1,2-Substituted 4-(1H)-Quinolones: Synthesis, Antimalarial and Antitrypanosomal Activities in Vitro
Abstract
:1. Introduction
2. Results and Discussion
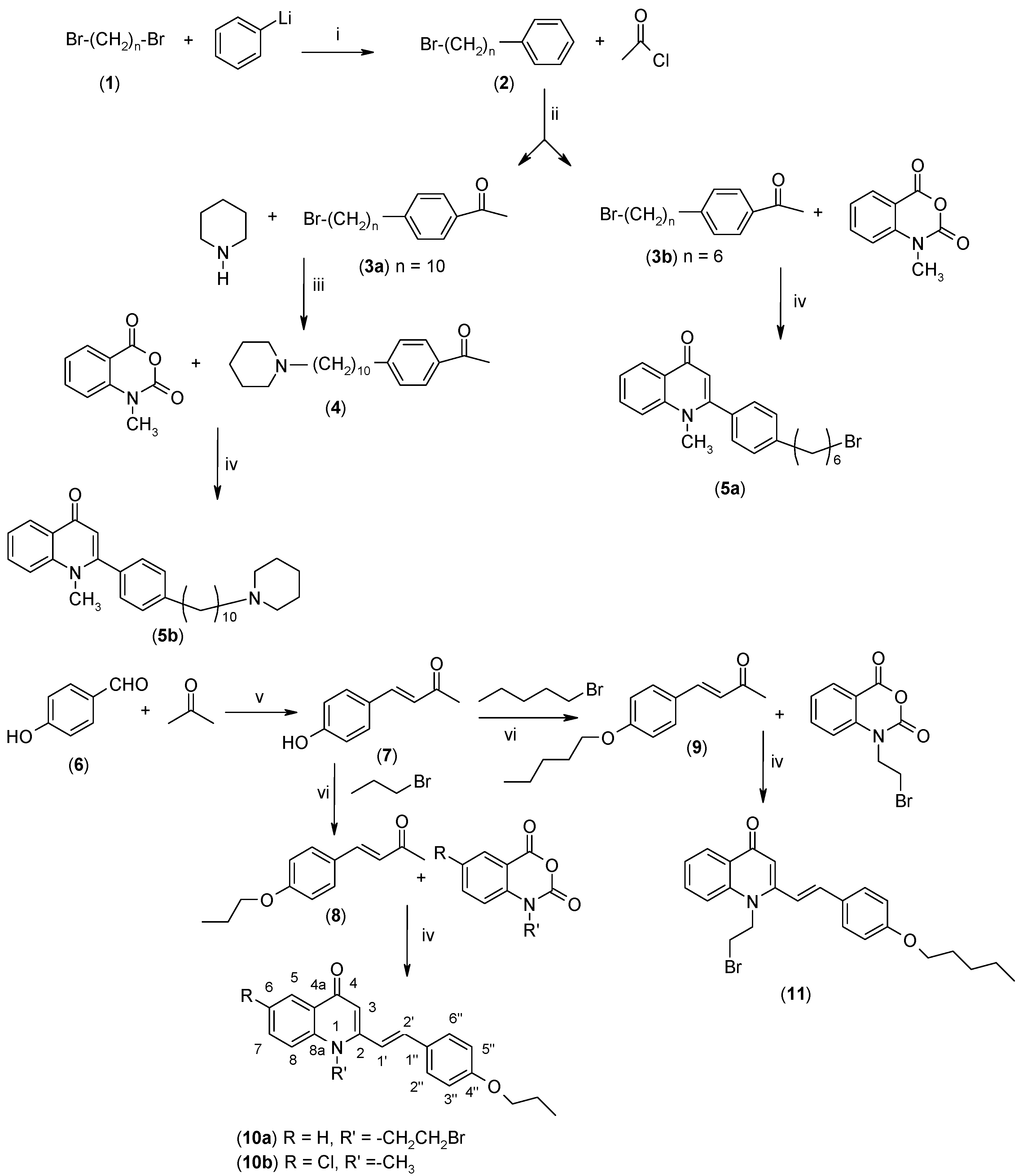
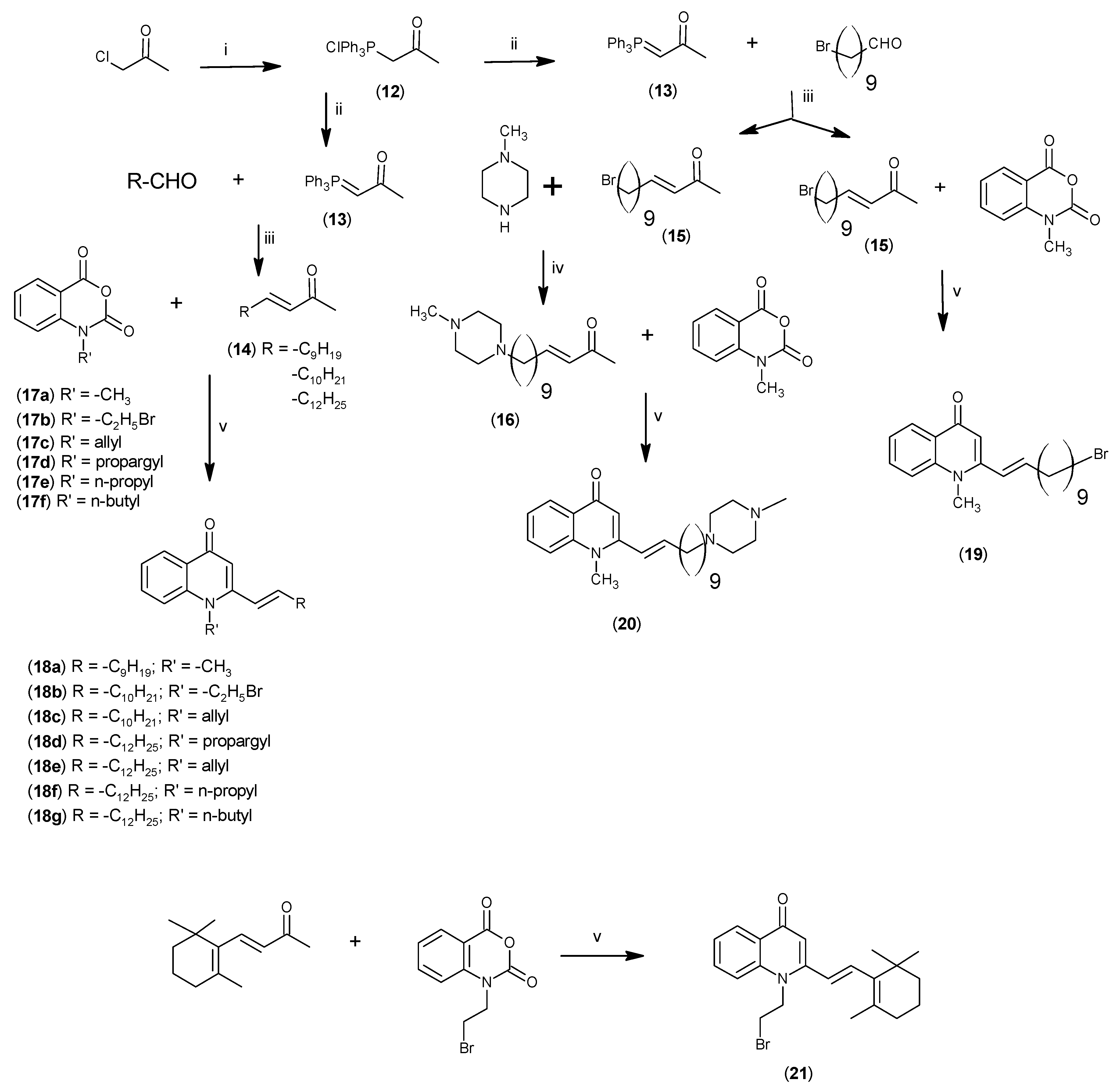
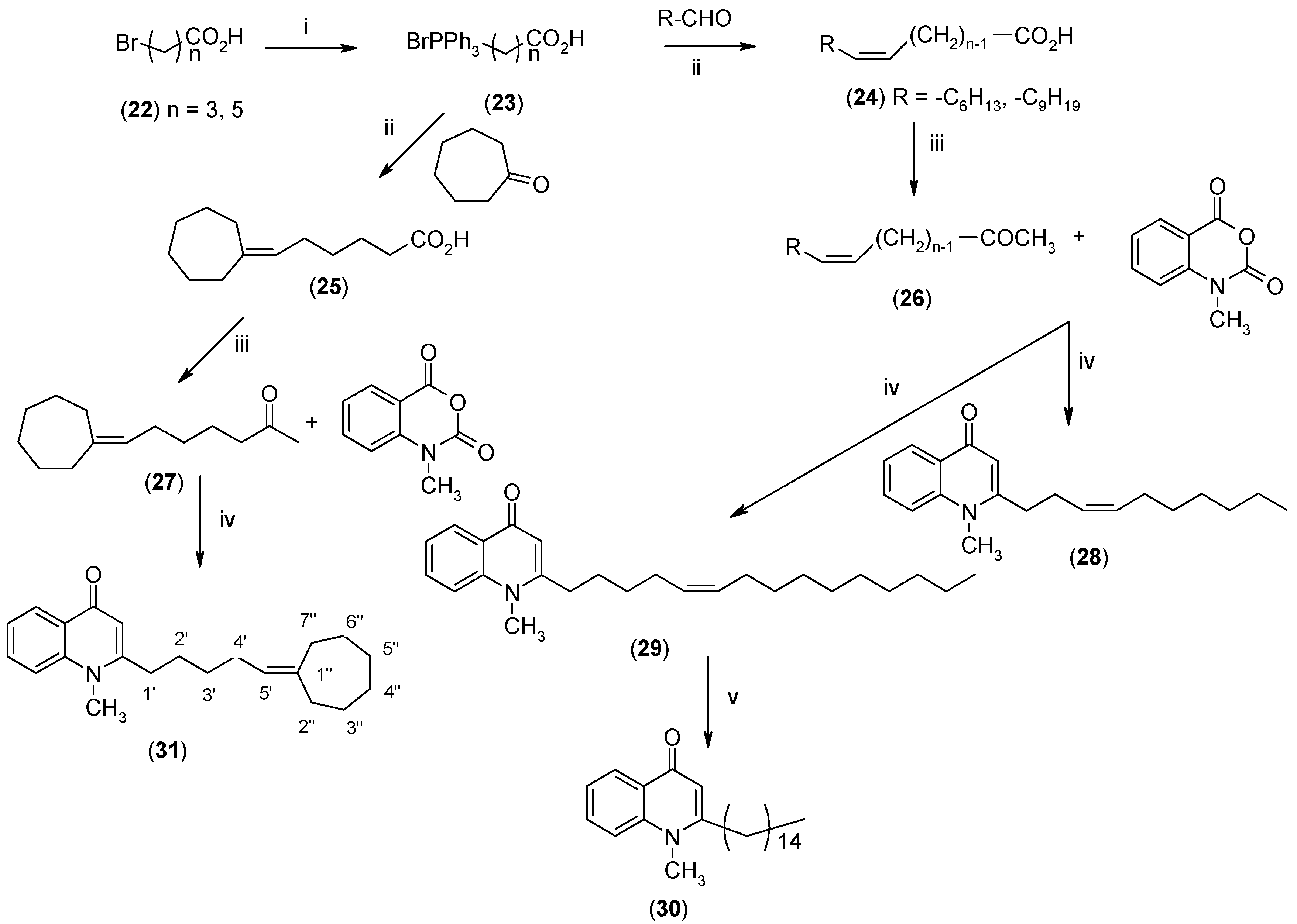
2.1. Synthesis
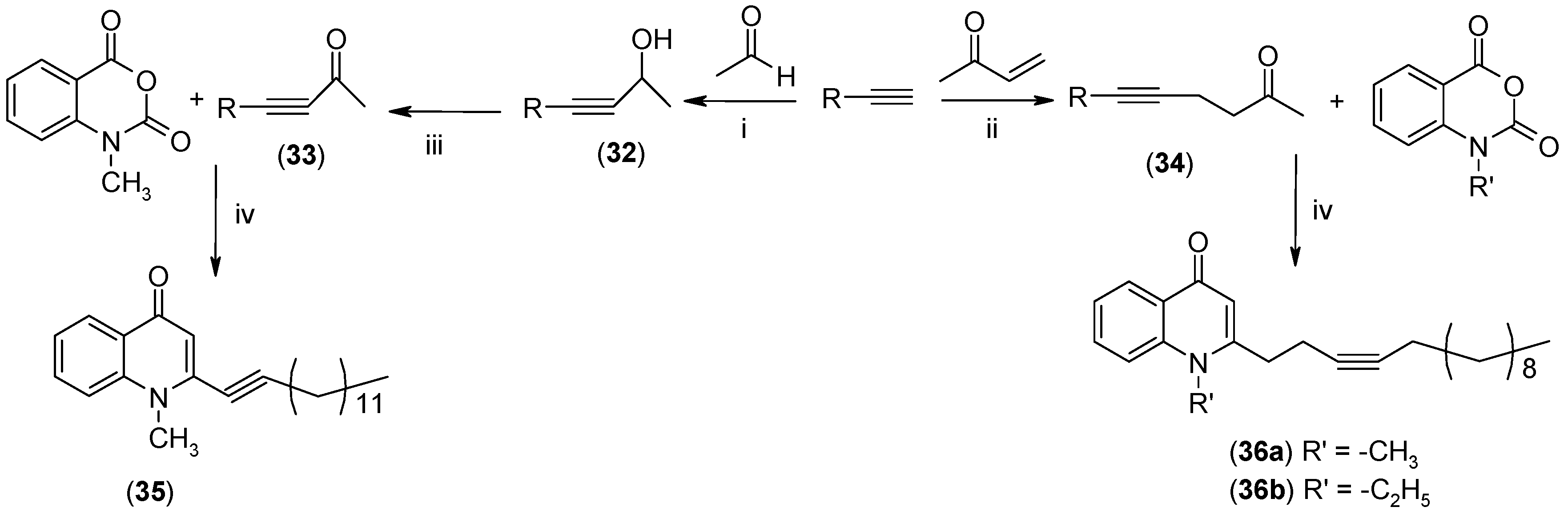
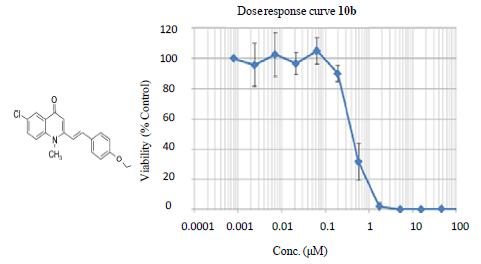
2.2. Biological Evaluations
| Comp. | Structure | IC50 (µM) | SI | |||
|---|---|---|---|---|---|---|
| NF54 | STIB900 | L-6 | L-6/NF54 | L-6/STIB900 | ||
| 5a |  | 0.09 | 1.64 | 6.63 | 73.33 | 4.04 |
| 5b |  | 0.82 | 3.41 | 9.63 | 11.73 | 2.83 |
| 10a |  | 1.58 | 1.25 | 25.83 | 16.45 | 20.58 |
| 10b |  | 0.47 | 21.89 | 161.2 | 342.89 | 7.36 |
| 11 |  | 1.56 | 3.82 | 42.39 | 27.11 | 11.10 |
| 18a |  | 12.40 | 52.22 | 44.72 | 3.61 | 0.86 |
| 18b |  | 2.30 | 8.28 | 38.35 | 16.69 | 4.63 |
| 18c |  | 2.90 | 21.23 | 19.82 | 6.82 | 0.93 |
| 18d |  | 4.64 | 24.48 | 24.68 | 5.32 | 1.01 |
| 18e |  | 0.31 | 8.18 | 14.01 | 45.78 | 1.71 |
| 18f |  | 1.19 | 19.08 | 13.04 | 10.99 | 0.68 |
| 18g |  > >
| 2.29 | 18.73 | 13.26 | 5.79 | 0.70 |
| 19 |  | 0.69 | 1.07 | 36.31 | 52.64 | 33.93 |
| 20 |  | 1.35 | 4.94 | 32.54 | 24.14 | 6.59 |
| 21 |  | 1.99 | 8.85 | 33.25 | 16.65 | 3.76 |
| 28 |  | 15.30 | 11.04 | 49.46 | 3.22 | 4.48 |
| 29 |  | 0.39 | 10.82 | 13.15 | 33.84 | 1.16 |
| 30 |  | 6.40 | 42.41 | 101.3 | 15.91 | 2.39 |
| 31 |  | 10.11 | 14.37 | 45.80 | 4.52 | 3.19 |
| 35 |  | 0.84 | 10.51 | 15.80 | 18.78 | 1.50 |
| 36a |  | 3.11 | 33.48 | 13.3 | 4.29 | 0.39 |
| 36b |  | 0.45 | 16.60 | 13.56 | 29.82 | 0.82 |
| Chloroquine | 0.0063 | |||||
| Melarsoprol | 0.0075 | |||||
| Podophyllotoxin | 0.017 | |||||
3. Experimental
3.1. General Information
3.2. Synthesis
3.2.1. Synthesis of 4-(ω-Bromoalkyl)acetophenone (3)
3.2.2. Synthesis of 1-(4-(10-Piperidinyldecyl)phenyl)ethanone (4)
3.2.3. Synthesis of (E)-4-(4-Hydroxyphenyl)-3-buten-2-one (7)
3.2.4. Synthesis of (E)-4-(4-Alkoxyphenyl)-3-buten-2-ones 8–9
3.2.5. Synthesis of (E)-13-(N-Methylpiperazinyl)-3-tridecen-2-one (15)
3.2.6. Synthesis of 7-Cycloheptylideneheptanoic Acid (24)
3.2.7. Synthesis of 7-Cycloheptylidene-2-heptanone (26)
3.2.8. Synthesis of 4-(1H)-Quinolones
3.3. Biological Evaluation
3.3.1. Antimalarial Assay
3.3.2. Antitrypanosomal Assay
3.3.3. Cytotoxicity Assay
4. Conclusions
Acknowledgments
Authors Contribution
Conflicts of Interest
References
- World Health Organization. World Malaria Report; Fact Sheet No. 94; World Health Organization: Geneva, Switzerland, 2013.
- Sachs, J.; Malaney, P. The economic and social burden of malaria. Nature 2002, 415, 680–685. [Google Scholar] [CrossRef]
- Langlands, B.W. The Sleeping Sickness Epidemic of Uganda,1900–1920: A Study in Historical Geography; Department of Geography,Makerere University College: Kampala, Uganda, 1967. [Google Scholar]
- Mäser, P.; Wittlin, S.; Rottmann, M.; Wenzler, T.; Kaiser, M.; Brun, R. Antiparasitic agents: New drugs on the horizon. Curr. Opin. Pharmacol. 2012, 12, 562–566. [Google Scholar] [CrossRef]
- Stephen, J.M.L.; Tonkin, L.M.; Walker, J. Tetrahydroacridones and related compounds as antimalarials. J. Chem. Soc. 1947, 1034–1039. [Google Scholar] [CrossRef]
- Winter, R.W.; Kelly, J.X.; Smilkstein, M.J.; Dodean, R.; Hinrichs, D.; Riscoe, M.K. Antimalarial quinolones: Synthesis, potency, and mechanistic studies. Exp. Parasitol. 2008, 118, 487–497. [Google Scholar] [CrossRef]
- Saenz, F.E.; LaCrue, A.N.; Cross, M.; Maignan, J.R.; Udenze, K.O.; Manetsch, R.; Kyle, D.E. 4-(1H)-Quinolones and 1,2,3,4-tetrahydroacridin-9-(10H)-ones prevent the transmission of Plasmodium falciparum to Anopheles freeborni. Antimicrob. Agents Chemother. 2013, 57, 6187–6195. [Google Scholar] [CrossRef]
- Saleh, A.; Friesen, J.; Baumeister, S.; Gross, U.; Bohne, W. Growth inhibition of Toxoplasma gondii and Plasmodium falciparum by nanomolar concentrations of 1-hydroxy-2-dodecyl-4-(1H)-quinolone, a high-affinity inhibitor of alternative (type II) NADH dehydrogenases. Antimicrob. Agents Chemother. 2007, 51, 1217–1222. [Google Scholar] [CrossRef]
- Cross, R.M.; Monastyrskyi, A.; Mutka, T.S.; Burrows, J.N.; Kyle, D.E.; Manetsch, R. Endochin optimization: Structure-activity and structure-property relationship studies of 3-substituted 2-methyl-4-(1H)-quinolones with antimalarial activity. J. Med. Chem. 2010, 53, 7076–7094. [Google Scholar] [CrossRef]
- Zhang, Y.; Clark, J.A.; Connelly, M.C.; Zhu, F.; Min, J.; Guiguemde, W.A.; Pradhan, A.; Iyer, L.; Furimsky, A.; Gow, J.; et al. Lead optimaization of 3-carboxy-4(1H)-quinolones to deliver orally bioavailable antimalarials. J. Med. Chem. 2012, 55, 4205–4219. [Google Scholar] [CrossRef]
- Pidathala, C.; Amewu, R.; Pacorel, B.; Nixon, G.L.; Gibbons, P.; Hong, W.D.; Leung, S.C.; Berry, N.G.; Sharma, R.; Stocks, P.A.; et al. Identification, design and biological evaluation of bisarray quinolones targeting Plasmodium falciparum type II NADH: Quinolone oxidoreductase (PfNDH2). J. Med. Chem. 2012, 55, 1831–1843. [Google Scholar] [CrossRef]
- Nenortas, E.; Burri, C.; Shapiro, T.A. Antitrypanosomal activity of fluoroquinolones. Antimicrob. Agents Chemother. 1999, 43, 2066–2068. [Google Scholar]
- Hiltensperger, G.; Jones, N.G.; Niedermeier, S.; Stich, A.; Kaiser, M.; Jung, J.; Puhl, S.; Damme, A.; Braunschweig, H.; Meinel, L.; et al. Synthesis and structure-activity relationships of new quinolone type molecules against Trypanosoma brucei. J. Med. Chem. 2012, 55, 2538–2548. [Google Scholar] [CrossRef]
- Wube, A.A.; Bucar, F.; Hochfellner, C.; Blunder, M.; Bauer, R.; Hüfner, A. Synthesis of N-substituted 2-[(1E)-alkenyl]-4-(1H)-quinolone derivatives as antimycobacterial agents against non-tubercular mycobacteria. Eur. J. Med. Chem. 2011, 46, 2091–2101. [Google Scholar]
- Wube, A.; Guzman, J.D.; Hüfner, A.; Hochfellner, C.; Blunder, M.; Bauer, R.; Gibbons, S.; Bhakta, S.; Bucar, F. Synthesis and antibacterial evaluation of a new series of N-alkyl-2-akynyl/(E)-alkenyl-4-(1H)-quinolones. Molecules 2012, 17, 8217–8240. [Google Scholar] [CrossRef]
- Ponnudurai, T.; Leeuwenberg, A.D.; Meuwissen, J.H. Chloroquine sensitivity of isolates of Plasmodium falciparum adapted to in vitro culture. Trop. Geogr. Med. 1981, 33, 50–54. [Google Scholar]
- Baltz, T.; Baltz, D.; Giroud, C.; Crockett, J. Cultivation in a semi-defined medium of animal infective forms of Trypanosoma brucei, T. equiperdum, T. evansi, T. rhodesiense and T. gambiense. EMBO J. 1985, 4, 1273–1277. [Google Scholar]
- Page, C.; Page, C.M.; Noel, C. A new fluorimetric assay for cytotoxicity measurements in vitro. Int. J. Oncol. 1993, 3, 473–476. [Google Scholar]
- Ahmed, S.A.; Gogal, R.M.; Walsh, J.E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: An alternative to [3H] thymidine incorporation assay. J. Immunol. Methods 1994, 170, 211–224. [Google Scholar]
- Owen, C.P.; Shahid, I.; Olusanjo, M.S.; Patel, C.H.; Dhanani, S.; Ahmed, S. Synthesis, biochemical evolution and rationalization of the inhibitory activity of a range of phenyl alkylimidazole-based compounds as potent inhibitory of the enzyme complex 17α-hydroxylase/17,20 lyase (P45017α). J. Steroid Biochem. Mol. Biol. 2008, 111, 117–127. [Google Scholar] [CrossRef]
- Pizzirani, D.; Roberti, M.; Grimaudo, S.; di Cristina, A.; Pipitone, R.M.; Tolomeo, M.; Recanatini, M. Identification of biphenyl-based hybrid molecules able to decrease the intracellular level of Bel-2 protein in Bcl-2 overexpression leukaemia cells. J. Med. Chem. 2009, 52, 6936–6940. [Google Scholar]
- Wube, A.A.; Hüfner, A.; Thomaschitz, C.; Blunder, M.; Kollroser, M.; Bauer, R.; Bucar, F. Design, synthesis and antimycobacterial activities of 1-methyl-2-alkenyl-4(1H)-quinolones. Bioorg. Med. Chem. 2011, 19, 567–579. [Google Scholar] [CrossRef]
- Desjardins, R.E.; Canfield, C.J.; Haynes, J.D.; Chulay, J.D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 1979, 16, 710–718. [Google Scholar] [CrossRef]
- Huber, W.; Koella, J.C. A comparison of the three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop. 1993, 55, 257–261. [Google Scholar] [CrossRef]
- Räz, B.; Iten, M.; Grether-Buhler, Y.; Kaminsky, R.; Brun, R. The Alamar Blue assay to determine drug sensitivity of African Trypanosomes (T. b. rhodesiense and T. b. gambiense) in vitro. Acta Trop. 1997, 68, 139–147. [Google Scholar]
- Sample Availability: Samples of 4(1H)-quinolone derivatives are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wube, A.; Hüfner, A.; Seebacher, W.; Kaiser, M.; Brun, R.; Bauer, R.; Bucar, F. 1,2-Substituted 4-(1H)-Quinolones: Synthesis, Antimalarial and Antitrypanosomal Activities in Vitro. Molecules 2014, 19, 14204-14220. https://doi.org/10.3390/molecules190914204
Wube A, Hüfner A, Seebacher W, Kaiser M, Brun R, Bauer R, Bucar F. 1,2-Substituted 4-(1H)-Quinolones: Synthesis, Antimalarial and Antitrypanosomal Activities in Vitro. Molecules. 2014; 19(9):14204-14220. https://doi.org/10.3390/molecules190914204
Chicago/Turabian StyleWube, Abraham, Antje Hüfner, Werner Seebacher, Marcel Kaiser, Reto Brun, Rudolf Bauer, and Franz Bucar. 2014. "1,2-Substituted 4-(1H)-Quinolones: Synthesis, Antimalarial and Antitrypanosomal Activities in Vitro" Molecules 19, no. 9: 14204-14220. https://doi.org/10.3390/molecules190914204
APA StyleWube, A., Hüfner, A., Seebacher, W., Kaiser, M., Brun, R., Bauer, R., & Bucar, F. (2014). 1,2-Substituted 4-(1H)-Quinolones: Synthesis, Antimalarial and Antitrypanosomal Activities in Vitro. Molecules, 19(9), 14204-14220. https://doi.org/10.3390/molecules190914204