsson, J.; Arnell, R.; Fornstedt, T. Potential of adsorption isotherm measurements for closer elucidating of binding in chiral liquid chromatographic phase systems. J. Sep. Sci. 2009, 32, 1491–1506. [Google Scholar] [CrossRef] [PubMed]
- Fornstedt, T. Chracterization of adsorption process in analytical liquid-solid chromatography. J. Chromatogr. A 2010, 1217, 792–812. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.V.; Reischl, R.; Maier, N.M.; Lämmerhofer, M.; Lindner, W. Investigations of mobile phase contributions to enantioselective anion- and zwitterion-exchange modes on quinine-based zwitterionic chiral stationary phases. J. Chromatogr. A 2009, 1216, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Ilisz, I.; Grecsó, N.; Aranyi, A.; Suchotin, P.; Tymecka, D.; Wilenska, B.; Misicka, A.; Fülöp, F.; Lindner, W.; Péter, A. Enantioseparation of β2-amino acids on Cinchona alkaloid-based zwitterionic chiral stationary phases. Structural and temperature effects. J. Chromatogr. A 2014, 1334, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Ilisz, I.; Gecse, Z.; Pataj, Z.; Fülöp, F.; Tóth, G.; Lindner, W.; Péter, A. Direct high-performance liquid chromatographic enantioseparation of secondary amino acids on Cinchona alkaloid-based chiral zwitterionic stationary phases. Unusual temperature behavior. J. Chromatogr. A 2014, 1363, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.Z. Molecular mechanics and molecular shape. Part 4. Shape and steric parameters. J. Chem. Soc. Perkin Trans. 1986, 2, 1567–1986. [Google Scholar]
- Gaucher, A.; Bintein, F.; Wakselman, M.; Mazaleyrat, J.P. Synthesis of α,α-disubstituted-β-aminoacids with axial chirality. Tetrahedron Lett. 1998, 39, 575–578. [Google Scholar]
- Gaucher, A.; Wakselman, M.; Mazaleyrat, J.P.; Crisma, M.; Formaggio, F.; Toniolo, C. β-Homo-peptides built from β2,2-hbip, a biphenyl-substituted 3-amino-2,2-dimethylpropanoic acid. Tetrahedron 2000, 56, 1715–1723. [Google Scholar] [CrossRef]
- Lee, J.; Gauthier, D.; Rivero, R.A. Solid-phase synthesis of 3,4,5-substituted 1,5-benzodiazepin-2-ones. J. Org. Chem. 1999, 64, 3060–3065. [Google Scholar] [CrossRef] [PubMed]
- Caddick, S.; Judd, D.B.; Lewis, A.K.K.; Reich, M.T.; Williams, M.R.V. A generic approach for the catalytic reduction of nitriles. Tetrahedron 2003, 59, 5417–5423. [Google Scholar] [CrossRef]
- Tymecka, D.; Kosson, P.; Lipkowski, A.W.; Misicka, A. Synthesis of β2-homo aromatic amino acids and their application in endomorphin analogues. In Peptides, Proceedings of 29th European Peptide Symposium, Gdańsk, Poland, 3–8 September 2006; Rolka, K., Rekowski, P., Silberring, J., Eds.; Kenes International: Geneva, Switzerland, 2007; p. 650. [Google Scholar]
- Zukha, A.; Rivlin, J. Syntheses of DL-β-aminobutyric acid and its N-alkyl derivatives. J. Org. Chem. 1958, 23, 94–96. [Google Scholar] [CrossRef]
- Gedey, S.; Liljeblad, A.; Lázár, L.; Fülöp, F.; Kanerva, L.T. Preparation of highly enantiopure β-amino esters by Candida antarctica lipase A. Tetrahedron: Asymmetry 2001, 2, 105–110. [Google Scholar] [CrossRef]
- Furukawa, M.; Okawara, T.; Terawaki, Y. Asymmetric syntheses of β-amino acids by the addition of chiral amines to C=C double bonds. Chem. Pharm. Bull. 1977, 25, 1319–1325. [Google Scholar] [CrossRef]
- Rodionov, W.M.; Malevinskaya, E.T. Zur Darstellung von Aryl-β-amino-fettsäuren. Berichte 1926, 59, 2952–2958. [Google Scholar]
- Lázár, L.; Martinek, T.; Bernáth, G.; Fülöp, F. A simple synthesis of β-alkyl-substituted β-amino acids. Synth. Commun. 1998, 28, 219–224. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilisz, I.; Grecsó, N.; Misicka, A.; Tymecka, D.; Lázár, L.; Lindner, W.; Péter, A. Comparison of the Separation Performances of Cinchona Alkaloid-Based Zwitterionic Stationary Phases in the Enantioseparation of β2- and β3-Amino Acids. Molecules 2015, 20, 70-87. https://doi.org/10.3390/molecules20010070
Ilisz I, Grecsó N, Misicka A, Tymecka D, Lázár L, Lindner W, Péter A. Comparison of the Separation Performances of Cinchona Alkaloid-Based Zwitterionic Stationary Phases in the Enantioseparation of β2- and β3-Amino Acids. Molecules. 2015; 20(1):70-87. https://doi.org/10.3390/molecules20010070
Chicago/Turabian StyleIlisz, István, Nóra Grecsó, Aleksandra Misicka, Dagmara Tymecka, László Lázár, Wolfgang Lindner, and Antal Péter. 2015. "Comparison of the Separation Performances of Cinchona Alkaloid-Based Zwitterionic Stationary Phases in the Enantioseparation of β2- and β3-Amino Acids" Molecules 20, no. 1: 70-87. https://doi.org/10.3390/molecules20010070






