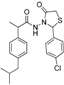
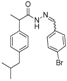
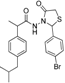
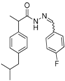
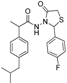
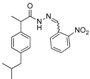
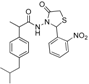
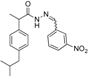
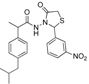
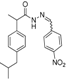
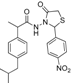
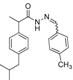
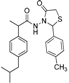
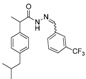
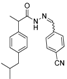
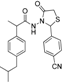
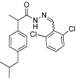
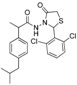
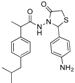
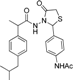
3.2.1. Preparation of the Acyl Hydrazones 3a–l
Preparation of the acyl hydrazones
3a–
l was realized according to a conventional method from the literature [
31]. To a solution of 2-(4-isobutylphenyl)propionic acid hydrazide
5 (13.6 mmol) in dry ethanol (50 mL) aromatic aldehydes (13.6 mmol) were added. The reaction mixtures were heated at 90–95 °C until completion of the reaction (TLC monitoring using dichloromethane-methanol, 9.8:0.2, v/v, UV light at 254 nm). The mixture was cooled to room temperature and the solvent was removed by rotary evaporator. The residue was purified on a silica gel column using dichloromethane-methanol (9.8:0.2, v/v) as eluent system. FT-IR and physical data for
3a–
g were reported in our previous paper [
22].
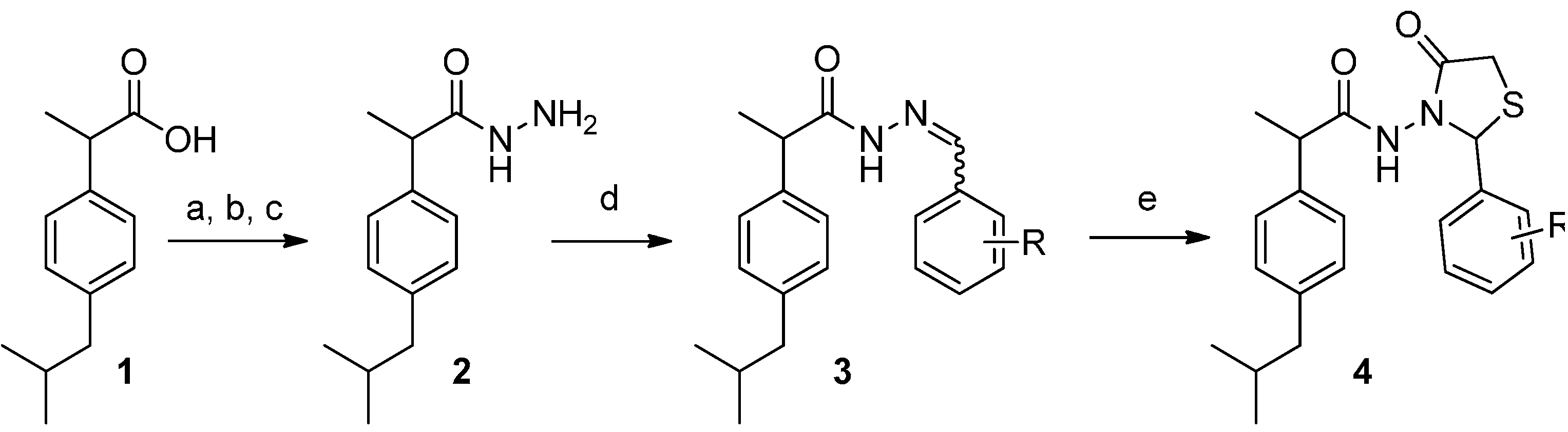
2-(4-Isobutylphenyl)propionic acid (benzylidene)hydrazide (3a). 50/50 racemic mixture. 1H-NMR (δ ppm): 11.50/11.25 (s, 1H, -NH-N=), 8.21/7.92 (s, 1H, -N=CH-), 7.76–7.56 (m, 2H, Ar-H ), 7.41 (d, J = 6.9 Hz, 3H, Ar-H), 7.27 (dd, J = 8.0, 4.8 Hz, 2H, Ar-H), 7.08 (dd, J = 14.6, 8.0 Hz, 2H, Ar-H), 4.65/3.67 (q, J = 6.9 Hz, 1H, -CH-CH3), 2.38 (dd, J = 13.8, 6.6 Hz, 2H, -CH2-CH(CH3)2), 1.85–1.69 (m, -CH2-CH(CH3)2), 1.39 (t, J = 6.9 Hz, 3H, -CH-CH3), 0.83 (dd, J = 11.5, 6.6 Hz, 6H, -CH2 CH(CH3)2); 13C-NMR (δ ppm): 175.48/170.27 (Cq), 146.94/142.92 (N=CH), 139.95/139.20 (Cq), 139.58 (Cq), 134.71 (Cq), 130.30/130.02 (CHAr), 129.23 (CHAr), 127.67 (CHAr), 127.38 (CHAr), 127.06 (CHAr), 44.63 (-CH2-CH(CH3)2), 44.05 (-CH-CH3)/40.59 (-CH-CH3), 29.99 (-CH(CH3)2), 22.56 (-CH(CH3)2), 18.87 (-CH-CH3); HRMS (EI-MS): m/z calculated 309.196140; [M+H]+ found 309.196400; Green chemistry metrics: E-factor 16.001, ME 0.059.
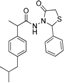
2-(4-Isobutylphenyl)propionic acid (4-chlorobenzylidene)hydrazide (3b). 50/50 racemic mixture. 1H-NMR (δ ppm): 11.57/11.31 (s, 1H, -NH-N=), 8.20/7.91 (s, 1H, -N=CH), 7.79–7.59 (m, 2H, Ar-H), 7.46 (d, J = 8.4 Hz, 2H, Ar-H), 7.26 (t, J = 7.6 Hz, 2H, Ar-H), 7.07 (dd, J = 14.9, 7.6 Hz, 2H, Ar-H), 4.63/3.67 (q, J = 7.0 Hz, 1H, -CH-CH3), 2.37 (dd, J = 13.6, 6.9 Hz, 2H, -CH2-CH(CH3)2), 1.84–1.69 (m, 1H, -CH2-CH(CH3)2), 1.38 (t, J = 7.0 Hz, 3H, -CH-CH3), 0.81 (dd, J = 11.5, 6.9 Hz, 6H, -CH2-CH(CH3)2); 13C-NMR (δ ppm): 175.59/170.41 (Cq), 145.68/141.68 (-N=CH-), 140.02/139.20 (Cq), 139.62 (Cq), 134.81/134.52 (Cq), 133.73 (Cq), 129.33 (2CHAr), 129.02/128.74 (CHAr), 127.72/127.47 (CHAr), 44.69 (-CH2-CH(CH3)2, 44.11 (-CH-CH3)/40.59 (-CH-CH3), 29.99 (-CH-(CH3)2), 22.60 (-CH(CH3)2), 18.85 (-CH-CH3); HRMS (EI-MS): m/z calculated 343.157167; [M+H]+ found 343.157343; Green chemistry metrics: E-factor 11.942, ME 0.077.
2-(4-Isobutylphenyl)propionic acid (4-bromobenzylidene)hydrazide (3c). 50/50 racemic mixture. 1H-NMR (δ ppm): 11.57/11.32 (s, 1H, -NH-N=), 8.18/7.89 (s, 1H, -N=CH), 7.60 (d, J = 2.2 Hz, 4H, Ar-H), 7.26 (t, J = 7.6 Hz, 2H, Ar-H), 7.07 (dd, J = 15.1, 7.6 Hz, 2H, Ar-H), 4.63/3.67 (q, J = 6.9 Hz, 1H, -CH-CH3), 2.37 (dd, J = 13.0, 6.8 Hz, 2H, -CH2-CH-(CH3)2), 1.77 (dq, J = 20.3, 6.8 Hz, 1H, -CH2-CH-(CH3)2), 1.38 (t, J = 6.9 Hz, 3H, -CH-CH3), 0.81 (dd, J = 13.0, 6.8 Hz, 6H, -CH2-CH-(CH3)2); 13C-NMR (δ ppm): 175.11/169.93 (Cq), 145.27/141.29 (-N=CH-), 139.53/138.70 (Cq), 139.12 (Cq), 133.59 (Cq), 131.72 (CHAr), 128.85 (CHAr), 128.50 (CHAr), 127.24/126.98 (CHAr), 123.10/122.77 (Cq), 44.21 (-CH2-CH(CH3)2), 43.64 (-CH-CH3)/40.18 (-CH-CH3), 29.56 (-CH-(CH3)2), 22.13 (-CH-(CH3)2), 18.43 (-CH-CH3); HRMS (EI-MS): m/z calculated 387.106652; [M+H]+ found 387.106804; Green chemistry metrics: E-factor 10.341, ME 0.088.
2-(4-Isobutylphenyl)propionic acid (4-fluorobenzylidene)hydrazide (3d). 50/50 racemic mixture. 1H-NMR (δ ppm): 11.50/11.25 (s, 1H, -NH-N=), 8.20/7.91 (s, 1H, -N=CH), 7.70 (dt, J = 9.0, 5.9 Hz, 2H, Ar-H), 7.27–7.23 (m, 4H, Ar-H), 7.08 (dd, J = 14.2, 9.0 Hz, 2H, Ar-H), 4.63/3.65 (q, J = 7.0 Hz, 1H, -CH-CH3), 2.38 (dd, J = 13.3, 6.7 Hz, 2H,-CH2-CH(CH3)2), 1.85–1.70 (m, 1H, -CH2-CH(CH3)2), 1.38 (t, J = 7.0 Hz, 3H, -CH-CH3), 0.82 (dd, J = 10.7, 6.7 Hz, 6H, -CH2-CH(CH3)2); 13C-NMR (δ ppm): 175.06/169.86 (Cq), 164.14/161.04 (Cq), 145.42/141.36 (-N=CH-), 139.54/138.78 (Cq), 139.17 (Cq), 131.32 (Cq), 129.09 (CHAr), 128.83 (CHAr), 127.25/126.99 (CHAr), 115.91/115.69 (CHAr), 44.63 (-CH2-CH(CH3)2), 44.02/40.58 (-CH-CH3), 30.00 (-CH(CH3)2), 22.56 (-CH(CH3)2), 18.87 (-CH-CH3); HRMS (EI-MS): m/z calculated 327.186718; [M+H]+ found 327.186906; Green chemistry metrics: E-factor 13.705, ME 0.068.
2-(4-Isobutylphenyl)propionic acid (2-nitrobenzylidene)hydrazide (3e). 50/50 racemic mixture. 1H-NMR (δ ppm): 11.69 (s, 1H, -NH-N=), 8.61/8.30 (s, 1H, -N=CH), 8.01 (td, J = 14.3, 7.7 Hz, 2H, Ar-H), 7.76 (t, J = 7.7 Hz, 1H, Ar-H), 7.63 (q, J = 7.7 Hz, 1H, Ar-H), 7.31–7.20 (m, 2H, Ar-H), 7.09 (dd, J = 12.0, 8.6 Hz, 2H, Ar-H), 4.59/3.68 (q, J = 8.0 Hz, 1H, -CH-CH3), 2.44–2.33 (m, 2H, -CH2-CH(CH3)2), 1.79 (m, 1H, -CH2-CH(CH3)2), 1.45–1.32 (m, 3H, -CH-CH3), 0.82 (dd, J = 11.3, 7.2 Hz, 6H, -CH2-CH(CH3)2); 13C-NMR (δ ppm): 175.40/170.20 (Cq), 148.01 (Cq), 141.76/138.62 (-N=CH-), 139.63/139.27 (Cq), 139.00/137.87 (Cq), 133.54 (CHAr), 130.34 (CHAr), 128.94 (CHAr), 128.75/128.40 (Cq), 127.86 (CHAr), 127.13 (CHAr), 124.56 (CHAr), 44.22 (-CH2-CH-(CH3)2), 43.71 (-CH-CH3)/40.24 (-CH-CH3), 29.58 (-CH(CH3)2), 22.14 (-CH(CH3)2), 18.52 (-CH-CH3); HRMS (EI-MS): m/z calculated 354.181218; [M+H]+ found 354.181363; Green chemistry metrics: E-factor 14.772, ME 0.063.
2-(4-Isobutylphenyl)propionic acid (3-nitrobenzylidene)hydrazide (3f). 50/50 racemic mixture. 1H-NMR (δ ppm): 11.75/11.48 (s, 1H, -NH-N=), 8.45 (d, J = 22.9 Hz, 1H, Ar-H), 8.31/8.00 (s, 1H, -N=CH), 8.20 (t, J = 8.0 Hz, 1H, Ar-H), 8.06 (dd, J = 11.2, 8.0 Hz, 1H, Ar-H), 7.78–7.60 (m, 1H, Ar-H), 7.27 (t, J = 7.5 Hz, 2H, Ar-H), 7.08 (dd, J = 12.9, 7.5 Hz, 2H, Ar-H), 4.60/3.70 (q, J = 6.9 Hz, 1H, -CH-CH3), 2.37 (dd, J = 17.0, 6.7 Hz, 2H, -CH2-CH-(CH3)2), 1.83–1.69 (m, 1H, -CH2-CH(CH3)2), 1.39 (dd, J = 9.1, 6.9 Hz, 3H, -CH-CH3), 0.80 (dd, J = 20.5, 6.7 Hz, 6H, -CH2-CH(CH3)2); 13C-NMR (δ ppm): 175.27/170.21 (Cq), 148.16 (Cq), 144.10/140.08 (-N=CH-), 139.60/139.20 (Cq), 138.62 (Cq), 136.20 (Cq), 133.13/132.79 (CHAr), 130.33 (CHAr), 128.93 (CHAr), 127.07 (CHAr ), 124.06/123.74 (CHAr), 120.90/120.67 (CHAr), 44.20 (-CH2-CH(CH3)2), 43.63/40.66 (-CH-CH3), 29.57 (-CH(CH3)2), 22.09 (-CH(CH3)2), 18.51 (-CH-CH3); HRMS (EI-MS): m/z calculated 354.181218; [M+H]+ found 354.181401; Green chemistry metrics: E-factor 14.123, ME 0.066.
2-(4-Isobutylphenyl)propionic acid (4-nitrobenzylidene)hydrazide (3g). 50/50 racemic mixture. 1H-NMR (δ ppm): 11.79/11.55 (s, 1H, -NH-N=), 8.30/8.00 (s, 1H, -N=CH), 8.25 (dd, J = 8.6, 3.5 Hz, 2H, Ar-H), 7.90 (dd, J = 8.6, 6.4 Hz, 2H, Ar-H), 7.27 (dd, J = 7.9, 1.8 Hz, 2H, Ar-H), 7.08 (dd, J = 13.2, 7.9 Hz, 2H, Ar-H), 4.65/3.70 (q, J = 6.9 Hz, 1H, -CH-CH3), 2.37 (dd, J = 15.1, 7.1 Hz, 2H, -CH2-CH(CH3)2), 1.88–1.66 (m, 1H, -CH2-CH(CH3)2), 1.39 (t, J = 6.9 Hz, 3H, -CH-CH3), 0.81 (dd, J = 14.2, 6.6 Hz, 6H, -CH2-CH(CH3)2); 13C-NMR (δ ppm): 175.44/170.27 (Cq), 147.73/147.51 (Cq), 144.07/140.64 (-N=CH-), 140.15 (Cq), 139.63/139.28 (Cq), 138.95/138.54 (Cq), 128.94 (CHAr), 127.84/127.55 (CHAr), 127.24/127.01 (CHAr), 123.97 (CHAr), 44.20 (-CH2-CH(CH3)2), 43.70/40.35 (-CH-CH3), 29.56 (-CH(CH3)2), 22.12 (-CH(CH3)2), 18.44 (-CH-CH3); HRMS (EI-MS): m/z calculated 354.181218; [M+H]+ found 354.181362; Green chemistry metrics: E-factor 15.231, ME 0.062.
2-(4-Isobutylphenyl)propionic acid (4-methylbenzylidene)hydrazide (3h). 50/50 racemic mixture. Yield: 81%, m.p. 145–147 °C; IR (ZnSe crystal, cm−1): 3181 (-NH-), 2953 (CHAr), 1666 (-CO-NH-), 1608 (-CH=N-); 1H-NMR (δ ppm): 11.44/11.18 (s, 1H, -NH-N=), 8.17/7.89 (s, 1H, -N=CH-), 7.54 (dd, J = 7.3, 5.2, 2H, Ar-H), 7.36–7.16 (m, 4H, Ar-H), 7.07 (dd, J = 15.5, 7.8 Hz, 2H, Ar-H), 4.64/3.66 (q, J = 6.8 Hz, 1H, -CH-CH3), 2.38 (dd, J = 13.8, 6.6 Hz, 2H, -CH2-CH(CH3)2), 2.31 (s, 3H, Ar-CH3), 1.77 (dd, J = 6.6, 3.6 Hz, 1H, -CH2-CH(CH3)2), 1.38 (t, J = 6.8 Hz, 3H, -CH-CH3), 0.82 (dd, J = 10.4, 6.6 Hz, 6H, -CH2-CH(CH3)2); 13C-NMR (δ ppm): 174.94/169.73 (Cq), 146.54/142.56 (-N=CH-), 139.66/139.49 (Cq), 139.33/138.83 (Cq), 139.16 (Cq), 131.60 (Cq), 129.35 (CHAr), 128.84 (CHAr), 127.26/126.61 (CHAr), 126.94 (CHAr), 44.22 (-CH2-CH(CH3)2), 43.62/40.13 (-CH(CH3), 29.58 (-CH(CH3)2), 22.14 (-CH(CH3)2), 20.97 (Ar-CH3), 18.43 (-CH-CH3); HRMS (EI-MS): m/z calculated 323.211790; [M+H]+ found 323.212005; Green chemistry metrics: E-factor 14.262, ME 0.065.
2-(4-Isobutylphenyl)propionic acid (3-trifluoromethylbenzylidene)hydrazide (3i). 50/50 racemic mixture. Yield: 69%, m.p. 120–123 °C; IR (ZnSe crystal, cm−1): 3180 (-NH-), 2956 (CHAr), 1668 (-CO-NH-), 1602 (-CH=N-), 1070 (C-F); 1H-NMR (δ ppm): 11.70/11.42 (s, 1H, -NH-N=), 8.28/7.99 (s, 1H, -N=CH-), 7.97–7.89 (m, 2 H, Ar-H), 7.74–7.61 (m, 2H, Ar-H), 7.25 (dd, J = 16.3, 7.6, 2H, Ar-H), 7.06 (dd, J = 19.3, 7.6 Hz, 2H, Ar-H), 4.58/3.69 (q, J = 6.9 Hz, 1H, -CH-CH3), 2.36 (dd, J = 17.9, 6.5 Hz, 2H, -CH2-CH(CH3)2), 1.85–1.66 (m, 1H, -CH2-CH(CH3)2), 1.38 (dd, J = 13.0, 6.9 Hz, 3H, ‑CH-CH3), 0.80 (dd, J = 20.8, 6.5 Hz, 6H, -CH2-CH(CH3)2); 13C-NMR (δ ppm): 175.27/170.21 (Cq), 144.81/140.72 (-N=CH-), 139.63 (Cq), 139.28 (Cq), 138.69 (Cq), 135.52 (Cq), 130.87/130.55 (CHAr), 129.91 (CHAr), 128.93 (CHAr), 127.08 (CHAr), 126.12/125.78 (CHAr), 125.38(Cq), 122.98/122.70 (CHAr), 44.24 (-CH2-CH(CH3)2), 43.65/40.78 (-CH-CH3), 29.60 (-CH(CH3)2), 22.11 (-CH(CH3)2), 18.54 (-CH-CH3); HRMS (EI-MS): m/z calculated 377.183524; [M+H]+ found 377.183680; Green chemistry metrics: E-factor 14.641, ME 0.064.
2-(4-Isobutylphenyl)propionic acid (4-trifluoromethylbenzylidene)hydrazide (3j). 50/50 racemic mixture. Yield: 69%, m.p. 160–162 °C; IR (ZnSe crystal, cm−1): 3185 (-NH-), 2957 (CHAr), 1669 (-CO-NH-), 1604 (-CH=N-), 1064 (C-F); 1H-NMR (δ ppm): 11.69/11.45 (s, 1H, -NH-N=), 8.28/7.98 (s, 1H, -N=CH-), 7.86 (t, J = 8.2 Hz, 2H, Ar-H), 7.75 (dd, J = 8.2, 2.4 Hz, 2H, Ar-H), 7.27 (dd, J = 7.9, 6.2, 2H, Ar-H), 7.07 (dd, J = 15.6, 7.9 Hz, 2H, Ar-H), 4.65/3.69 (q, J = 6.9 Hz, 1H, -CH-CH3), 2.37 (dd, J = 16.0, 6.7 Hz, 2H, -CH2-CH(CH3)2), 1.76 (m, 1H, -CH2-CH(CH3)2), 1.39 (t, J = 6.9 Hz, 3H, ‑CH-CH3), 0.81 (dd, J = 16.6, 6.7 Hz, 6H, -CH2-CH(CH3)2); 13C-NMR (δ ppm): 175.31/170.15 (Cq), 144.79/140.81 (-N=CH-), 139.60 (Cq), 139.14 (Cq), 138.64 (Cq), 138.29 (Cq), 128.91 (CHAr), 127.50/127.01 (CHAr), 127.22 (CHAr), 125.60 (CHAr), 122.73 (Cq) 44.21 (-CH2-CH(CH3)2), 43.68/40.33 (-CH-CH3), 29.57 (-CH(CH3)2), 22.09 (-CH(CH3)2), 18.43 (-CH-CH3); HRMS (EI-MS): m/z calculated 377.183524; [M+H]+ found 377.183689; Green chemistry metrics: E-factor 14.822, ME 0.063.
2-(4-Isobutylphenyl)propionic acid (4-cianobenzylidene)hydrazide (3k). 50/50 racemic mixture. Yield: 52%, m.p. 179–180 °C; IR (ZnSe crystal, cm−1): 3180 (-NH-), 2954 (CHAr), 2227 (C≡N), 1666 (-CO-NH-), 1596 (-CH=N-); 1H-NMR (δ ppm): 11.75/11.47 (s, 1H, -NH-N=), 8.25/7.95 (s, 1H, -N=CH-), 7.92–7.73 (m, 4H, Ar-H), 7.26 (t, J = 7.4, 2H, Ar-H), 7.07 (dd, J = 15.2, 7.4 Hz, 2H, Ar-H), 4.64/3.68 (q, J = 7.1 Hz, 1H, -CH-CH3), 2.37 (dd, J = 14.4, 6.8 Hz, 2H, -CH2-CH(CH3)2), 1.76 (dd, J = 12.5, 6.8 Hz, 1H, -CH2-CH(CH3)2), 1.38 (t, J = 7.1 Hz, 3H, -CH-CH3), 0.81 (dd, J = 13.8, 6.8 Hz, 6H, -CH2-CH(CH3)2); 13C-NMR (δ ppm): 175.45/170.29 (Cq), 144.67/140.69 (-N=CH-), 139.68/139.32 (Cq), 139.03/138.60 (Cq), 138.80 (Cq), 132.70 (CHAr), 128.98 (CHAr), 127.54/127.05 (CHAr), 127.27 (CHAr), 118.69 (Cq), 111.79/111.51 (Cq), 44.24 (-CH2-CH(CH3)2), 43.72/40.34 (-CH-CH3), 29.61 (-CH(CH3)2), 22.17 (-CH(CH3)2), 18.48 (-CH-CH3); HRMS (EI-MS): m/z calculated 334.191389; [M+H]+ found 334.191499; Green chemistry metrics: E-factor 22.513, ME 0.042.
2-(4-Isobutylphenyl)propionic acid (2,6-dichlorobenzylidene)hydrazide (3l). 60/40 racemic mixture. Yield: 60%, m.p. 151–153 °C; IR (ZnSe crystal, cm−1): 3185 (-NH-), 2951 (CHAr), 1662 (-CO-NH-), 1605 (-CH=N-), 776 (C-Cl); 1H-NMR (δ ppm): 11.77/11.52 (s, 1H, -NH-N=), 8.41/8.22 (s, 1H, -N=CH-), 7.51 (d, J = 7.9 Hz, 2H, Ar-H), 7.43–7.34 (m, 1H, Ar-H), 7.24 (dd, J =29.5, 8.0 Hz, 2H, Ar-H), 7.07 (dd, J = 29.5, 8.0, 2H, Ar-H), 4.59/3.67 (q, J = 7.0 Hz, 1H, -CH-CH3), 2.37 (dd, J = 14.8, 6.8 Hz, 2H, -CH2-CH(CH3)2), 1.86–1.67 (m, 1H, -CH2-CH(CH3)2), 1.38 (dd, J = 15.8, 7.0 Hz, 3H, -CH-CH3), 0.82 (dd, J = 10.2, 6.8 Hz, 6H, -CH2-CH(CH3)2); 13C-NMR (δ ppm): 175.55/170.12 (Cq), 141.97/137.97 (-N=CH-), 139.70/139.29 (Cq), 138.76/138.61 (Cq), 133.88 (Cq), 131.07/130.87 (CHAr), 130.47 (Cq), 129.74 (Cq), 129.34 (CHAr), 128.93 (CHAr), 127.20 (CHAr), 44.26 (-CH2-CH(CH3)2), 43.78/39.85 (-CH-CH3), 29.62 (-CH(CH3)2), 22.16 (-CH(CH3)2), 18.64 (-CH-CH3); HRMS (EI-MS): m/z calculated 377.118195; [M+H]+ found 377.118261; Green chemistry metrics: E-factor 16.891, ME 0.056.
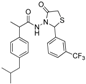
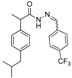
3.2.2. Preparation of the Acetamidothiazolidin-4-One Derivatives 4a–l
The acyl hydrazones of ibuprofen
3a–
l (3.2 mmol) were reacted with mercaptoacetic acid (14.4 mmol) and the reaction mixtures was heated at 80–85 °C for 2–8 h using an oil bath, according to a procedure described for other compounds [
32]. After the completion of the reaction (TLC monitoring using dichloromethane-methanol, 9.8:0.2, v/v, UV light at 254 nm), the mixture was diluted with ethyl acetate (200 mL) and then neutralized with a saturated solution of sodium carbonate. After stirring for 3–4 h the organic layer was separated and washed with saturated solution of sodium bicarbonate, then with saturated solution of sodium chloride and finally with water. The organic layers were collected, dried with MgSO
4 and concentrated by rotary evaporator. The residue was purified on a silica gel column using dichloromethane-methanol (9.8:0.2, v/v) as the eluent system.
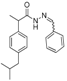
2-(Phenyl)-3-[2-(4-(isobutyl)phenyl)-2-methyl]acetamidothiazolidine-4-one (4a). 60/40 racemic mixture. Yield: 57%, m.p. 125 °C; IR (ZnSe crystal, cm−1): 3243 (-NH-), 2959 (CHAr), 1716 (C=O, thiazolidine-4-one), 1666 (-CO-NH-), 1203 (C-N), 694 (C-S); 1H-NMR (δ ppm): 10.26 (d, J = 3.1 Hz, 1H, -NH-), 7.50–7.35 (m, 2H, Ar-H), 7.34–7.21 (m, 3H, Ar-H), 7.11 (d, J = 7.6 Hz, 1H, Ar-H), 7.01 (dd, J = 14.8, 7.6 Hz, 3H, Ar-H), 5.82/5.67 (s, 1H, CH thiazolidine-4-one), 3.86 (t, J = 14.9 Hz, 1H, CH2 thiazolidine-4-one), 3.72 (dd, J = 14.9, 9.0 Hz, 1H, CH2 thiazolidine-4-one), 3.61–3.47 (m, 1H, -CH-CH3), 2.39 (d, J = 6.7 Hz, 2H, -CH2-CH(CH3)2), 1.79 (dt, J = 13.4, 6.7 Hz, 1H, -CH2-CH(CH3)2), 1.25 (dd, J = 24.2, 7.0 Hz, 3H, -CH-CH3), 0.85 (d, J = 6.7 Hz, 6H, -CH2-CH(CH3)2); 13C-NMR (δ ppm): 172.23 (Cq), 168.85/168.69 (Cq), 139.31 (Cq), 138.15 (Cq), 137.95 (Cq), 128.98/128.88 (CHAr), 128.69 (CHAr), 128.54/128.39 (CHAr), 127.80 (CHAr), 127.01/126.73 (CHAr), 61.43 (CH thiazolidine-4-one), 44.22 (-CH2-CH(CH3)2), 42.12 (-CH-CH3), 29.62 (-CH(CH3)2), 29.27 (CH2 thiazolidine-4-one), 22.13 (-CH(CH3)2), 18.81/17.90 (-CH-CH3); HRMS (EI-MS): m/z calculated 383.178776; [M+H]+ found 383.178716; Green chemistry metrics: E-factor 2.361, ME 0.297.
2-(4-Chlorophenyl)-3-[2-(4-(isobutyl)phenyl)-2-methyl]acetamidothiazolidine-4-one (4b). 50/50 racemic mixture. Yield: 76%, m.p. 80 °C; IR (ZnSe crystal, cm−1): 3238 (-NH-), 2954 (CHAr), 1718 (C=O, thiazolidine-4-one), 1672 (-CO-NH-), 1215 (C-N), 810 (C-Cl), 656 (C-S); 1H-NMR (δ ppm): 10.23 (d, J = 7.1 Hz, 1H, -NH-), 7.43 (q, J = 8.6 Hz, 2H, Ar-H), 7.28 (dd, J = 18.7, 8.6 Hz, 2H, Ar-H), 7.16–6.88 (m, 4H, Ar-H), 5.80/5.71 (s, 1H, CH thiazolidine-4-one), 3.87 (dd, J = 15.8, 8.3, 1H, CH2 thiazolidine-4-one), 3.72 (dd, J = 15.8, 8.3 Hz, 1H, CH2 thiazolidine-4-one), 3.52 (dq, J = 14.1, 7.0 Hz, 1H, -CH-CH3), 2.46–2.33 (m, 2H, -CH2-CH(CH3)2), 1.79 (dt, J = 13.5, 6.7 Hz, 1H, -CH2-CH(CH3)2), 1.25 (dd, J = 17.9, 7.0 Hz, 3H, -CH-CH3), 0.85 (d, J = 6.7 Hz, 6H, -CH2-CH(CH3)2); 13C-NMR (δ ppm): 172.17 (Cq), 168.70/168.45 (Cq), 139.36 (Cq), 138.17/137.94 (Cq), 137.25/137.07 (Cq), 133.56/133.44 (Cq), 129.77 (CHAr), 128.70 (CHAr), 128.49/128.38 (CHAr), 126.92/126.71 (CHAr), 60.86/60.50 (CH thiazolidine-4-one), 44.22 (-CH2-CH(CH3)2), 42.21 (-CH-CH3), 29.61 (-CH(CH3)2), 29.21 (CH2 thiazolidine-4-one), 22.15 (-CH(CH3)2), 18.76/17.89 (-CH-CH3); HRMS (EI-MS): m/z calculated 417.139803; [M+H]+ found 417.139745; Green chemistry metrics: E-factor 1.423, ME 0.413.
2-(4-Bromophenyl)-3-[2-(4-(isobutyl)phenyl)-2-methyl]acetamidothiazolidine-4-one (4c). 50/50 racemic mixture. Yield: 77%, m.p. 70–72 °C; IR (ZnSe crystal, cm−1): 3238 (-NH), 2954 (CHAr), 1718 (C=O, thiazolidine-4-one), 1672 (-CO-NH-), 1215 (C-N), 810 (C-Cl), 656 (C-S); 1H-NMR (δ ppm): 10.23 (d, J = 8.1 Hz, 1H, -NH-), 7.55 (d, J = 8.4 Hz, 1H, Ar-H), 7.41 (dd, J = 21.1, 8.4 Hz, 2H, Ar-H), 7.19 (d, J = 8.4 Hz, 1H, Ar-H), 7.11–6.93 (m, 4H, Ar-H), 5.79/5.70 (s, 1H, CH thiazolidine-4-one), 3.87 (dd, J = 15.8, 8.4 Hz, 1H, CH2 thiazolidine-4-one), 3.72 (dd, J = 15.8, 8.4 Hz, 1H, CH2 thiazolidine-4-one), 3.52 (dq, J = 14.1, 7.0 Hz, 1H, -CH-CH3), 2.40 (dd, J = 6.9, 2.3 Hz, 2H, -CH2-CH(CH3)2), 1.79 (td, J = 13.4, 6.9 Hz, 1H, -CH2-CH(CH3)2), 1.25 (dd, J = 18.2, 7.0 Hz, 3H, -CH-CH3), 0.85 (d, J = 6.9 Hz, 6H, -CH2-CH(CH3)2); 13C-NMR (δ ppm): 172.16 (Cq), 168.71/168.46 (Cq), 139.35 (Cq), 138.16/137.95 (Cq), 137.68/137.48 (Cq), 131.36 (CHAr), 130.06 (CHAr), 128.70 (CHAr), 126.91/126.71 (CHAr), 122.22/122/08 (Cq), 60.94/60.56 (CH thiazolidine-4-one), 44.24 (-CH2-CH(CH3)2), 42.21 (-CH-CH3), 29.62 (-CH(CH3)2), 29.22 (CH2 thiazolidine-4-one), 22.19 (-CH(CH3)2), 18.77/17.89 (-CH-CH3); HRMS (EI-MS): m/z calculated 461.089288; [M+H]+ found 461.089204; Green chemistry metrics: E-factor 1.122, ME 0.471.
2-(4-Fluorophenyl)-3-[2-(4-(isobutyl)phenyl)-2-methyl]acetamidothiazolidine-4-one (4d). 50/50 racemic mixture. Yield: 66%, m.p. 72 °C; IR (ZnSe crystal, cm−1): 3252 (-NH-), 2954 (CHAr), 1716 (C=O, thiazolidine-4-one), 1672 (-CO-NH-), 1224 (C-N), 1095 (C-F), 659 (C-S); 1H-NMR (δ ppm): 10.22 (d, J = 4.4 Hz, 1H, -NH-), 7.48 (dd, J = 8.8, 5.5 Hz, 1H, Ar-H), 7.28 (dd, J = 8.6, 5.5 Hz, 1H, Ar-H), 7.18 (t, J = 8.8 Hz, 1H, Ar-H), 7.14–6.90 (m, 5H, Ar-H), 5.82/5.71 (s, 1H, CH thiazolidine-4-one), 3.86 (dd, J = 15.8, 8.6, 1.4 Hz, 1H, CH2 thiazolidine-4-one), 3.72 (dd, J = 15.8, 8.6 Hz, 1H, CH2 thiazolidine-4-one), 3.52 (dq, J = 14.1, 7.0 Hz, 1H, -CH-CH3), 2.39 (dd, J = 6.7, 1.2 Hz, 2H, -CH2-CH(CH3)2), 1.79 (dt, J = 13.5, 6.7 Hz, 1H, -CH2-CH(CH3)2), 1.25 (dd, J = 17.2, 7.0 Hz, 3H, -CH-CH3), 0.85 (d, J = 6.7 Hz, 6H, -CH2-CH(CH3)2); 13C-NMR (δ ppm): 172.18 (Cq), 168.70/168.47 (Cq), 163.54/161.10 (Cq), 139.35 (Cq), 138.19/137.95 (Cq), 134.21(Cq), 130.16 (CHAr), 128.70 (CHAr), 126.85 (CHAr), 115.27 (CHAr), 60.90/60.59 (CH thiazolidine-4-one), 44.22 (-CH2-CH(CH3)2), 42.21 (-CH-CH3), 29.62 (-CH(CH3)2), 29.26 (CH2 thiazolidine-4-one), 22.15 (CH(CH3)2), 18.76/17.89 (-CH-CH3); HRMS (EI-MS): m/z calculated 401.169354; [M+H]+ found 401.169387; Green chemistry metrics: E-factor 1.821, ME 0.354.
2-(2-Nitrophenyl)-3-[2-(4-(isobutyl)phenyl)-2-methyl]acetamidothiazolidine-4-one (4e). 70/30 racemic mixture. Yield: 73%, m.p. 69–71 °C; IR (ZnSe crystal, cm−1): 3246 (-NH), 2953 (CHAr), 1721 (C=O, thiazolidine-4-one), 1674 (-CO-NH-), 1216 (C-N), 661 (C-S); 1H-NMR (δ ppm): 10.45/10.38 (d, 1H, -NH-), 8.05/7.96 (d, J = 8.2 Hz, 1H, Ar-H), 7.90–7.49 (m, 3H, Ar-H), 7.12/7.03 (d, J = 8.1 Hz, 3H, Ar-H), 6.90 (d, J = 6.5 Hz, 1H, Ar-H), 6.14/6.02 (d, J = 1.4 Hz, 1H, CH thiazolidine-4-one), 3.93–3.86 (m, 1H, CH2 thiazolidine-4-one), 3.70 (dd, J = 20.0, 15.8, 1H, CH2 thiazolidine-4-one), 3.57–3.48 (m, 1H, -CH-CH3), 2.37 (dd, J = 20.3, 6.8 Hz, 2H, -CH2-CH(CH3)2), 1.84–1.69 (m, 1H, -CH2-CH(CH3)2), 1.24 (dd, J = 23.1, 7.0 Hz, 3H, -CH-CH3), 0.83 (dd, J = 13.4, 6.8 Hz, 6H, -CH2-CH(CH3)2); 13C-NMR (δ ppm): 172.38 (Cq), 169.07/168.89 (Cq), 147.67/147.45 (Cq), 139.38/139.31 (Cq), 138.01/137.92 (Cq), 134.55 (CHAr), 134.33 (Cq), 129.75 (CHAr), 128.72 (CHAr), 128.16/127.94 (CHAr), 126.93 (CHAr), 126.35 (CHAr), 124.79 (CHAr), 56.98/56.48 (CH thiazolidine-4-one), 44.19 (-CH2-CH(CH3)2), 42.26 (-CH-CH3), 29.57 (-CH(CH3)2), 28.45 (-CH2 thiazolidine-4-one), 22.14 (-CH(CH3)2), 18.68/17.83 (-CH-CH3); HRMS (EI-MS): m/z calculated 428.163854; [M+H]+ found 428.163864; Green chemistry metrics: E-factor 1.503, ME 0.405.
2-(3-Nitrophenyl)-3-[2-(4-(isobutyl)phenyl)-2-methyl]acetamidothiazolidine-4-one (4f). 50/50 racemic mixture. Yield: 62%, m.p. 70–72 °C; IR (ZnSe crystal, cm−1): 3247 (-NH-), 2953 (CHAr), 1721 (C=O, thiazolidine-4-one), 1674 (-CO-NH-), 1216 (C-N), 679 (C-S); 1H-NMR (δ ppm): 10.31/10.25 (d, 1H, -NH-), 8.37–8.04 (m, 2H, Ar-H), 7.91/7.72 (d, J = 7.7 Hz, 1H, Ar-H), 7.60 (dt, J = 29.4, 7.7 Hz, 1H, Ar-H), 7.16–6.76 (m, 4H, Ar-H), 5.96/5.90 (s, 1H, CH thiazolidine-4-one), 3.97–3.92 (m, 1H, CH2 thiazolidine-4-one), 3.77 (dd, J = 15.8, 8.3, 1H, CH2 thiazolidine-4-one), 3.49 (dq, J = 14.1, 6.9 Hz, 1H, -CH-CH3), 2.35 (t, J = 7.5 Hz, 2H, -CH2-CH(CH3)2), 1.83–1.67 (m, 1H, -CH2-CH(CH3)2), 1.23 (dd, J = 20.2, 6.9 Hz, 3H, -CH-CH3), 0.81 (t, J = 7.1 Hz, 6H, -CH2-CH(CH3)2); 13C-NMR (δ ppm): 172.16 (Cq), 168.71/168.39 (Cq), 147.66 (Cq), 140.70 (Cq), 139.37 (Cq), 138.01 (Cq), 134.80/134.55 (CHAr), 130.08 (CHAr), 128.65 (CHAr), 126.71 (CHAr), 123.82/122.69 (CHAr), 60.66/60.20 (CH thiazolidine-4-one), 44.17 (-CH2-CH(CH3)2), 42.29 (-CH-CH3), 29.56 (-CH(CH3)2), 29.21 (CH2 thiazolidine-4-one), 22.15 (-CH(CH3)2), 18.70/17.76 (-CH-CH3); HRMS (EI-MS): m/z calculated 428.163854; [M+H]+ found 428.163708; Green chemistry metrics: E-factor 1.874, ME 0.352.
2-(4-Nitrophenyl)-3-[2-(4-(isobutyl)phenyl)-2-methyl]acetamidothiazolidine-4-one (4g). 50/50 racemic mixture. Yield: 72%, m.p. 70–72 °C; IR (ZnSe crystal, cm−1): 3233 (-NH-), 2955 (CHAr), 1716 (C=O, thiazolidine-4-one), 1672 (-CO-NH-), 1217 (C-N), 692 (C-S); 1H-NMR (δ ppm): 10.31 (d, J = 11.5 Hz, 1H, -NH-), 8.17 (d, J = 8.4 Hz, 1H, Ar-H), 8.09 (d, J = 8.6 Hz, 1H, Ar-H), 7.70 (d, J = 8.4 Hz, 1H, Ar-H), 7.53 (d, J = 8.6 Hz, 1H, Ar-H), 7.16–6.82 (m, 4H, Ar-H), 5.93/5.89 (s, 1H, CH thiazolidine-4-one), 3.93 (d, J = 15.8, 1H, CH2 thiazolidine-4-one), 3.76 (dd, J = 15.8, 10.4, 1H, CH2 thiazolidine-4-one), 3.60–3.42 (m, 1H, -CH-CH3), 2.37 (d, J = 7.0 Hz, 2H, -CH2-CH(CH3)2), 1.82–1.69 (m, 1H, -CH2-CH(CH3)2), 1.24 (dd, J = 17.2, 6.9 Hz, 3H, -CH-CH3), 0.82 (t, J = 7.0 Hz, 6H, ‑CH2-CH(CH3)2); 13C-NMR (δ ppm): 172.20 (Cq), 168.74/168.44 (Cq), 147.64/147.50 (Cq), 145.88 (Cq), 139.38 (Cq), 138.19/137.91 (Cq), 129.06 (CHAr), 128.66 (CHAr), 126.77 (CHAr), 123.53 (CHAr), 60.55/60.01 (CH thiazolidine-4-one), 44.15 (-CH2-CH(CH3)2), 42.28 (-CH-CH3), 29.58 (-CH(CH3)2), 29.14 (CH2 thiazolidine-4-one), 22.13 (-CH(CH3)2), 18.60/17.91 (-CH-CH3); HRMS (EI-MS): m/z calculated 428.163854; [M+H]+ found 428.163805; Green chemistry metrics: E-factor 1.506, ME 0.403.
2-(4-Methylphenyl)-3-[2-(4-(isobutyl)phenyl)-2-methyl]acetamidothiazolidine-4-one (4h). 50/50 racemic mixture. Yield: 41%, m.p. 67 °C; IR (ZnSe crystal, cm−1): 3254 (-NH-), 2953 (CHAr), 1715 (C=O thiazolidine-4-one), 1673 (-CO-NH-), 1213 (C-N), 659 (C-S); 1H-NMR (δ ppm): 10.21 (d, J = 4.0 Hz, 1H, -NH-), 7.31 (d, J = 7.9 Hz, 1H, Ar-H), 7.19 (d, J = 7.9 Hz, 1H, Ar-H), 7.16–7.05 (m, 3H, Ar-H), 7.05–6.96 (m, 3H, Ar-H), 5.78/5.64 (s, 1H, CH thiazolidine-4-one), 3.92–3.77 (m, 1H, CH2 thiazolidine-4-one), 3.71 (dd, J = 15.8, 9.0, 1H, CH2 thiazolidine-4-one), 3.53 (dq, J = 20.4, 7.0 Hz, 1H, -CH-CH3), 2.40 (dd, J = 7.0, 2.0 Hz, 2H, -CH2-CH(CH3)2), 2.30 (d, J = 21.4 Hz, 3H, Ar-CH3), 1.85–1.75 (m, 1H, -CH2-CH(CH3)2), 1.25 (dd, J = 20.4, 7.0 Hz, 3H, -CH-CH3), 0.85 (dd, J = 7.0, 2.9 Hz, 6H, -CH2-CH(CH3)2); 13C-NMR (δ ppm): 172.19 (Cq), 168.82/168.63 (Cq), 139.30 (Cq), 138.46/138.31 (Cq), 138.19.137.93 (Cq), 135.05/134.88 (Cq), 129.02 (CHAr), 128.68 (CHAr), 127.82 (CHAr), 127.02/126.75 (CHAr), 61.39/61.21 (CH thiazolidine-4-one), 44.22 (-CH2-CH(CH3)2), 42.11 (-CH-CH3), 29.63 (-CH(CH3)2), 29.28 (CH2 thiazolidine-4-one), 22.13 (-CH(CH3)2), 20.77 (Ar-CH3), 18.81/17.99 (-CH-CH3); HRMS (EI-MS): m/z calculated 397.194426; [M+H]+ found 397.194552; Green chemistry metrics: E-factor 3.767, ME 0.213.
2-(3-trifluoromethylphenyl)-3-[2-(4-(isobutyl)phenyl)-2-methyl]acetamidothiazolidine-4-one (4i). 50/50 racemic mixture. Yield: 53%, m.p. 68 °C; IR (ZnSe crystal, cm−1): 3233 (-NH-), 2955 (CHAr), 1720 (C=O, thiazolidine-4-one), 1673 (-CO-NH-), 1216 (C-N), 1071 (C-F), 656 (C-S); 1H-NMR (δ ppm): 10.32 (d, J = 19.4 Hz, 1H, -NH-), 7.88–7.46 (m, 4H, Ar-H), 7.20–6.86 (m, 4H, Ar-H), 5.96/5.86 (s, 1H, CH thiazolidine-4-one), 3.96–3.90 (m, 1H, CH2 thiazolidine-4-one), 3.76 (dd, J = 15.8, 8.2, 1H, CH2 thiazolidine-4-one), 3.60–3.48 (m, 1H, -CH-CH3), 2.37 (dd, J = 6.8, 3.5 Hz, 2H, -CH2-CH(CH3)2), 1.85–1.70 (m, 1H, -CH2-CH(CH3)2), 1.26 (dd, J = 18.7, 7.0 Hz, 3H, -CH-CH3), 0.83 (dd, J = 6.8, 3.5 Hz, 6H, -CH2-CH(CH3)2); 13C-NMR (δ ppm): 172.33 (Cq), 168.88/.16862 (Cq), 139.86 (Cq), 139.38 (Cq), 138.07 (Cq), 132.06 (CHAr), 129.61 (CHAr), 128.71 (CHAr), 126.92/126.68 (CHAr), 125.72/124.55 (CHAr), 125.36 (Cq), 122.66 (Cq), 61.01/60.77 (CH thiazolidine-4-one), 44.29 (-CH2-CH(CH3)2, 42.33 (-CH-CH3), 29.62 (-CH(CH3)2), 29.32 (CH2 thiazolidine-4-one), 22.13 (-CH(CH3)2), 18.80/17.87 (-CH-CH3); HRMS (EI-MS): m/z calculated 451.166160; [M+H]+ found 451.166296; Green chemistry metrics: E-factor 2.079, ME 0.325.
2-(4-Trifluoromethylphenyl)-3-[2-(4-(isobutyl)phenyl)-2-methyl]acetamidothiazolidine-4-one (4j). 50/50 racemic mixture. Yield: 38%, m.p. 74–75 °C; IR (ZnSe crystal, cm−1): 3246 (-NH-), 2956 (CHAr), 1721 (C=O, thiazolidine-4-one), 1673 (-CO-NH-), 1217 (C-N), 1066 (C-F), 664 (C-S); 1H-NMR (δ ppm): 10.28 (d, J = 9.6 Hz, 1H, -NH-), 7.77–7.54 (m, 3H, Ar-H), 7.48 (d, J = 8.1 Hz, 1H, Ar-H), 7.14‒6.89 (m, 4H, Ar-H), 5.89/5.82 (s, 1H, CH thiazolidine-4-one), 3.91 (dd, J = 15.7, 5.5 Hz, 1H, CH2 thiazolidine-4-one), 3.75 (dd, J = 15.7, 10.6, 1H, CH2 thiazolidine-4-one), 3.53 (dq, J =13.9, 7.0 Hz, 1H, -CH-CH3), 2.38 (d, J = 6.8 Hz, 2H, -CH2-CH(CH3)2), 1.85–1.69 (m, 1H, -CH2-CH(CH3)2), 1.25 (dd, J = 17.5, 7.0 Hz, 3H, -CH-CH3), 0.83 (dd, J = 6.8, 3.9 Hz, 6H, -CH2-CH(CH3)2); 13C-NMR (δ ppm): 172.22 (Cq), 168.81/168.56 (Cq), 143.19 (Cq), 142.95 (Cq), 139.34 (Cq), 138.21 (Cq), 137.97 (Cq), 128.66 (CHAr), 126.90 (CHAr), 126.69 (CHAr), 125.35 (CHAr), 60.86/60.41 (CH thiazolidine-4-one), 44.20 (-CH2-CH(CH3)2), 42.24 (-CH-CH3), 29.58 (-CH(CH3)2), 29.19 (CH2 thiazolidine-4-one), 22.13 (-CH(CH3)2), 18.72/17.88 (-CH-CH3); HRMS (EI-MS): m/z calculated 451.166160; [M+H]+ found 451.166066; Green chemistry metrics: E-factor 3.687, ME 0.2134.
2-(4-Cyanophenyl)-3-[2-(4-(isobutyl)phenyl)-2-methyl]acetamidothiazolidine-4-one (4k). 50/50 racemic mixture. Yield: 87%, m.p. 75 °C; IR (ZnSe crystal, cm−1): 3256 (-NH-), 2953 (CHAr), 2230 (C≡N), 1720 (C=O, thiazolidine-4-one), 1677 (-CO-NH-), 1204 (C-N), 661 (C-S); 1H-NMR (δ ppm): 10.28 (d, J = 10.7 Hz, 1H, -NH-), 7.81 (d, J = 8.0 Hz, 1H, Ar-H), 7.72 (d, J = 8.2 Hz, 1H, Ar-H), 7.61 (d, J = 8.2 Hz, 1H, Ar-H), 7.45 (d, J = 8.0 Hz, 1H, Ar-H), 7.11–6.90 (m, 4H, Ar-H), 5.87/5.81 (s, 1H, CH thiazolidine-4-one), 3.91 (dd, J = 15.8, 4.4 Hz, 1H, CH2 thiazolidine-4-one), 3.73 (dd, J = 15.8, 10.1, 1H, CH2 thiazolidine-4-one), 3.50 (dq, J = 14.1, 7.0 Hz, 1H, -CH-CH3), 2.39 (dd, J = 6.8, 2.5 Hz, 2H, -CH2-CH(CH3)2), 1.84–1.71 (m, 1H, -CH2-CH(CH3)2), 1.24 (dd, J = 16.6, 7.0 Hz, 3H, -CH-CH3), 0.83 (dd, J = 6.8, 2.5 Hz, 6H, -CH2-CH(CH3)2); 13C-NMR (δ ppm): 172.23 (Cq), 168.77/168.49 (Cq), 144.02/143.87 (Cq), 139.40 (Cq), 138.15/137.92 (Cq), 132.41 (CHAr), 128.69 (CHAr), 126.90 (CHAr), 126.68 (CHAr), 118.48 (Cq), 111.52 (Cq), 60.85/60.39 (CH thiazolidine-4-one), 44.20 (-CH2-CH(CH3)2), 42.27 (-CH-CH3), 29.62 (-CH(CH3)2), 29.14 (CH2 thiazolidine-4-one), 22.15 (-CH(CH3)2), 18.68/17.91 (-CH-CH3); HRMS (EI-MS): m/z calculated 408.174025; [M+H]+ found 408.173935; Green chemistry metrics: E-factor 1.218, ME 0.452.
2-(2,6-Dichlorophenyl)-3-[2-(4-(isobutyl)phenyl)-2-methyl]acetamidothiazolidine-4-one (4l). 60/40 racemic mixture. Yield: 38%, m.p. 74 °C; IR (ZnSe crystal, cm−1): 3239 (-NH), 2953 (CHAr), 1716 (C=O, thiazolidine-4-one), 1673 (-CO-NH-), 1218 (C-N), 778 (C-Cl), 663 (C-S); 1H-NMR (δ ppm): 10.38 (d, J = 10.9 Hz, 1H, -NH-), 7.61–7.24 (m, 3H, Ar-H), 7.22–6.89 (m, 4H, Ar-H), 6.71/6.57 (s, 1H, CH thiazolidine-4-one), 3.96–3.71 (m, 2H, CH2 thiazolidine-4-one), 3.68–3.50 (m, 1H, -CH-CH3), 2.38 (dd, J = 10.5, 6.5 Hz, 2H, -CH2-CH(CH3)2), 1.88–1.67 (m, 1H, -CH2-CH(CH3)2), 1.26 (dd, J = 22.0, 6.9 Hz, 3H, -CH-CH3), 0.84 (dd, J = 6.5, 2.8 Hz, 6H, -CH2-CH(CH3)2); 13C-NMR (δ ppm): 172.84/172.68 (Cq), 168.19/168.01 (Cq), 139.48/138.25 (Cq), 138.06/137.74 (Cq), 135.58/135.48 (Cq), 135.25/134.72 (Cq), 130.96 (Cq), 131.38 (CHAr), 131.14 (CHAr), 130.89 (CHAr), 128.68 (CHAr), 127.08/126.46 (CHAr), 55.87/55.72 (CH thiazolidine-4-one), 44.23 (-CH2-CH(CH3)2), 41.90 (-CH-CH3), 30.27 (-CH(CH3)2), 29.64 (CH2 thiazolidine-4-one), 22.18 (-CH(CH3)2), 18.84/17.49 (-CH-CH3); HRMS (EI-MS): m/z calculated 451.100831; [M+H]+ found 451.100760; Green chemistry metrics: E-factor 3.418, ME 0.226.