Cytotoxic Illudalane Sesquiterpenes from the Wood-Decay Fungus Granulobasidium vellereum (Ellis & Cragin) Jülich
Abstract
:1. Introduction
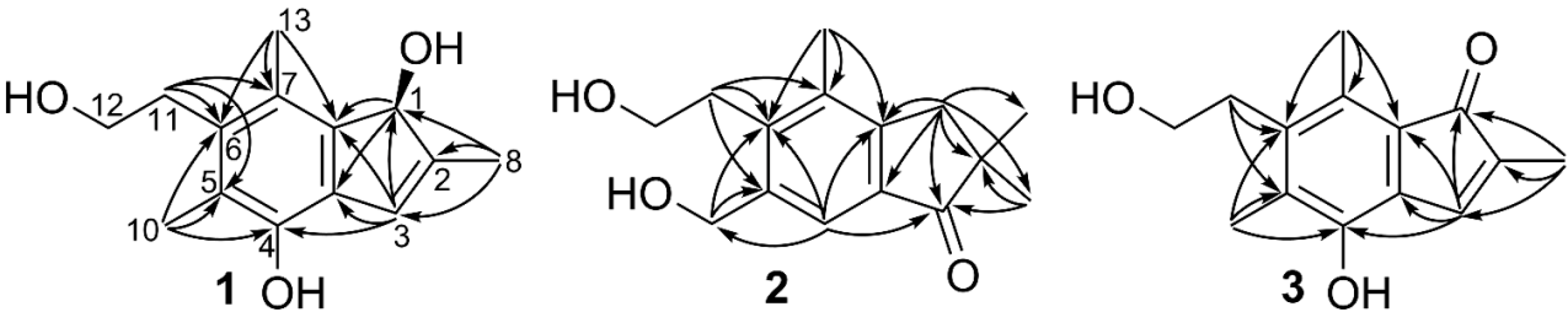
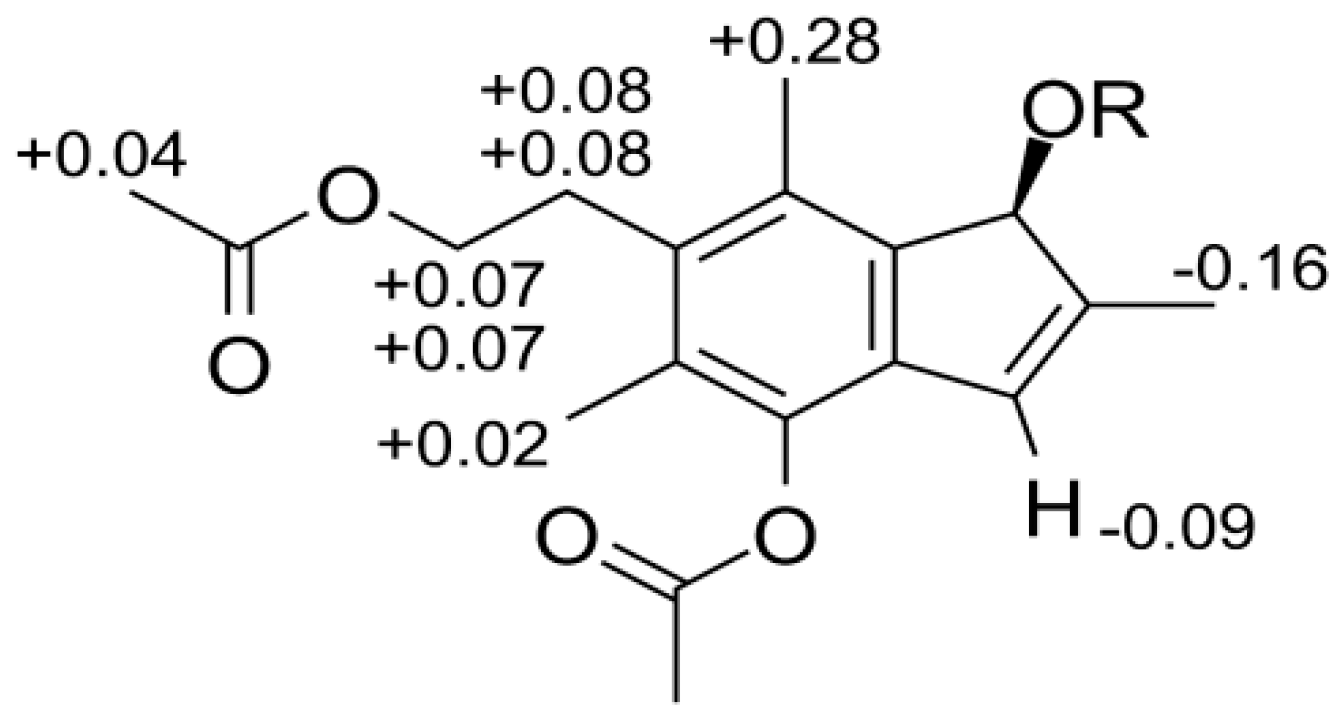
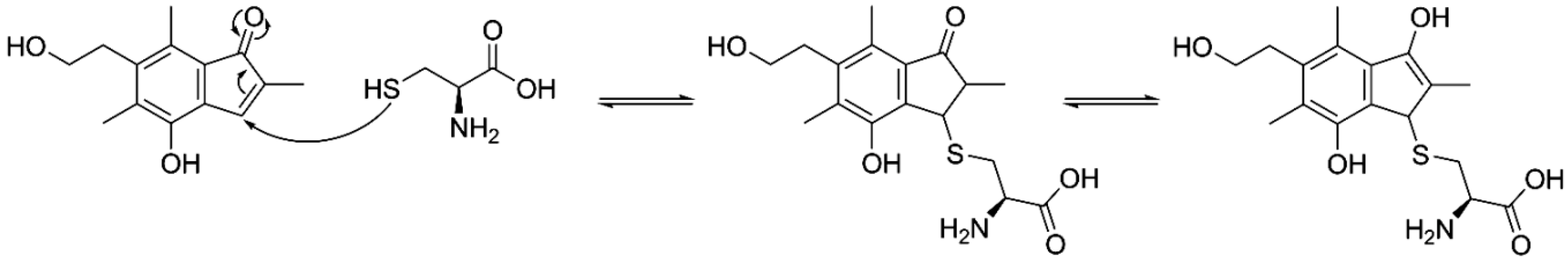
2. Results and Discussion
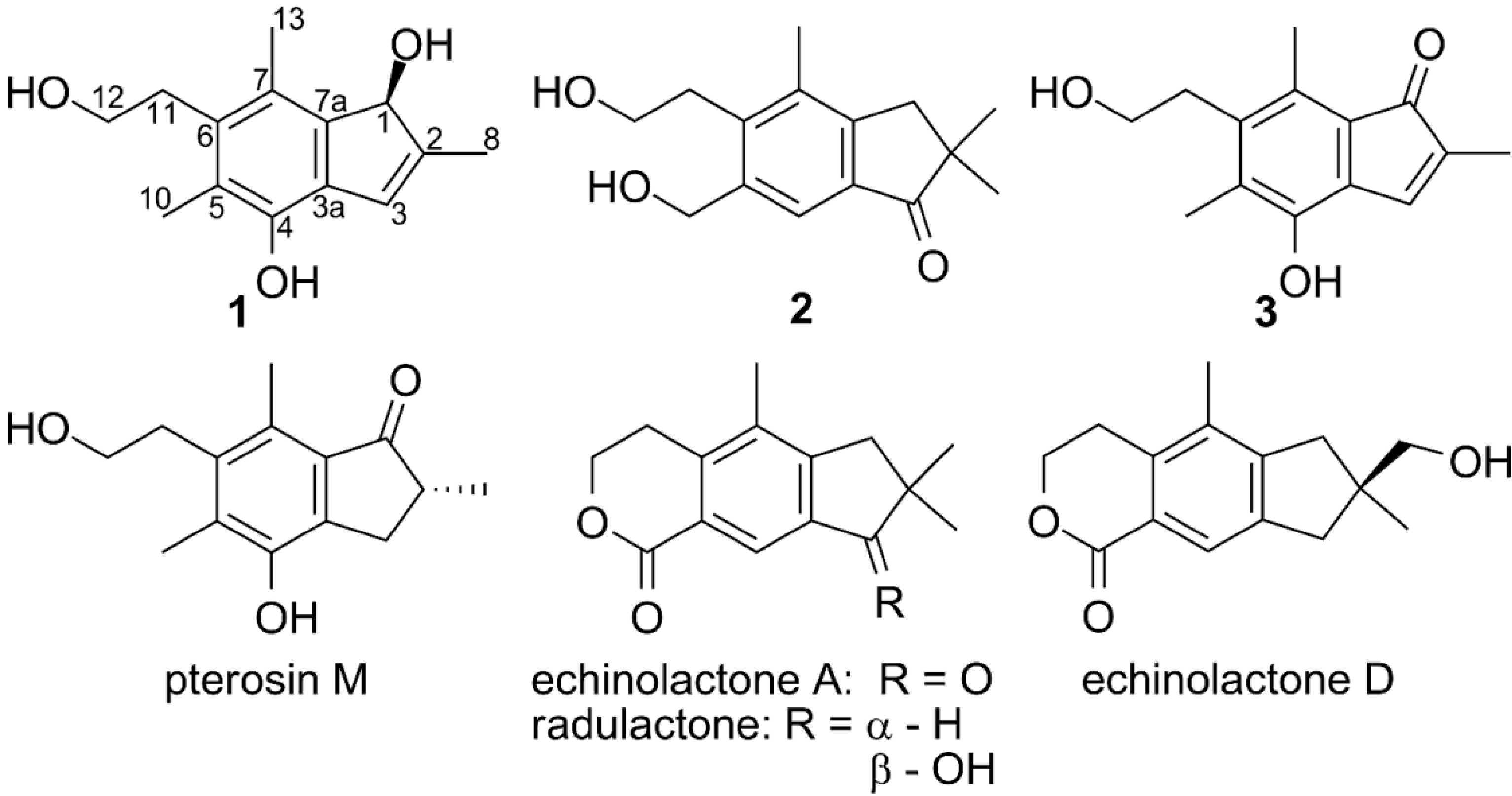
| pos. | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δC, mult | δH ( J in Hz) | δC, mult | δH (J in Hz) | δC, mult. | δH (J in Hz) | |
| 1 | 73.5, CH | 4.81, m | 43.3, CH2 | 2.94, s | 201.8, | C |
| 2 | 146.5, C | 46.7 | 134.4, C | |||
| 3 | 123.9, CH | 6.47, dd (1.7, 2.7) | 214.2, | C | 140.8, CH | 7.38, q (1.8) |
| 3a | 128.9, C | 134.1, C | 127.5, C | |||
| 4 | 146.3, C | 122.4, CH, | 7.61, s | 147.6, C | ||
| 5 | 125.8, C | 141.6, C, | 134.4, C | |||
| 6 | 134.0, C | 144.6, C, | 138.8, C | |||
| 7 | 126.5, C | 136.3, C, | 131.2, C | |||
| 7a | 143.1, C | 153.0, C, | 127.2, C | |||
| 8 | 13.9, CH3 | 2.01, dd (0.9, 1.7) | 25.6, CH3 | 1.20, s | 10.0, CH3 | 1.76, d (1.8) |
| 9 | 25.6, CH3 | 1.20, s | ||||
| 10 | 12.3, CH3 | 2.20, s | 63.5, CH2 | 4.73, s | 12.9, CH3 | 2.19, s |
| 11 | 34.0, CH2 | 2.91, m | 33.6, CH2 | 3.10, t (7.4) | 33.4, CH2 | 2.87, t (7.5) |
| 12 | 62.2, CH2 | 3.55, m | 62.1, CH2 | 3.72, t (7.4). | 61.8, CH2 | 3.56, t (7.5) |
| 13 | 14.9, CH3 | 2.35, s | 14.7, CH3 | 2.35, s | 12.8, CH3 | 2.42, s |
| Cell Line | CC50 (µM) | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Huh7 | >400 | >400 | 6.7 |
| MT4 | 55 | 180 | 0.15 |
3. Experimental Section
3.1. General Procedures
3.2. Fungal Cultivation
3.3. Bioassay Procedure
3.4. Isolation of Compounds from Liquid Cultures
3.4.1. Granuloinden A (1)
3.4.2. Dihydrogranuloinden (2)
3.4.3. Granuloinden B (3)
3.4.4. Reaction of 3 with Cysteine
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tanaka, N.; Satake, T.; Takahashi, A.; Mochizuki, M.; Murakami, T.; Saiki, Y.; Yang, J.Z.; Chen, C.M. Chemical studies on the constituents of Pteris bella Tagawa and Pteridium aquilinum subsp. wightianum (Wall) Shich. Chem. Pharm. Bull. 1982, 30, 3640–3646. [Google Scholar] [CrossRef]
- Fabian, K.; Lorenzen, K.; Anke, T.; Johansson, M.; Sterner, O. Five new bioactive sesquiterpenes from the fungus Radulomyces confluens (Fr.) Christ. Z. Naturforsch. 1998, 53, 939–945. [Google Scholar]
- Suzuki, S.; Murayama, T.; Shiono, Y. Illudalane sesquiterpenoids, echinolactones A and B, from a mycelial culture of Echinodontium japonicum. Phytochemistry 2005, 66, 2329–2333. [Google Scholar] [CrossRef]
- Suzuki, S.; Murayama, T.; Shiono, Y. Echinolactones C and D: Two illudalane sesquiterpenoids isolated from the cultured mycelia of the fungus Echinodontium japonicum. Phytochemistry 2006, 61b, 1295–1298. [Google Scholar]
- Harinantenaina, L.; Matsunami, K.; Otsuka, H. Chemical and biologically active constituents of Pteris multifida. J. Nat. Med. 2008, 62, 452–455. [Google Scholar] [CrossRef]
- Shu, J.; Liu, J.; Zhong, Y.; Pan, J.; Liu, L.; Zhang, R. Two new pterosin sesquiterpenes from Pteris multifida Poir. Phytochem. Lett. 2012, 5, 276–279. [Google Scholar] [CrossRef]
- Ouyang, D.W.; Ni, X.; Xu, H.Y.; Chen, J.; Yang, P.M.; Kong, D.Y. Pterosins from Pteris multifida. Planta Med. 2010, 76, 1896–1900. [Google Scholar] [CrossRef]
- Nord, C.L.; Menkis, A.; Vasaitis, R.; Broberg, A. Protoilludane sesquiterpenes from the wood decomposing fungus Granulobasidium vellereum (Ellis & Cragin) Jülich. Phytochemistry 2013, 90, 128–134. [Google Scholar] [CrossRef]
- Nord, C.L.; Menkis, A.; Lendel, C.; Vasaitis, R.; Broberg, A. Sesquiterpenes from the saprotrophic fungus Granulobasidium vellereum (Ellis & Cragin) Jülich. Phytochemistry 2014, 102, 197–204. [Google Scholar] [CrossRef]
- Hasegawa, M.; Akabori, Y. New indanone compounds from Onychium japonicum. Phytochemistry 1974, 13, 509–511. [Google Scholar] [CrossRef]
- Kuroyanagi, M.; Fukuoka, M.; Yoshihira, K.; Natori, S. The absolute configurations of pterosins, 1-indanone derivates from bracken, Pteridium aquilinum var latiusculum. Chem. Pharm. Bull. 1974, 22, 723–726. [Google Scholar] [CrossRef]
- Ohtani, I.; Kusumi, T.; Kashman, Y.; Kakisawa, H. High-field FT NMR application of Mosher’s method.The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 1991, 113, 4092–4096. [Google Scholar] [CrossRef]
- McMorris, T.C.; Kelner, M.J.; Wang, W.; Estes, L.A.; Montoya, M.A.; Taetle, R. Structure-activity relationships of illudins: Analogs with improved therapeutic index. J. Org. Chem. 1992, 57, 6876–6883. [Google Scholar] [CrossRef]
- McMorris, T.C.; Kelner, M.J.; Wang, W.; Moon, S.; Taetle, R. On the mechanism of toxicity of illudins: The role of glutathione. Chem. Res. Toxicol. 1990, 3, 574–579. [Google Scholar] [CrossRef]
- Stenlid, J. Population-structure of Heterobasidion annosum as determined by somatic incompatibility, sexual incompatibility, and isoenzyme patterns. Can. J. Bot. 1985, 63, 2268–2273. [Google Scholar] [CrossRef]
- Weislow, O.S.; Kiser, R.; Fine, D.L.; Bader, J.; Shoemaker, R.H.; Boyd, M.R. New soluble-formazan assay for HIV-1 cytopathic effects: Application to high-flux screening of synthetic and natural products for AIDS-antiviral activity. J. Natl. Cancer Inst. 1989, 81, 577–586. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nord, C.L.; Menkis, A.; Broberg, A. Cytotoxic Illudalane Sesquiterpenes from the Wood-Decay Fungus Granulobasidium vellereum (Ellis & Cragin) Jülich. Molecules 2014, 19, 14195-14203. https://doi.org/10.3390/molecules190914195
Nord CL, Menkis A, Broberg A. Cytotoxic Illudalane Sesquiterpenes from the Wood-Decay Fungus Granulobasidium vellereum (Ellis & Cragin) Jülich. Molecules. 2014; 19(9):14195-14203. https://doi.org/10.3390/molecules190914195
Chicago/Turabian StyleNord, Christina L., Audrius Menkis, and Anders Broberg. 2014. "Cytotoxic Illudalane Sesquiterpenes from the Wood-Decay Fungus Granulobasidium vellereum (Ellis & Cragin) Jülich" Molecules 19, no. 9: 14195-14203. https://doi.org/10.3390/molecules190914195
APA StyleNord, C. L., Menkis, A., & Broberg, A. (2014). Cytotoxic Illudalane Sesquiterpenes from the Wood-Decay Fungus Granulobasidium vellereum (Ellis & Cragin) Jülich. Molecules, 19(9), 14195-14203. https://doi.org/10.3390/molecules190914195





