Curcumin Differs from Tetrahydrocurcumin for Molecular Targets, Signaling Pathways and Cellular Responses
Abstract
:1. Introduction
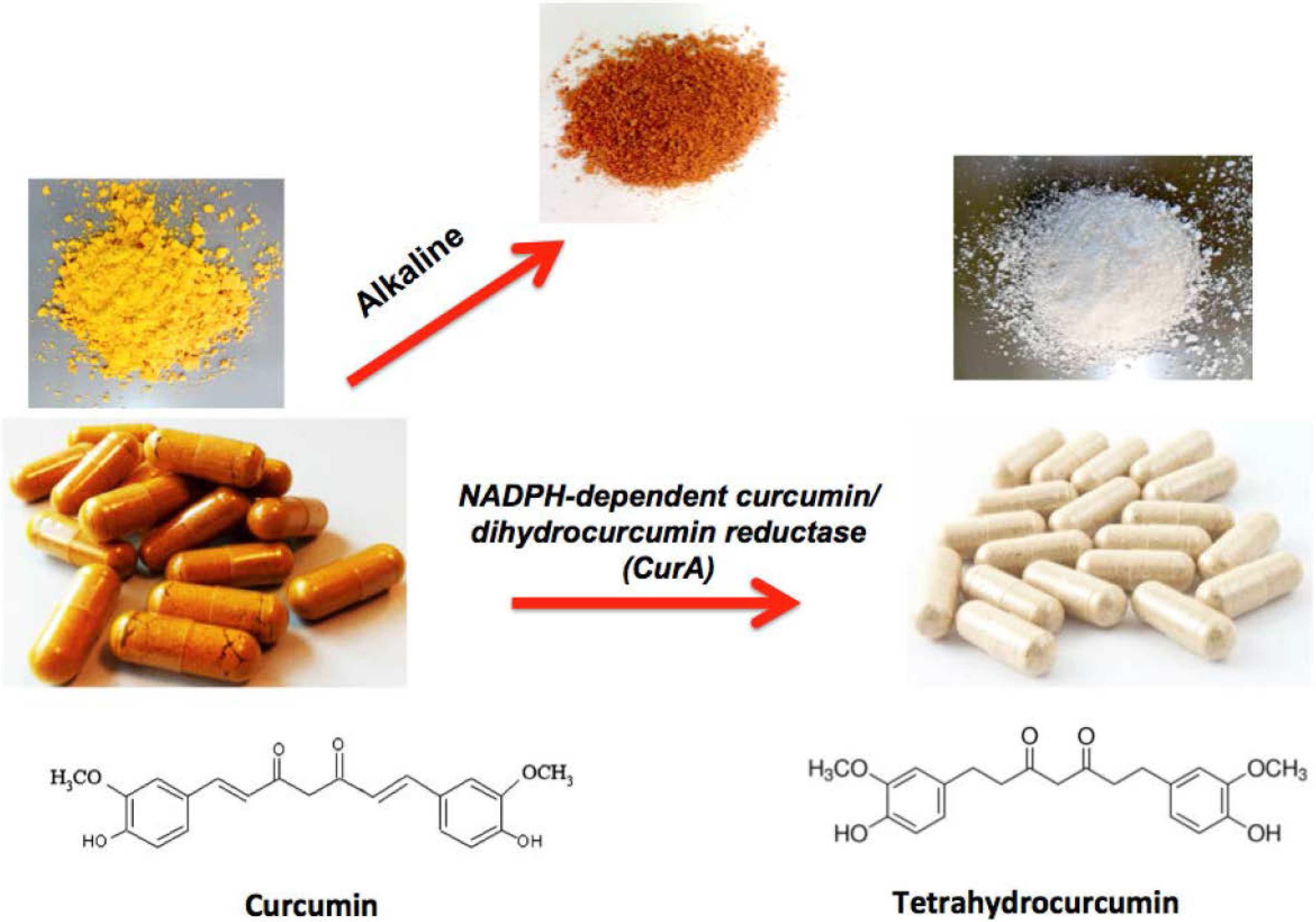
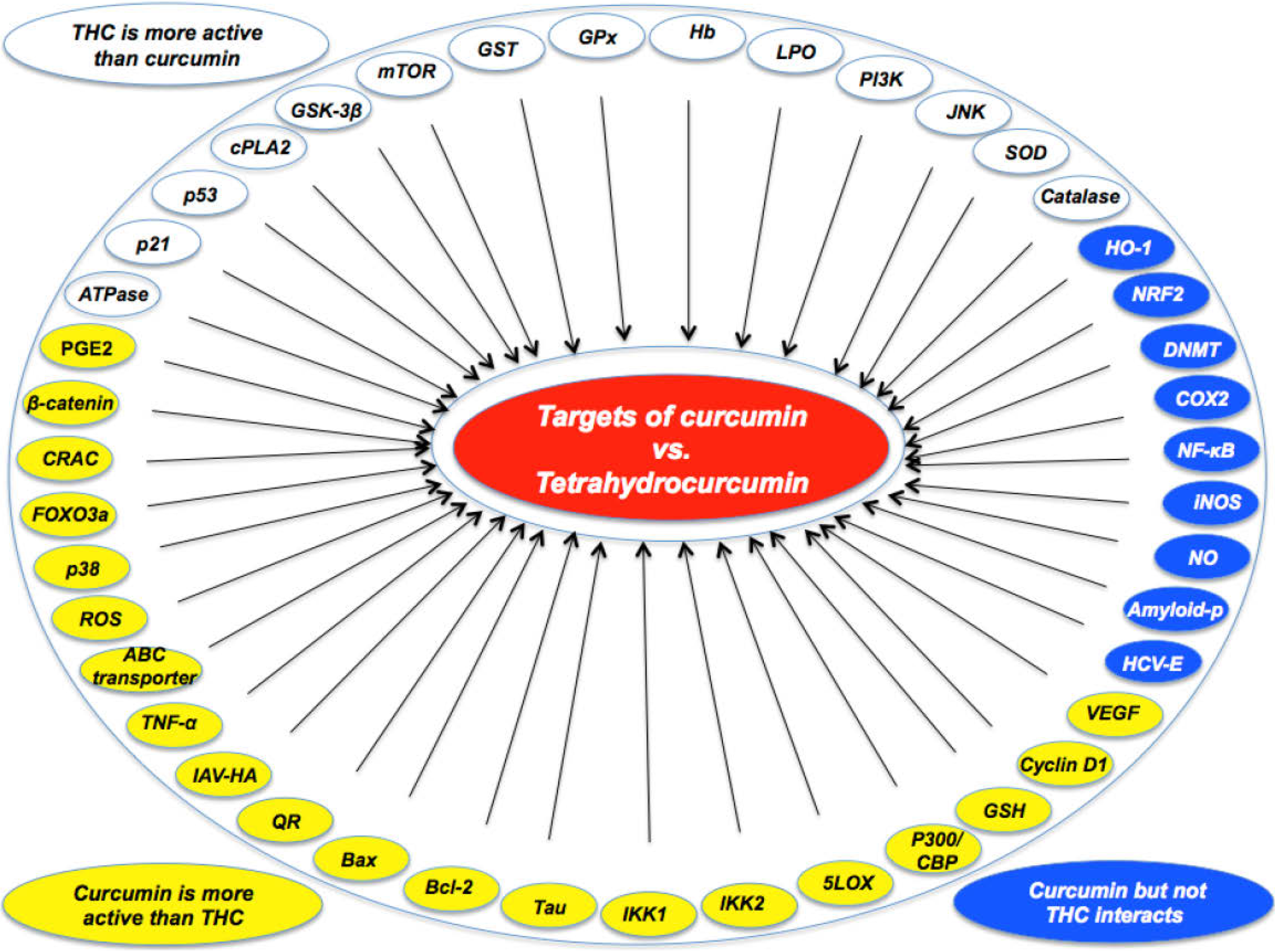
2. Studies Showing Curcumin to Be More Active than THC
| •Curcumin was more active than THC in suppressing carrageenin-induced inflammation [21]. |
| •Curcumin was more effective than THC in preventing PMA-induced skin tumor promotion in mice [22]. |
| •Curcumin was more effective than THC as an antioxidant [23]. |
| •Under aerated conditions, curcumin was more active than THC [24]. |
| •Curcumin was more effective than THC in suppressing NF-κB activation [19,25,26]. |
| •Curcumin was more effective than THC in down-modulating PMA-induced COX2 expression and PGE2 production [15]. |
| •Curcumin was more effective than THC in inhibiting 5-LOX activity [27]. |
| •Curcumin was more active than THC in ROS production and as a membrane mobility coefficient [28]. |
| •Curcumin was more effective than THC in modulating ABC drug transporters [29]. |
| •Curcumin induced apoptosis of HL-60 (decrease of bcl-2; increase of bax) but THC did not [30]. |
| •Curcumin induced HO-1 expression through activation of ARE but THC did not [31]. |
| During induction of cell death, curcumin induced ROS and GSH; THC did not [32] |
| •Curcumin, but not THC, inhibited NO production and iNOS expression [26]. |
| •Curcumin was more effective than THC in inhibiting the Wnt/beta-catenin pathway by decreasing the amount of the transcriptional coactivator p300 [33,34]. |
| •Curcumin, but not THC, inhibited LPS-stimulated NF-κB and COX-2 gene expression [35]. |
| •Curcumin, but not THC, was effective in reducing amyloid plaque burden and amyloid aggregation [36]. |
| •Curcumin, but not THC, induced HO-1 expression and Nrf2 nuclear translocation [37] |
| •Curcumin, but not THC, covalently blocked the catalytic thiolate of C1226 of DNMT1 [38]. |
| •Curcumin, but not THC, inhibited Ca(2+) influx through CRAC for activating immune cells [39]. |
| •Curcumin was more effective than THC in inducing FOXO3a-mediated gene expression by inducing FOXO3a phosphorylation and nuclear translocation [40]. |
| •Curcumin was more effective than THC in reducing β-amyloid and phosphorylated Tau protein burden in Alzheimer transgenic mice [41]. |
| •Curcumin was more active than THC in suppressing LPS-induced production of TNF-α [42]. |
| •Curcumin, but not THC, inhibited entry of hepatitis C virus genotypes into human liver cells [43]. |
| •Curcumin, but not THC, was taken up and increased lipid accumulation in monocytic cell line THP-1 [44]. |
| •Curcumin was more effective than THC in inhibiting TNF-induced expression of cyclin D1 and VEGF [25]. |
| •Curcumin inhibited type A influenza virus infection to a greater extent than THC by interfering with viral hemagglutination activity [45]. |
| •Curcumin inhibited IKK1 and IKK2 activities induced by LPS to a greater extent than THC [19]. |
2.1. Antioxidant Activities
2.2. Pro-Oxidant Activities
2.3. Anti-Inflammatory Activities
2.4. Anticancer Agent
2.6. Neurologic Effects
2.7. Immunological Effects
3. Studies Showing THC to Be More Active than Curcumin
| •THC was more active than curcumin in the carrageenin-induced rat paw edema test for anti-inflammatory activity [21] |
| •THC was more active than curcumin as an antioxidant [20,58,59,60,61] |
| •THC was more active than curcumin for suppression of lipid peroxidation of erythrocyte membrane ghosts [62]. |
| •THC was more active than curcumin for prevention of DMH-induced ACF formation in mice [63]. |
| •THC was more active than curcumin for suppression of radiation-induced lipid peroxidation [24]. |
| •THC was more active than curcumin for suppression of nitrilotriacetate-induced oxidative renal damage [64]. |
| •THC was more active than curcumin for suppression of LDL oxidation [65]. |
| •THC was more active than curcumin for inhibition of COX2-dependent arachidonic acid metabolism [27]. |
| •THC was equal to curcumin in potency for suppression of histamine release [46]. |
| •THC was more active than curcumin for inhibition of JNK activation [36]. |
| •THC was more active than curcumin for protection from chloroquine-induced hepatotoxicity in rats [66]. |
| •THC was more active than curcumin in normalizing blood glucose and improvement of altered carbohydrate metabolic enzymes in diabetic animals [67]. |
| •THC was more active than curcumin for antidiabetic effects in rats [59]. |
| •THC was more active than curcumin in increasing plasma insulin in diabetic rats [59,67,68] |
| •THC was more active than curcumin in preventing brain lipid peroxidation in diabetic rats [69]. |
| •THC was more active than curcumin in increasing tissue sialic acid [67]. |
| •THC was more active than curcumin for antidiabetic and antihyperlipidemic effects [70] |
| •THC was more active than curcumin in reducing accumulation and cross-linking of collagen in diabetic rats [71]. |
| THC was more active than curcumin in modulating renal and hepatic functional markers in diabetic rats [72] |
| •THC was more active than curcumin in modulating erythrocyte TBARS in diabetic rats [59]. |
| •THC was more active than curcumin in a hepatoprotective role in CCL4-induced liver damage in rats and alcoholic liver disease model rats [73]. |
| •THC was more effective than curcumin in improving the specific insulin binding to the receptors on erythrocytes [68]. |
| •THC was more active than curcumin in binding to phospholipase (PLA) 2 [74] |
| •THC was more active than curcumin in preventing azoxymethane-induced colon carcinogenesis [75]. |
| •THC was more active than curcumin as an antihypertensive [61]. |
| •THC activated p53 and p21 more effectively than curcumin [51]. |
3.1. Antioxidant Activities
3.2. Anti-Inflammatory Activities
3.3. Anticancer Effects
3.4. Neurologic Effects
3.5. Antidiabetic Effects
3.6. Other Effects
4. Bioavailability of Curcumin and THC
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Prasad, S.; Gupta, S.C.; Tyagi, A.K.; Aggarwal, B.B. Curcumin, a component of golden spice: From bedside to bench and back. Biotechnol. Adv. 2014, 32, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Sundaram, C.; Malani, N.; Ichikawa, H. Curcumin: the Indian solid gold. In The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Aggarwal, B.B., Surh, Y.J., Shishodia, S., Eds.; Advances in Experimental Medicine and Biology, Series Volume 595; Springer: New York, NY, USA, 2007; pp. 1–75. [Google Scholar]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar] [PubMed]
- Aggarwal, B.B. Targeting inflammation-induced obesity and metabolic diseases by curcumin and other nutraceuticals. Ann. Rev. Nutr. 2010, 30, 173–199. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sung, B. Pharmacological basis for the role of curcumin in chronic diseases: An age-old spice with modern targets. Trends Pharmacol. Sci. 2009, 30, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Jhurani, S.; Aggarwal, B.B. Multi-targeted therapy by curcumin: How spicy is it? Mol. Nutr. Food Res. 2008, 52, 1010–1030. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, E.M. Spectrophotometric determination of boron in iron and steel with curcumin after separation by 2-ethyl-1,3-hexanediol-chloroform extraction. Talanta 1981, 28, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Prasad, S.; Kim, J.H.; Patchva, S.; Webb, L.J.; Priyadarsini, I.K.; Aggarwal, B.B. Multitargeting by curcumin as revealed by molecular interaction studies. Nat. Prod. Rep. 2011, 28, 1937–1955. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Sung, B.; Kunnumakkara, A.B.; Rajasekharan, K.N.; Aggarwal, B.B. Suppression of pro-inflammatory and proliferative pathways by diferuloylmethane (curcumin) and its analogues dibenzoylmethane, dibenzoylpropane, and dibenzylideneacetone: Role of Michael acceptors and Michael donors. Biochem. Pharmacol. 2011, 82, 1901–1909. [Google Scholar] [CrossRef] [PubMed]
- Holder, G.M.; Plummer, J.L.; Ryan, A.J. Metabolism and Excretion of Curcumin (1,7-Bis-(4-Hydroxy-3-Methoxyphenyl)-1,6-Heptadiene-3,5-Dione) in the Rat. Xenobiotica 1978, 8, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Hassaninasab, A.; Hashimoto, Y.; Tomita-Yokotani, K.; Kobayashi, M. Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism. Proc. Natl. Acad. Sci. USA 2011, 108, 6615–6620. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Huang, T.M.; Lin, J.K. Biotransformation of curcumin through reduction and glucuronidation in mice. Drug Metab. Dispos. 1999, 27, 486–494. [Google Scholar] [PubMed]
- Ireson, C.R.; Jones, D.J.L.; Orr, S.; Coughtrie, M.W.H.; Boocock, D.J.; Williams, M.L.; Farmer, P.B.; Steward, W.P.; Gescher, A.J. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 105–111. [Google Scholar] [PubMed]
- Prasad, S.; Tyagi, A.K.; Aggarwal, B.B. Recent developments in delivery, bioavailability, absorption and metabolism of curcumin: the golden pigment from golden spice. Cancer Res. Treat. 2014, 46, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Ireson, C.; Orr, S.; Jones, D.J.; Verschoyle, R.; Lim, C.K.; Luo, J.L.; Howells, L.; Plummer, S.; Jukes, R.; Williams, M.; et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001, 61, 1058–1064. [Google Scholar] [PubMed]
- Li, F.; Nitteranon, V.; Tang, X.; Liang, J.; Zhang, G.; Parkin, K.L.; Hu, Q. In vitro antioxidant and anti-inflammatory activities of 1-dehydro-[6]-gingerdione, 6-shogaol, 6-dehydroshogaol and hexahydrocurcumin. Food Chem. 2012, 135, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Yang, W.L.; Kuo, S.Y. Cytotoxic activity and cell cycle analysis of hexahydrocurcumin on SW 480 human colorectal cancer cells. Nat. Prod. Commun. 2011, 6, 1671–1672. [Google Scholar] [CrossRef] [PubMed]
- Srimuangwong, K.; Tocharus, C.; Yoysungnoen Chintana, P.; Suksamrarn, A.; Tocharus, J. Hexahydrocurcumin enhances inhibitory effect of 5-fluorouracil on HT-29 human colon cancer cells. World J. Gastroenterol. 2012, 18, 2383–2389. [Google Scholar]
- Pan, M.H.; Lin-Shiau, S.Y.; Lin, J.K. Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through down-regulation of I kappa B kinase and NF kappa B activation in macrophages. Biochem. Pharmacol. 2000, 60, 1665–1676. [Google Scholar] [CrossRef] [PubMed]
- Somparn, P.; Phisalaphong, C.; Nakornchai, S.; Unchern, S.; Morales, N.P. Comparative antioxidant activities of curcumin and its demethoxy and hydrogenated derivatives. Biol. Pharm. Bull. 2007, 30, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, A.; Basu, N.; Ghatak, N.; Gujral, P.K. Anti-Inflammatory and Irritant Activities of Curcumin Analogs in Rats. Agents Actions 1982, 12, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.T.; Ma, W.; Lu, Y.P.; Chang, R.L.; Fisher, C.; Manchand, P.S.; Newmark, H.L.; Conney, A.H. Effects of Curcumin, Demethoxycurcumin, Bisdemethoxycurcumin and Tetrahydrocurcumin on 12-O-Tetradecanoylphorbol-13-Acetate-Induced Tumor Promotion. Carcinogenesis 1995, 16, 2493–2497. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Ohto, Y.; Murakami, A.; Osawa, T.; Ohigashi, H. Inhibitory effects of curcumin and tetrahydrocurcuminoids on the tumor promoter-induced reactive oxygen species generation in leukocytes in vitro and in vivo. Jpn. J. Cancer Res. 1998, 89, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Khopde, S.M.; Priyadarsini, K.I.; Guha, S.N.; Satav, J.G.; Venkatesan, P.; Rao, M.N.A. Inhibition of radiation-induced lipid peroxidation by tetrahydrocurcumin: Possible mechanisms by pulse radiolysis. Biosci. Biotechnol. Biochem. 2000, 64, 503–509. [Google Scholar] [CrossRef]
- Sandur, S.K.; Pandey, M.K.; Sung, B.; Ahn, K.S.; Murakatni, A.; Sethi, G.; Limtrakul, P.; Badmaev, V.; Aggarwal, B.B. Curcumin, demethtoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 2007, 28, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Pae, H.O.; Jeong, S.O.; Kim, H.S.; Kim, S.H.; Song, Y.S.; Kim, S.K.; Chai, K.Y.; Chung, H.T. Dimethoxycurcumin, a synthetic curcumin analogue with higher metabolic stability, inhibits NO production, inducible NO synthase expression and NF-kappaB activation in RAW264.7 macrophages activated with LPS. Mol. Nutr. Food Res. 2008, 52, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.I.; Bose, M.; Ju, J.Y.; Ryu, J.H.; Chen, X.X.; Sang, S.M.; Lee, M.J.; Yang, C.S. Modulation of arachidonic acid metabolism by curcumin and related beta-diketone derivatives: Effects on cytosolic phospholipase A, cyclooxygenases and 5-lipoxygenase. Carcinogenesis 2004, 25, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, T.; Fujisawa, S.; Tonosaki, K. Relationship between intracellular ROS production and membrane mobility in curcumin- and tetrahydrocurcumin-treated human gingival fibroblasts and human submandibular gland carcinoma cells. Oral Dis. 2005, 11, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Limtrakul, P.; Chearwae, W.; Shukla, S.; Phisalphong, C.; Ambudkar, S.V. Modulation of function of three ABC drug transporters, P-glycoprotein (ABCB1), mitoxantrone resistance protein (ABCG2) and multidrug resistance protein 1 (ABCC1) by tetrahydrocurcumin, a major metabolite of curcumin. Mol. Cell. Biochem. 2007, 296, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Pae, H.O.; Jeong, S.O.; Jeong, G.S.; Kim, K.M.; Kim, H.S.; Kim, S.A.; Kim, Y.C.; Kang, S.D.; Kim, B.N.; Chung, H.T. Curcumin induces pro-apoptotic endoplasmic reticulum stress in human leukemia HL-60 cells. Biochem. Biophys. Res. Commun. 2007, 353, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Pae, H.O.; Jeong, G.S.; Jeong, S.O.; Kim, H.S.; Kim, S.A.; Kim, Y.C.; Yoo, S.J.; Kim, H.D.; Chung, H.T. Roles of heme oxygenase-1 in curcumin-induced growth inhibition in rat smooth muscle cells. Exp. Mol. Med. 2007, 39, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, T.; Tonosaki, K.; Fujisawa, S. Comparative cytotoxicity and ROS generation by curcumin and tetrahydrocurcumin following visible-light irradiation or treatment with horseradish peroxidase. Anticancer Res. 2007, 27, 363–371. [Google Scholar] [PubMed]
- Ryu, M.J.; Cho, M.; Song, J.Y.; Yun, Y.S.; Choi, I.W.; Kim, D.E.; Park, B.S.; Oh, S. Natural derivatives of curcumin attenuate the Wnt/beta-catenin pathway through down-regulation of the transcriptional coactivator p300. Biochem. Biophys. Res. Commun. 2008, 377, 1304–1308. [Google Scholar] [CrossRef] [PubMed]
- Marcu, M.G.; Jung, Y.J.; Lee, S.; Chung, E.J.; Lee, M.J.; Trepel, J.; Neckers, L. Curcumin is an Inhibitor of p300 Histone Acetylatransferase. Med. Chem. 2006, 2, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Ishii, H.; Takada, N.; Tanaka, S.; Machin, M.; Ito, S.; Fujisawa, S. Comparative anti-inflammatory activities of curcumin and tetrahydrocurcumin based on the phenolic O-H bond dissociation enthalpy, ionization potential and quantum chemical descriptor. Anticancer Res. 2008, 28, 699–707. [Google Scholar] [PubMed]
- Begum, A.N.; Jones, M.R.; Lim, G.P.; Morihara, T.; Kim, P.; Heath, D.D.; Rock, C.L.; Pruitt, M.A.; Yang, F.; Hudspeth, B.; et al. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2008, 326, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.O.; Oh, G.S.; Ha, H.Y.; Soon Koo, B.; Sung Kim, H.; Kim, Y.C.; Kim, E.C.; Lee, K.M.; Chung, H.T.; Pae, H.O. Dimethoxycurcumin, a Synthetic Curcumin Analogue, Induces Heme Oxygenase-1 Expression through Nrf2 Activation in RAW264.7 Macrophages. J. Clin. Biochem. Nutr. 2009, 44, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xie, Z.; Jones, W.; Pavlovicz, R.E.; Liu, S.; Yu, J.; Li, P.K.; Lin, J.; Fuchs, J.R.; Marcucci, G.; et al. Curcumin is a potent DNA hypomethylation agent. Bioorg. Med. Chem. Lett. 2009, 19, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.H.; Nam, J.H.; Lee, E.S.; Zhang, Y.; Kim, S.J. Inhibition of Ca2+ release-activated Ca2+ channel (CRAC) by curcumin and caffeic acid phenethyl ester (CAPE) via electrophilic addition to a cysteine residue of Orai1. Biochem. Biophys. Res. Commun. 2012, 428, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Zingg, J.M.; Hasan, S.T.; Cowan, D.; Ricciarelli, R.; Azzi, A.; Meydani, M. Regulatory effects of curcumin on lipid accumulation in monocytes/macrophages. J. Cell. Biochem. 2012, 113, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Shytle, R.D.; Tan, J.; Bickford, P.C.; Rezai-Zadeh, K.; Hou, L.; Zeng, J.; Sanberg, P.R.; Sanberg, C.D.; Alberte, R.S.; Fink, R.C.; et al. Optimized turmeric extract reduces beta-Amyloid and phosphorylated Tau protein burden in Alzheimer's transgenic mice. Curr. Alzheimer Res. 2012, 9, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Nishida, M.; Nishiumi, S.; Mizushina, Y.; Fujishima, Y.; Yamamoto, K.; Masuda, A.; Mizuno, S.; Fujita, T.; Morita, Y.; Kutsumi, H.; et al. Monoacetylcurcumin strongly regulates inflammatory responses through inhibition of NF-kappaB activation. Int. J. Mol. Med. 2010, 25, 761–767. [Google Scholar] [PubMed]
- Anggakusuma; Colpitts, C.C.; Schang, L.M.; Rachmawati, H.; Frentzen, A.; Pfaender, S.; Behrendt, P.; Brown, R.J.; Bankwitz, D.; Steinmann, J.; et al. Turmeric curcumin inhibits entry of all hepatitis C virus genotypes into human liver cells. Gut 2014, 63, 1137–1149. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Zingg, J.M.; Kim, S.H.; Thomas, M.J.; Dolnikowski, G.G.; Azzi, A.; Miyazawa, T.; Meydani, M. Differential cellular uptake and metabolism of curcuminoids in monocytes/macrophages: Regulatory effects on lipid accumulation. Br. J. Nutr. 2014, 112, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.L.; Mizushina, Y.; Wang, S.Y.; Chuang, D.Y.; Nadar, M.; Hsu, W.L. Structure-activity relationship analysis of curcumin analogues on anti-influenza virus activity. FEBS J. 2013, 280, 5829–5840. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Nakamura, T.; Iyoki, S.; Fujiwara, A.; Watanabe, Y.; Mohri, K.; Isobe, K.; Ono, K.; Yano, S. Elucidation of anti-allergic activities of curcumin-related compounds with a special reference to their anti-oxidative activities. Biol. Pharm. Bull. 2005, 28, 1438–1443. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Chu, L.C.; Hua, K.F.; Chao, L.K. Heme oxygenase-1 mediates the anti-inflammatory effect of curcumin within LPS-stimulated human monocytes. J. Cell Physiol. 2008, 215, 603–612. [Google Scholar] [CrossRef]
- Farombi, E.O.; Shrotriya, S.; Na, H.K.; Kim, S.H.; Surh, Y.J. Curcumin attenuates dimethylnitrosamine-induced liver injury in rats through Nrf2-mediated induction of heme oxygenase-1. Food Chem. Toxicol. 2008, 46, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Yoysungnoen, P.; Wirachwong, P.; Changtam, C.; Suksamram, A.; Patumraj, S. Anti-cancer and anti-angiogenic effects of curcumin and tetrahydrocurcumin on implanted hepatocellular carcinoma in nude mice. World J. Gastroenterol. 2008, 14, 2003–2009. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Wang, M.M.; Wang, Y.H.; Zhang, Z.N.; Cao, H.R.; Lv, Y.H.; Yang, Y.; Fan, P.H.; Qiu, F.; Gao, X.M. Tetrahydrocurcumin induces G2/M cell cycle arrest and apoptosis involving p38 MAPK activation in human breast cancer cells. Food Chem. Toxicol. 2014, 67, 193–200. [Google Scholar] [CrossRef]
- Ayli, E.E.; Dugas-Breit, S.; Li, W.; Marshall, C.; Zhao, L.; Meulener, M.; Griffin, T.; Gelfand, J.M.; Seykora, J.T. Curcuminoids activate p38 MAP kinases and promote UVB-dependent signalling in keratinocytes. Exp. Dermatol. 2010, 19, 493–500. [Google Scholar] [PubMed]
- Barry, J.; Fritz, M.; Brender, J.R.; Smith, P.E.S.; Lee, D.K.; Ramamoorthy, A. Determining the Effects of Lipophilic Drugs on Membrane Structure by Solid-State NMR Spectroscopy: The Case of the Antioxidant Curcumin. J. Am. Chem. Soc. 2009, 131, 4490–4498. [Google Scholar] [CrossRef] [PubMed]
- Lupberger, J.; Zeisel, M.B.; Xiao, F.; Thumann, C.; Fofana, I.; Zona, L.; Davis, C.; Mee, C.J.; Turek, M.; Gorke, S.; et al. EGFR and EphA2 are host factors for hepatitis C virus entry and possible targets for antiviral therapy. Nat. Med. 2011, 17, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.Y.; Shien, J.H.; Tiley, L.; Chiou, S.S.; Wang, S.Y.; Chang, T.J.; Lee, Y.J.; Chan, K.W.; Hsu, W.L. Curcumin inhibits influenza virus infection and haemagglutination activity. Food Chem. 2010, 119, 1346–1351. [Google Scholar]
- Chen, T.Y.; Chen, D.Y.; Wen, H.W.; Ou, J.L.; Chiou, S.S.; Chen, J.M.; Wong, M.L.; Hsu, W.L. Inhibition of Enveloped Viruses Infectivity by Curcumin. PLoS One 2013, 8, e62482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, B.Y.; Song, Y.J.; Kim, K.M.; Choe, Y.K.; Hwang, S.Y.; Kim, T.S. Curcumin inhibits Th1 cytokine profile in CD4(+) T cells by suppressing interleukin-12 production in macrophages. Br. J. Pharmacol. 1999, 128, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Rajeswari, A.; Sabesan, M. Inhibition of monoamine oxidase-B by the polyphenolic compound, curcumin and its metabolite tetrahydrocurcumin, in a model of Parkinson’s disease induced by MPTP neurodegeneration in mice. Inflammopharmacology 2008, 16, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Osawa, T.; Kato, Y. Protective role of antioxidative food factors in oxidative stress caused by hyperglycemia. Ann. N. Y. Acad. Sci. 2005, 1043, 440–451. [Google Scholar] [CrossRef] [PubMed]
- Murugan, P.; Pari, L. Influence of tetrahydrocurcumin on erythrocyte membrane bound enzymes and antioxidant status in experimental type 2 diabetic rats. J. Ethnopharmacol. 2007, 113, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Kadoma, Y.; Fujisawa, S. Comparative radical-scavenging activity of curcumin and tetrahydrocurcumin with thiols as measured by the induction period method. In Vivo 2007, 21, 979–982. [Google Scholar] [PubMed]
- Nakmareong, S.; Kukongviriyapan, U.; Pakdeechote, P.; Donpunha, W.; Kukongviriyapan, V.; Kongyingyoes, B.; Sompamit, K.; Phisalaphong, C. Antioxidant and vascular protective effects of curcumin and tetrahydrocurcumin in rats with l-NAME-induced hypertension. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2011, 383, 519–529. [Google Scholar] [CrossRef]
- Sugiyama, Y.; Kawakishi, S.; Osawa, T. Involvement of the beta-diketone moiety in the antioxidative mechanism of tetrahydrocurcumin. Biochem. Pharmacol. 1996, 52, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Araki, S.; Kim, D.J.; Park, C.B.; Takasuka, N.; Baba-Toriyama, H.; Ota, T.; Nir, Z.; Khachik, F.; Shimidzu, N.; et al. Chemopreventive effects of carotenoids and curcumins on mouse colon carcinogenesis after 1,2-dimethylhydrazine initiation. Carcinogenesis 1998, 19, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Wangpoengtrakul, C.; Tanaka, T.; Toyokuni, S.; Uchida, K.; Osawa, T. Curcumin and especially tetrahydrocurcumin ameliorate oxidative stress-induced renal injury in mice. J. Nutr. 2001, 131, 2090–2095. [Google Scholar] [PubMed]
- Naito, M.; Wu, X.; Nomura, H.; Kodama, M.; Kato, Y.; Kato, Y.; Osawa, T. The protective effects of tetrahydrocurcumin on oxidative stress in cholesterol-fed rabbits. J. Atheroscler. Thromb. 2002, 9, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Pari, L.; Amali, D.R. Protective role of tetrahydrocurcumin (THC) an active principle of turmeric on chloroquine induced hepatotoxicity in rats. J. Pharm. Pharm. Sci. 2005, 8, 115–123. [Google Scholar] [PubMed]
- Pari, L.; Murugan, P. Changes in glycoprotein components in streptozotocin-nicotinamide induced type 2 diabetes: Influence of tetrahydrocurcumin from Curcuma longa. Plant Food Hum. Nutr. 2007, 62, 25–29. [Google Scholar] [CrossRef]
- Murugan, P.; Pari, L.; Rao, C.A. Effect of tetrahydrocurcumin on insulin receptor status in type 2 diabetic rats: Studies on insulin binding to erythrocytes. J. Biosci. 2008, 33, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Pari, L.; Murugan, P. Tetrahydrocurcumin prevents brain lipid peroxidation in Streptozotocin-induced diabetic rats. J. Med. Food. 2007, 10, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Pari, L.; Murugan, P. Antihyperlipidemic effect of curcumin and tetrahydrocurcumin in experimental type 2 diabetic rats. Renal Fail. 2007, 29, 881–889. [Google Scholar] [CrossRef]
- Pari, L.; Murugan, P. Influence of tetrahydrocurcumin on tail tendon collagen contents and its properties in rats with streptozotocin-nicotinamide-induced type 2 diabetes. Fund Clin. Pharmacol. 2007, 21, 665–671. [Google Scholar] [CrossRef]
- Murugan, P.; Pari, L. Influence of tetrahydrocurcumin on hepatic and renal functional markers and protein levels in experimental type 2 diabetic rats. Basic Clin. Pharmacol. 2007, 101, 241–245. [Google Scholar]
- Osawa, T. Nephroprotective and hepatoprotective effects of curcuminoids. Adv. Exp. Med. Biol. 2007, 595, 407–423. [Google Scholar] [PubMed]
- Dileep, K.V.; Tintu, I.; Sadasivan, C. Molecular docking studies of curcumin analogs with phospholipase A2. Interdiscip. Sci. Comput. Life Sci. 2011, 3, 189–197. [Google Scholar] [CrossRef]
- Lai, C.S.; Wu, J.C.; Yu, S.F.; Badmaev, V.; Nagabhushanam, K.; Ho, C.T.; Pan, M.H. Tetrahydrocurcumin is more effective than curcumin in preventing azoxymethane-induced colon carcinogenesis. Mol. Nutr. Food Res. 2011, 55, 1819–1828. [Google Scholar] [CrossRef] [PubMed]
- Arunkhamkaew, S.; Athipornchai, A.; Apiratikul, N.; Suksamrarn, A.; Ajavakom, V. Novel racemic tetrahydrocurcuminoid dihydropyrimidinone analogues as potent acetylcholinesterase inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 2880–2882. [Google Scholar] [CrossRef] [PubMed]
- Pari, L.; Murugan, P. Tetrahydrocurcumin: Effect on chloroquine-mediated oxidative damage in rat kidney. Basic Clin. Pharmacol. 2006, 99, 329–334. [Google Scholar] [CrossRef]
- Asai, A.; Miyazawa, T. Occurrence of orally administered curcuminoid as glucuronide and glucuronide/sulfate conjugates in rat plasma. Life Sci. 2000, 67, 2785–2793. [Google Scholar] [CrossRef] [PubMed]
- Neyrinck, A.M.; Alligier, M.; Memvanga, P.B.; Nevraumont, E.; Larondelle, Y.; Preat, V.; Cani, P.D.; Delzenne, N.M. Curcuma longa extract associated with white pepper lessens high fat diet-induced inflammation in subcutaneous adipose tissue. PLoS One 2013, 8, e81252. [Google Scholar] [CrossRef]
- Pfeiffer, E.; Hoehle, S.I.; Walch, S.G.; Riess, A.; Solyom, A.M.; Metzler, M. Curcuminoids form reactive glucuronides in vitro. J. Agric. Food Chem. 2007, 55, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Vijaya Saradhi, U.V.; Ling, Y.; Wang, J.; Chiu, M.; Schwartz, E.B.; Fuchs, J.R.; Chan, K.K.; Liu, Z. A liquid chromatography-tandem mass spectrometric method for quantification of curcuminoids in cell medium and mouse plasma. J. Chromatogr. B 2010, 878, 3045–3051. [Google Scholar] [CrossRef]
- Hong, J.; Lambert, J.D.; Lee, S.H.; Sinko, P.J.; Yang, C.S. Involvement of multidrug resistance-associated proteins in regulating cellular levels of (−)-epigallocatechin-3-gallate and its methyl metabolites. Biochem. Biophys. Res. Commun. 2003, 310, 222–227. [Google Scholar] [CrossRef]
- Shoba, G.; Joy, D.; Joseph, T.; Majeed, M.; Rajendran, R.; Srinivas, P.S. Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Med. 1998, 64, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M.; et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Bi, C.; Chan, H.M.; Sun, S.; Zhang, Q.; Zheng, Y. Curcumin-loaded solid lipid nanoparticles have prolonged in vitro antitumour activity, cellular uptake and improved in vivo bioavailability. Colloids Surf. B 2013, 111, 367–375. [Google Scholar] [CrossRef]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aggarwal, B.B.; Deb, L.; Prasad, S. Curcumin Differs from Tetrahydrocurcumin for Molecular Targets, Signaling Pathways and Cellular Responses. Molecules 2015, 20, 185-205. https://doi.org/10.3390/molecules20010185
Aggarwal BB, Deb L, Prasad S. Curcumin Differs from Tetrahydrocurcumin for Molecular Targets, Signaling Pathways and Cellular Responses. Molecules. 2015; 20(1):185-205. https://doi.org/10.3390/molecules20010185
Chicago/Turabian StyleAggarwal, Bharat B., Lokesh Deb, and Sahdeo Prasad. 2015. "Curcumin Differs from Tetrahydrocurcumin for Molecular Targets, Signaling Pathways and Cellular Responses" Molecules 20, no. 1: 185-205. https://doi.org/10.3390/molecules20010185






