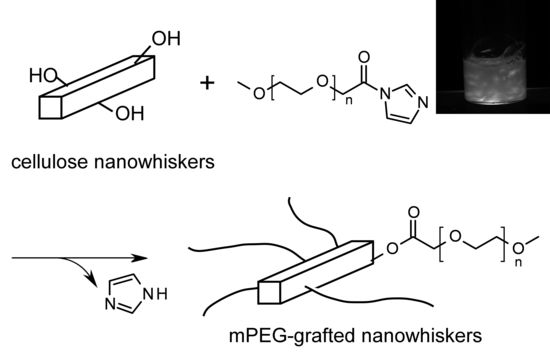
Steric Stabilization of “Charge-Free” Cellulose Nanowhiskers by Grafting of Poly(ethylene glycol)
Abstract
:1. Introduction

2. Results and Discussion
2.1. Strategy for PEG-Grafting onto “Charge-Free” Cellulose Nanowhiskers
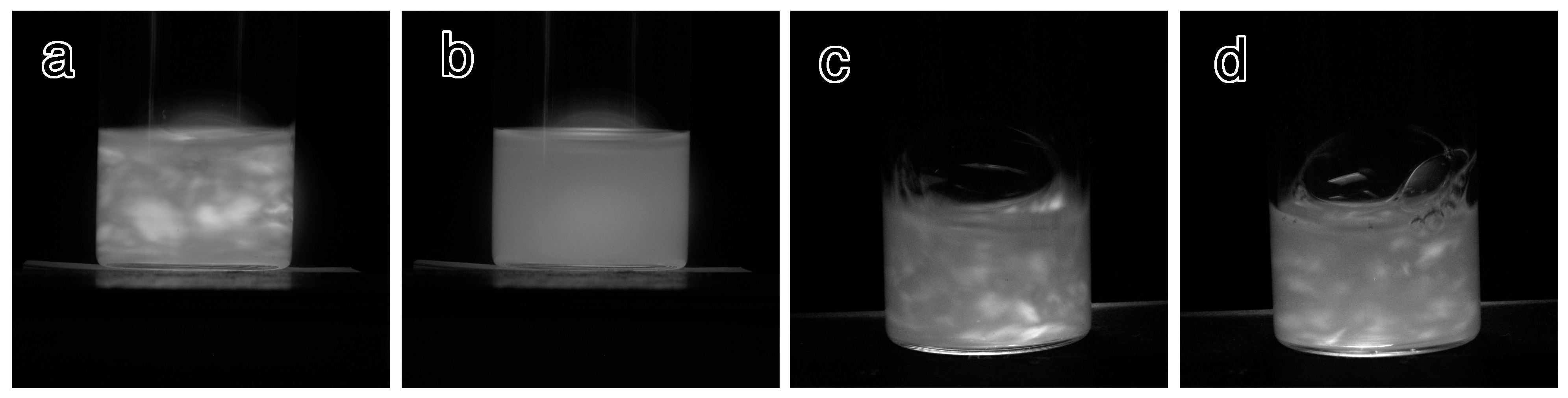
2.2. Qualitative Evaluation of Dispersion Stability of the PEG-Grafted Suspensions
| Sample Name | Flow Birefringence a | Dispersion Stability after 24 h b | ||
|---|---|---|---|---|
| in Water | in 0.1 M NaCl | in Water | in 0.1 M NaCl | |
| ungrafted | + | - | a | a |
| 1k-DMAc-80C-1d | + | + | a | a |
| 1k-DMSO-80C-1d | + | + | a | a |
| 1k-DMAc-80C-3d | + | + | a | a |
| 1k-DMSO-80C-3d | + | + | a | a |
| 1k-DMAc-100C-3d | + | + | d | a |
| 1k-DMAc-100C-7d | + | + | d | d c |

2.3. Evaluation of Amount of Bound mPEG
| Samples | The Amount of Grafted mPEG-COOH1000, g/g Cellulose | |
|---|---|---|
| from Weight Increase | from Weight Loss after Alkali Cleavage | |
| ungrafted | – | 0.03 |
| 1k-DMAc-100C-3d | 0.30 | 0.26 |
| 1k-DMAc-100C-7d | 0.32 | 0.25 |
2.4. Confirmation of mPEG Binding by ATR-FTIR Spectrometry

2.5. Transmission Electron Microscopy (TEM)

3. Experimental Section
3.1. Materials
3.2. Preparation of Cellulose Nanowhiskers
3.3. Synthesis of Terminal-Carboxylated mPEG (mPEG-COOH) via Oxidation Using Silica Gel Supported TEMPO
3.4. Grafting of mPEG-COOH onto Surfaces of Cellulose Nanowhiskers via CDI-Mediated Esterification
| Sample Name | Reaction Medium | Reaction Temperature, °C | Reaction Time, day(s) | Weight of Nanowhiskers, g | Weight of mPEG1000–COOH, g (mmol) | Weight of CDI, g (mmol) |
|---|---|---|---|---|---|---|
| 1k-DMAc-80C-1d | DMAc | 80 | 1 | 0.464 | 1.0 (1.00) | 0.200 (1.23) |
| 1k-DMSO-80C-1d | DMSO | 80 | 1 | 0.450 | 1.0 (1.00) | 0.200 (1.23) |
| 1k-DMAc-80C-3d | DMAc | 80 | 3 | 0.394 | 0.5 (0.500) | 0.100 (0.616) |
| 1k-DMSO-80C-3d | DMSO | 80 | 3 | 0.424 | 0.5 (0.500) | 0.100 (0.616) |
| 1k-DMAc-100C-3d | DMAc | 100 | 3 | 0.500 | 0.5 (0.500) | 0.100 (0.616) |
| 1k-DMAc-100C-7d | DMAc | 100 | 7 | 0.500 | 0.5 (0.500) | 0.100 (0.616) |
3.5. Qualitative Evaluation of Dispersion Stability of the PEG-Grafted Suspensions
3.6. Evaluation of Amount of Grafted PEG
3.7. Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Araki, J. Electrostatic or steric?—preparations and characterizations of well-dispersed systems containing rod-like nanowhiskers of crystalline polysaccharides. Soft Matter 2013, 9, 4125–4141. [Google Scholar] [CrossRef]
- Azizi Samir, M.A.; Alloin, F.; Dufresne, A. Review of recent research into cellulosic whiskers, their properties and their application in nanocomposite field. Biomacromolecules 2005, 6, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose nanocrystals: Chemistry, self-assembly, and applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, S.J. Cellulose nanowhiskers: Promising materials for advanced applications. Soft Matter 2011, 7, 303–315. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Grey, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Araki, J.; Wada, M.; Kuda, S.; Okano, T. Flow properties of microcrystalline cellulose suspension prepared by acid treatment of native cellulose. Colloids Surf. A 1998, 142, 75–82. [Google Scholar] [CrossRef]
- Araki, J.; Wada, M.; Kuga, S.; Okano, T. Influence of surface charge on viscosity behavior of cellulose microcrystal suspension. J. Wood Sci. 1999, 45, 258–261. [Google Scholar] [CrossRef]
- Dong, X-M.; Kimura, T.; Revol, J.-F.; Gray, D.G. Effects of ionic strength on the isotropic-chiral nematic phase transition of suspensions of cellulose crystallites. Langmuir 1996, 12, 2076–2082. [Google Scholar]
- Revol, J.-F.; Godbout, L.; Dong, X.-M.; Gray, D.G.; Chanzy, H.; Maret, G. Chiral nematic suspensions of cellulose crystallites; phase separation and magnetic field orientation. Liquid Cryst. 1994, 16, 127–134. [Google Scholar] [CrossRef]
- Revol, J.-F.; Marchessault, R.H. In vitro chiral nematic ordering of chitin crystallites. Int. J. Biol. Macromol. 1993, 15, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Revol, J.-F.; Marchessault, R.H. Rheological properties of aqueous suspensions of chitin crystallites. J. Colloid Interface Sci. 1996, 183, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Hirai, A.; Inui, O.; Horii, F.; Tsuji, M. Phase separation behavior in aqueous suspensions of bacterial cellulose nanocrystals prepared by sulfuric acid treatment. Langmuir 2009, 25, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Chanzy, H.; Henrissat, B. Electron microscopy study of the enzymic hydrolysis of Valonia cellulose. Carbohydr. Polym. 1983, 3, 161–173. [Google Scholar] [CrossRef]
- Sugiyama, J.; Chanzy, H.; Maret, G. Orientation of cellulose microcrystals by strong magnetic fields. Macromolecules 1992, 25, 4232–4234. [Google Scholar] [CrossRef]
- Araki, J.; Kuga, S. Effect of trace electrolyte on liquid crystal type of cellulose microcrystals. Langmuir 2001, 17, 4493–4496. [Google Scholar] [CrossRef]
- Araki, J.; Wada, M.; Kuga, S.; Okano, T. Birefringent glassy phase of a cellulose microcrystal suspension. Langmuir 2000, 16, 2413–2415. [Google Scholar] [CrossRef]
- Kimura, F.; Kimura, T.; Tamura, M.; Hirai, A.; Ikuno, M.; Horii, F. Magnetic alignment of the chiral nematic phase of a cellulose microfibril suspension. Langmuir 2005, 21, 2034–2037. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, S.; Kai, W.; Isogai, A.; Iwata, T. Elastic modulus of single cellulose microfibrils from tunicate measured by atomic force microscopy. Biomacromolecules 2009, 10, 2571–2576. [Google Scholar] [CrossRef] [PubMed]
- Paillet, M.; Dufresne, A. Chitin whisker reinforced thermoplastic nanocomposites. Macromolecules 2001, 34, 6527–6530. [Google Scholar] [CrossRef]
- Favier, V.; Chanzy, H.; Cavaillé, J.Y. Polymer nanocomposites reinforced by cellulose whiskers. Macromolecules 1995, 28, 6365–6367. [Google Scholar] [CrossRef]
- Herbert, W.; Cavaillé, J.Y.; Dufresne, A. Thermoplastic nanocomposites filled with wheat straw cellulose whiskers. Part I: Processing and Mechanical Behavior. Polym. Compos. 1996, 17, 604–611. [Google Scholar] [CrossRef]
- Uddin, A.J.; Araki, J.; Gotoh, Y. Towards “strong” green nanocomposites: Polyvinyl alcohol reinforced with extremely oriented cellulose whiskers. Biomacromolecules 2011, 12, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Uddin, A.J.; Araki, J.; Gotoh, Y.; Takatera, M. A novel approarch to reduce fibrillation of PVA fibers using cellulose whiskers. Textile Res. J. 2011, 81, 447–458. [Google Scholar] [CrossRef]
- Uddin, A.J.; Araki, J.; Gotoh, Y. Characterization of the poly(vinyl alcohol)/cellulose whisker gel spun fibers. Composites Part A 2011, 42, 741–747. [Google Scholar] [CrossRef]
- Uddin, A.J.; Araki, J.; Gotoh, Y. Interfacial interaction and mechanical properties of chitin whiskers/PVA gel-spun nanocomposite fibers. Polym. Int. 2011, 60, 1230–1239. [Google Scholar] [CrossRef]
- Araki, J.; Yamanaka, Y.; Ohkawa, K. Chitin-chitosan nanocomposite gels: Reinforcement of chitosan hydrogels with rod-like chitin nanowhiskers. Polym. J. 2012, 44, 713–717. [Google Scholar] [CrossRef]
- Araki, J.; Yamanaka, Y. Anionic and Cationic Nanocomposite Hydrogels Reinforced with Cellulose and Chitin Nanowhiskers: Effect of Electrolyte Concentration on Mechanical Properties and Swelling Behaviors. Polym. Adv. Technol. 2014, 25, 1108–1115. [Google Scholar] [CrossRef]
- Peresin, M.S.; Habibi, Y.; Zoppe, J.O.; Pawlak, J.J.; Rojas, O.J. Nanofiber composites of polyvinyl alcohol and cellulose nanocrystals: Manufacture and characterization. Biomacromolecules 2010, 11, 674–681. [Google Scholar] [CrossRef] [PubMed]
- Viet, D.; Beck-Candanedo, S.; Gray, D.G. Dispersion of cellulose nanocrystals in polar organic solvents. Cellulose 2007, 14, 109–113. [Google Scholar] [CrossRef]
- Napper, D.H. Steric stabilization. J. Colloid Interface Sci. 1977, 58, 390–407. [Google Scholar] [CrossRef]
- Heux, L.; Chauve, G.; Bonini, C. Nonflocculating and chiral-nematic self-ordering of cellulose microcrystals suspensions in nonpolar solvents. Langmuir 2000, 16, 8210–8212. [Google Scholar] [CrossRef]
- Bonini, C.; Heux, L.; Cavaillé, J.Y.; Lindner, P.; Dewhurst, C.; Terech, P. Rodlike cellulose whiskers coated with surfactant: A small-angle neutron scattering characterization. Langmuir 2002, 18, 3311–3314. [Google Scholar] [CrossRef]
- Araki, J.; Wada, M.; Kuga, S. Steric stabilization of a cellulose microcrystal suspension by poly(ethylene glycol) grafting. Langmuir 2001, 17, 21–27. [Google Scholar] [CrossRef]
- Araki, J.; Kuga, S.; Magoshi, J. Influence of reagent addition on carbodiimide-mediated amidation for poly(ethylene glycol) grafting. J. Appl. Polym. Sci. 2002, 85, 1349–1352. [Google Scholar] [CrossRef]
- Lönnberg, H.; Fogelström, L.; Berglund, M.A.S.A.S.L.; Malmström, E.; Hult, A. Surface grafting of microfibrillated cellulose with poly(ε-caprolactone)—Synthesis and characterization. Eur. Polym. J. 2008, 44, 2991–2997. [Google Scholar] [CrossRef]
- Lin, N.; Chen, G.; Huang, J.; Dufresne, A.; Chang, P. R. Effects of polymer-grafted natural nanocrystals on thestructure and mechanical properties of poly(lactic acid): A case of cellulose whisker-graft-polycaprolactone. J. Appl. Polym. Sci. 2009, 113, 3417–3425. [Google Scholar] [CrossRef]
- Yi, J.; Xu, Q.; Zhang, X.; Zhang, H. Chiral-nematic self-ordering of rodlike cellulose nanocrystals grafted with poly(styrene) in both thermotropic and lyotropic states. Polymer 2008, 49, 4406–4412. [Google Scholar] [CrossRef]
- Yi, J.; Xu, Q.; Zhang, X.; Zhang, H. Temperature-induced chiral nematic phase changes of suspensions of poly(N,N-dimethylaminoethyl methacrylate)-grafted cellulose nanocrystals. Cellulose 2009, 16, 989–997. [Google Scholar] [CrossRef]
- Zoppe, J.O.; Habibi, Y.; Rojas, O.J.; Venditti, R.A.; Johansson, L.-S.; Efimenko, K.; Österberg, M.; Laine, J. Poly(N-isopropylacrylamide) brushes grafted from cellulose nanocrystals via surface-initiated single-electron transfer living radical polymerization. Biomacromolecules 2010, 11, 2683–2691. [Google Scholar] [CrossRef] [PubMed]
- Azzam, F.; Heux, L.; Putaux, J.-L.; Jean, B. Preparation by grafting onto, characterization, and properties of thermally responsive polymer-decorated cellulose nanocrystals. Biomacromolecules 2010, 11, 3652–3659. [Google Scholar] [CrossRef] [PubMed]
- Araki, J.; Kurihara, M. Preparation of sterically stabilized chitin nanowhisker dispersions by grafting of poly(ethylene glycol) and evaluation of their dispersion stability. Biomacromolecules 2015, in press. [Google Scholar]
- Kloser, E.; Gray, D.G. Surface grafting of cellulose nanocrystals with poly(ethylene oxide) in aqueous media. Langmuir 2010, 26, 13450–13456. [Google Scholar] [CrossRef] [PubMed]
- Battista, O.A. Hydrolysis and crystallization of cellulose. Ind. Eng. Chem. 1950, 42, 502–507. [Google Scholar] [CrossRef]
- Edelson, M.R.; Hermans, J. Flow gels of cellulose microcrystals. II. Effect of added electrolyte. J. Polym. Sci. C 1960, 2, 145–152. [Google Scholar] [CrossRef]
- Liebert, T.F.; Heinze, T. Tailored cellulose esters: Synthesis and structure determination. Biomacromolecules 2005, 6, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Araki, J.; Kagaya, K.; Ohkawa, K. Synthesis and characterization of polyrotaxane-amino acid conjugates: A new synthetic pathway for amino-functionalized polyrotaxanes. Biomacromolecules 2009, 10, 1947–1954. [Google Scholar] [CrossRef] [PubMed]
- Araki, J.; Ohkawa, K.; Uchida, Y.; Murakami, Y. Synthesis of a “molecular rope curtain”: Preparation and characterization of a sliding graft copolymer with grafted peg side chains by the “grafting onto” strategy. J. Polym. Sci. A Polym. Chem. 2012, 50, 488–494. [Google Scholar] [CrossRef]
- Araki, J.; Zhao, C.-M.; Ito, K. Efficient production of polyrotaxanes from α-cyclodextrin and poly(ethylene glycol). Macromolecules 2005, 38, 7524–7527. [Google Scholar] [CrossRef]
- Araki, J. Effect of preparation conditions for poly(ethylene glycol)/cyclodextrin polyrotaxane on modes of end-capping reactions and decomposition of the yielded polyrotaxane. J. Polym. Sci. A Polym. Chem. 2010, 48, 5258–5264. [Google Scholar] [CrossRef]
- Fey, T.; Fischer, H.; Bachmann, S.; Albert, K.; Bolm, C. Silica-supported tempo catalysts: Synthesis and application in the anelli oxidation of alcohols. J. Org. Chem. 2011, 66, 8154–8159. [Google Scholar] [CrossRef]
- De Vos, R.; Goethals, E.J. End group analysis of commercial poly(ethylene glycol) monomethyl ether’s. Polym. Bull. 1986, 15, 547–549. [Google Scholar] [CrossRef]
- Murayama, H.; Imran, A.B.; Nagano, S.; Seki, T.; Kidowaki, M.; Ito, K.; Takeoka, Y. Chromic Slide-Ring Gel Based on Reflection from Photonic Bandgap. Macromolecules 2008, 41, 1808–1814. [Google Scholar] [CrossRef]
- Theodorou, V.; Skobridis, K.; Tzakos, A.G.; Ragoussis, V. Tetrahedron Lett. 2007, 48, 8230–8233.
- Liang, C.Y.; Marchessault, R.H. Infrared spectra of crystalline polysaccharides. I. Hydrogen bonds in native cellulose. J. Polym. Sci. 1959, 37, 385–395. [Google Scholar] [CrossRef]
- Matsuura, H.; Miyazawa, T. Vibrational analysis of molten poly(ethylene glycol). J. Polym. Sci. A-2 1969, 7, 1735–1744. [Google Scholar] [CrossRef]
- Sample Availability: Samples in the presente paper are not available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araki, J.; Mishima, S. Steric Stabilization of “Charge-Free” Cellulose Nanowhiskers by Grafting of Poly(ethylene glycol). Molecules 2015, 20, 169-184. https://doi.org/10.3390/molecules20010169
Araki J, Mishima S. Steric Stabilization of “Charge-Free” Cellulose Nanowhiskers by Grafting of Poly(ethylene glycol). Molecules. 2015; 20(1):169-184. https://doi.org/10.3390/molecules20010169
Chicago/Turabian StyleAraki, Jun, and Shiho Mishima. 2015. "Steric Stabilization of “Charge-Free” Cellulose Nanowhiskers by Grafting of Poly(ethylene glycol)" Molecules 20, no. 1: 169-184. https://doi.org/10.3390/molecules20010169