Several protocols with scanning probe microscopy were conducted to investigate the self-assembly and surface morphology of BMPHA on Au(111) in a liquid environment. Our goal was to obtain side-by-side views of the morphology of BMPHA and n-alkanethiols films using AFM characterizations of grafted nanopatterns. The known height of n-octadecanethiol (ODT) was used as a nanoscale ruler to evaluate the thickness of BMPHA films prepared under selected conditions. Depending on the concentration and immersion intervals, either single or double layers of BMPHA could be generated.
2.1. Nanoshaving within a Monolayer Film of BMPHA
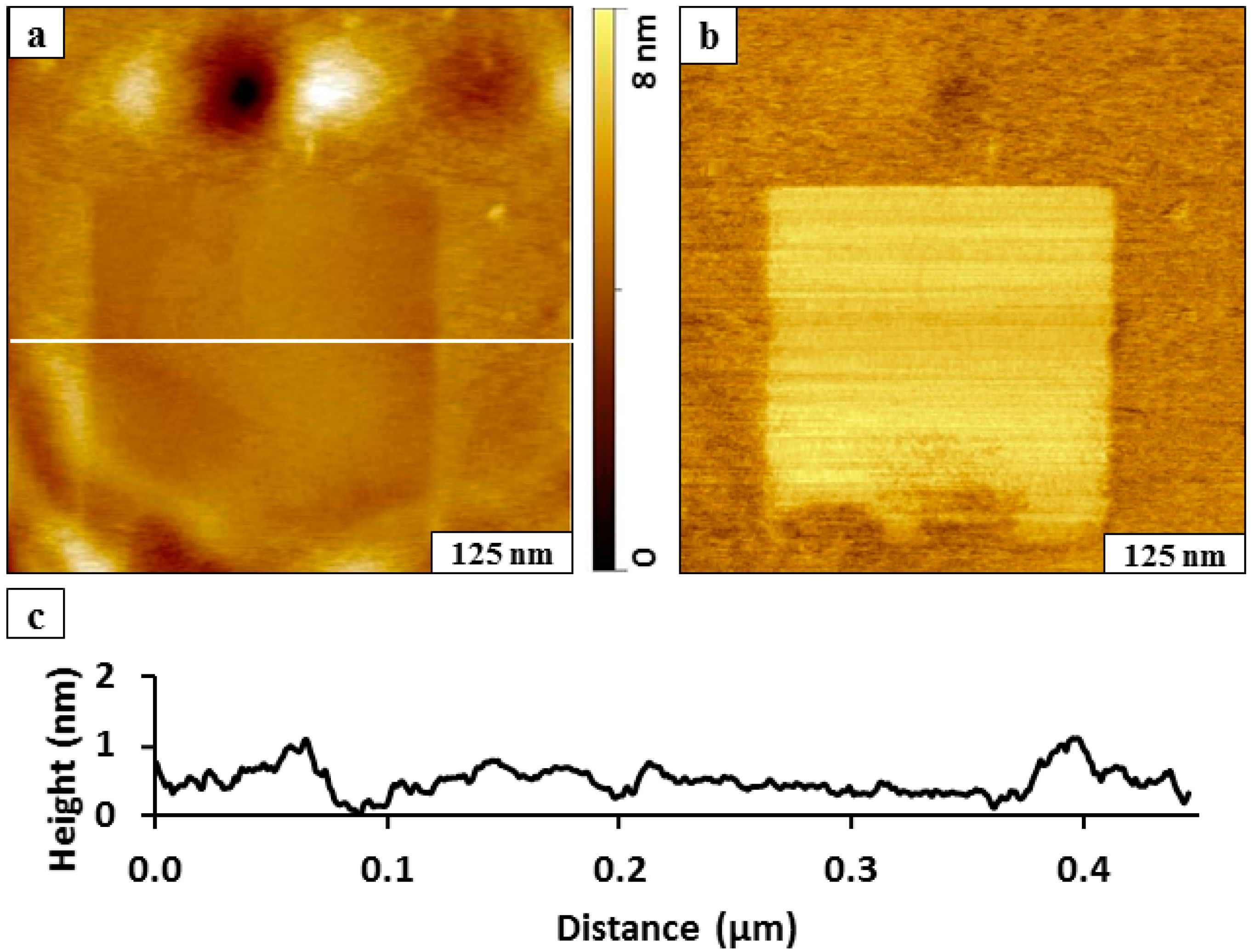
A square nanoshaved pattern (200 × 200 nm
2) within a SAM of BMPHA is shown in
Figure 2. The BMPHA film of the matrix area surrounding the nanoshaved hole was formed by 24 h immersion in 0.1 mM solution. The nanoshaved area is the dark square in the topography image (
Figure 2a) and reveals the underlying gold substrate. The irregularly shaped bright patches at the top and bottom of the nanoshaved square are residues of BMPHA removed by the AFM probe. To assess how effective the nanoshaving parameters were for removal of BMPHA, the simultaneously acquired lateral force image is shown in
Figure 2b. The bright area of the square nanopattern located at the center of the image indicates the exposed areas of the substrate, which indicates clean removal of BMPHA. The tip-surface interactions mapped in lateral force frames are distinct for the gold surface and carboxylic endgroups of BMPHA. The thickness of the BMPHA film can be evaluated with a local cursor measurement, referencing the uncovered area of the substrate as a baseline at the bottom of the nanoshaved square pattern (
Figure 2c). The thickness of the BMPHA SAM measured 2.0 ± 0.2 nm, which is in close agreement with the value obtained by ellipsometry [
15]. A conservative error term of 0.2 nm is reported due to the roughness of the underlying gold substrate, which is based on the height of a single gold terrace step.
Figure 2.
Nanoshaving within a BMPHA monolayer. (a) Topography view of nanoshaved square; (b) corresponding lateral force image; (c) height profile for the white line in a.
Figure 2.
Nanoshaving within a BMPHA monolayer. (a) Topography view of nanoshaved square; (b) corresponding lateral force image; (c) height profile for the white line in a.
Compared to monodentate
n-alkanethiols, a higher force was required to shave away the multidentate BMPHA layer. For the example in
Figure 1, the area was swept 20 times with 5 nN/m applied force. Typically,
n-alkanethiols can be nanoshaved with 4-6 sweeps at forces between 0.2 and 10 nN/M depending on the sharpness of the AFM probe. When nanoshaving
n-alkanethiol SAMs in ethanol, the displaced molecules are often dissolved in the surrounding liquid media so that no residues are present at the edges of the patterns. However, with the bidentate example shown in
Figure 2, BMPHA molecules formed aggregate assemblies that failed to dissolve fully in ethanolic media. Intermolecular associations between the carboxylic acid groups and π-π interactions of the aromatic moiety of BMPHA are sufficiently strong to induce aggregation, as indicated by surface residues at the edges of the nanoshaved pattern.
2.2. Nanografting n-Alkanethiols within a Monolayer of BMPHA
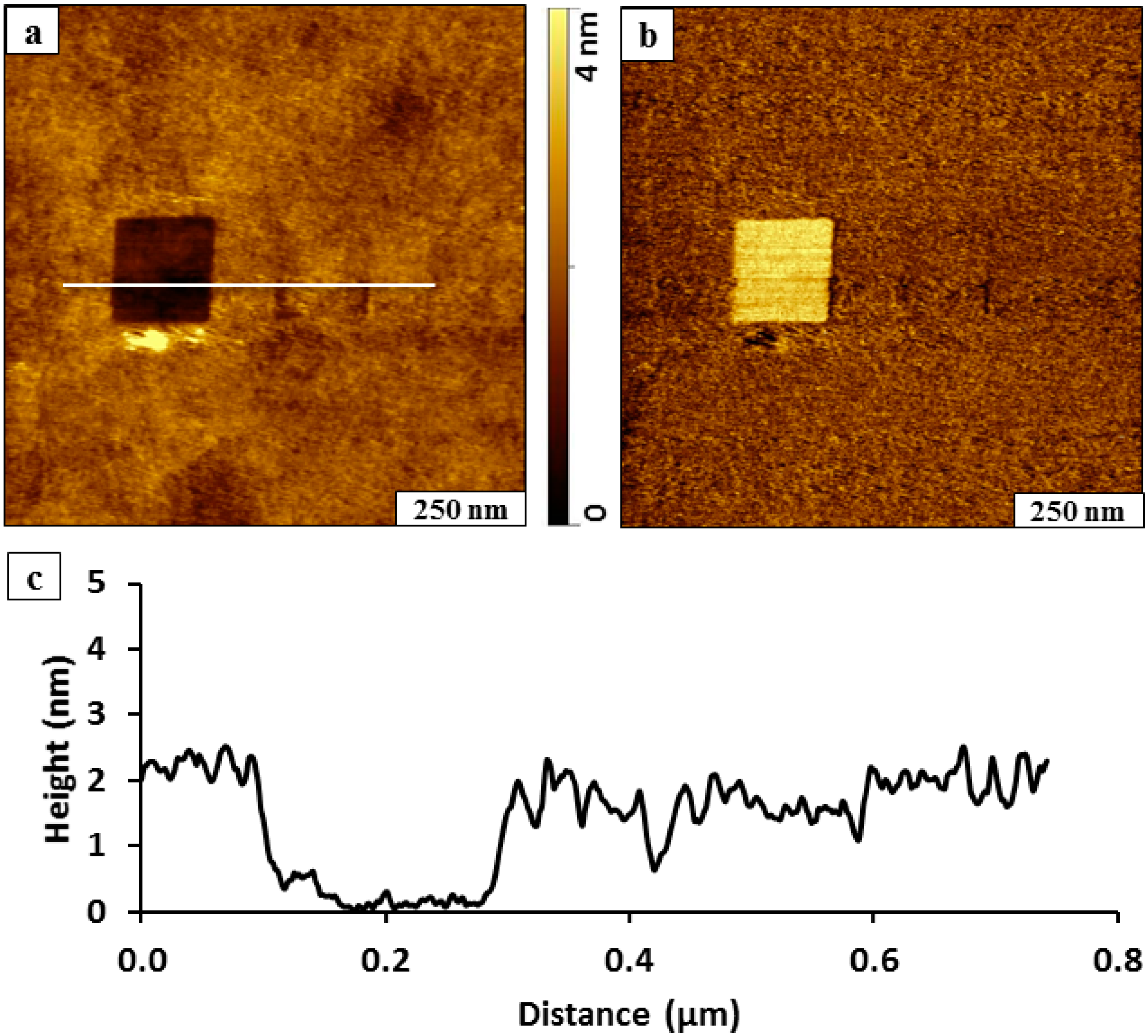
The thickness and quality of the bidentate BMPHA films were examined further using protocols of nanografting. Films of BMPHA were prepared on Au(111) by immersing a gold substrate into an ethanolic solution (0.1 mM) for 24 h. A nanopattern of
n-octadecanethiol (ODT) was inscribed within a matrix of BMPHA (
Figure 3) to enable a side-by-side comparison of the surface morphology. The nanopattern of ODT appears to be slightly shorter in height than the surrounding areas of BMPHA (
Figure 3a) revealing a recessed square region containing overlapping gold steps. The concurrently acquired lateral force image (
Figure 3b) reveals distinct changes in the surface chemistry for the regions of the ODT nanopattern and BMPHA matrix. The horizontal lines are produced by the left and right raster pattern of the AFM probe. The expected height for a densely packed ODT monolayer is 2.1 nm, assuming a tilt angle of 28°. Residues of BMPHA are piled at the edges of the nanografted pattern and provide a distinct boundary around the inscribed region of ODT; therefore, comparison of the height differences at the pattern edges cannot be used to provide a reliable estimate of the BMPHA film thickness for this example. Comparing the cursor measurement in areas beyond the edges of the nanopattern reveals that the thickness of the BMPHA film is approximately the same as ODT, which would correspond to a monolayer.
Figure 3.
Side-by-side comparison of the surface morphology of ODT and a BMPHA monolayer prepared on Au(111). (a) Nanografted pattern of ODT (300 × 300 nm2) viewed with a contact-mode AFM topography image acquired in ethanol; (b) corresponding lateral force image; (c) cursor profile for the line in a.
Figure 3.
Side-by-side comparison of the surface morphology of ODT and a BMPHA monolayer prepared on Au(111). (a) Nanografted pattern of ODT (300 × 300 nm2) viewed with a contact-mode AFM topography image acquired in ethanol; (b) corresponding lateral force image; (c) cursor profile for the line in a.
The areas of BMPHA appear to have a rougher morphology in the areas surrounding the nanografted ODT pattern (
Figure 3a). A few small adsorbates are present on the BMPHA areas, and the density of BMPHA is consistent with a loosely packed tailgroup assembly [
31]. The differences in tip-surface adhesive interactions are quite distinct in the lateral force image of
Figure 3b. A monolayer of BMPHA would present carboxylic acid moieties at the surface, whereas the nanopattern of ODT is terminated with methyl groups, which produces distinct changes in color contrast for the lateral force frame.
Protocols of nanoshaving and nanografting can be accomplished within the same experiment by rinsing and exchanging solutions with the AFM liquid sample cell. This protocol requires a stable imaging environment, since it is easy to perturb the sample to displace the tip away from the nanofabricated region. In the next
in situ experiment, a 200 × 200 nm
2 square of BMPHA molecules was nanografted within a naturally grown BMPHA SAM (
Figure 4). The nanopattern can be vaguely distinguished near the center of the image, because the bottom corners of the pattern did not fill in completely. Otherwise, the height of the nanografted area matches the height of BMPHA and is not visible in the topography frame. After nanografting, the BMPHA solution was removed and replaced with clean ethanol to enable nanoshaving. A square area (200 × 200 nm
2) was swept at high force to disclose the underlying gold substrate at the left side of the image. The nanoshaved area is easy to distinguish as a dark square at the left side of the topography frame of
Figure 4a.
Figure 4.
Fabrication of a nanoshaved square and nanografted pattern of BMPHA side-by-side within a monolayer film of BMPHA. (a) Contact-mode topography image acquired in ethanol; (b) lateral force frame; (c) cursor profile for the line in a drawn across both nanopatterns.
Figure 4.
Fabrication of a nanoshaved square and nanografted pattern of BMPHA side-by-side within a monolayer film of BMPHA. (a) Contact-mode topography image acquired in ethanol; (b) lateral force frame; (c) cursor profile for the line in a drawn across both nanopatterns.
Although mostly indistinguishable, the square pattern of BMPHA was nanografted immediately to the right of the nanoshaved pattern. The area in the center of the pattern with a nanografted pattern of BMPHA has the same surface chemistry (‑COOH) as the surrounding matrix and cannot be clearly detected in the lateral force image (
Figure 4b). However the nanoshaved square on the left side of the frame is readily identifiable with brighter color.
Combining protocols of nanografting followed by nanoshaving is particularly useful to test if the nanografting solution interacts to bind to the surface of the matrix layer. A representative line profile across the nanoshaved area of
Figure 4a shows that the thickness of the film measures 2.1 ± 0.2 nm, referencing the bottom of the nanoshaved area as a baseline (
Figure 4c). This thickness corresponds to the expected height for a monolayer of BMPHA; consequently, there is no evidence of adsorption of the nanografting solution to the BMPHA matrix. For the nanografted area, no difference in height was detected for the nanografted patch of BMPHA
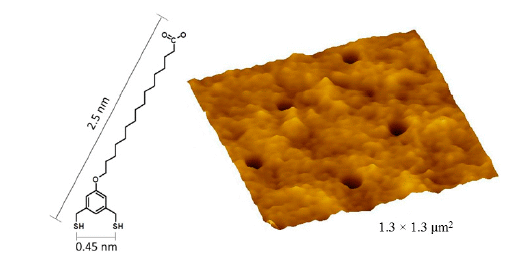
versus the surrounding BMPHA film. This area can be detected using the vague dark outline of the bottom corners of the nanografted square. Considering the overall theoretical molecular length of BMPHA (2.53 nm); the tilt angle for a BMPHA monolayer would measure ~34 degrees, assuming that both sulfurs of the bidentate molecule bind to the surface of gold.
2.3. Nanografting ODT within a Double Layer of BMPHA
A double layer film of BMPHA on Au(111) can be produced by changing the experimental parameters of concentration and immersion intervals. A bilayer of BMPHA was formed by immersion of a gold substrate in 5 mM ethanolic solution for 30 h. Nanopatterns were fabricated within a BMPHA bilayer using sequential steps of nanoshaving and nanografting prepared as shown in
Figure 5. First, a rectangular area (200 × 300 nm
2) was nanoshaved within the BMPHA film. Next, a solution of ODT (1 mM) was injected into the sample cell, and a smaller rectangle was nanografted to the right of the original nanoshaved area. At this concentration, we were unable to detect the growth of ODT within the nanoshaved area over a period of less than one hour. Growth of ODT within nanoshaved regions is greatly influenced by whether the area of the gold substrate is cleanly shaved. The nanoshaved area is not a pristine gold surface for self-assembly and is not well-suited for studying adsorption kinetics. For the rectangular nanopattern on the right side of
Figure 5a, an aggregate of residue removed from the nanoshaved area persists near the bottom of the rectangle. The nanografted pattern of ODT has a taller mound of removed adsorbates at the top edge of the feature. Differences in surface adhesion are mapped in the lateral force frame of
Figure 5b, revealing different color contrast for the matrix of BMPHA, the nanoshaved area, the nanografted ODT pattern, and adsorbate residues. The patterns exhibit different depths within the BMPHA matrix, as shown with a representative line profile in
Figure 5c. The nanofabricated areas have heights that are shorter than the surrounding BMPHA film. The thickness of the BMPHA matrix film measured using the depth of the nanoshaved rectangle is 4.2 ± 0.2 nm, which corresponds to a bilayer. The difference in thickness for the nanografted pattern on the right measured 2.1 ± 0.2 nm, which is consistent with the known thickness for a monolayer of ODT. Additional examples of nanoshaved and nanografted patterns produced within a BMPHA bilayer are provided in supporting information (Figure S1).
A model for the heights of the nanoshaved and nanografted patterns is presented in
Figure 5d. In the proposed model, a bilayer is formed by interactions between –COOH functional groups of BMPHA to form a head-to-head arrangement. Previously, double layers were detected with extended immersion or higher concentrations of acid-terminated
n-alkanethiol SAMs, such as 11-mercaptoundecanoic acid and mercaptohexadecanoic acid [
19]. For this model the bilayer of BMPHA presents dithiol moieties at the interface. Notably, no BMPHA bilayer structures were observed when the SAMs were formed from 1 mM ethanolic BMPHA followed by
ex situ rinsing with water, THF, and ethanol [
31].
The optimized threshold force was not determined for each experiment, and depends sensitively on the sharpness of the AFM probe. The amount of force used for fabrication using either nanografting or nanoshaving protocols were comparable for the monolayer or bilayer films of BMPHA. The monolayer film in
Figure 3 was fabricated using an applied force of 5.2 nN, whereas the bilayer film shown in
Figure 5 was accomplished with a force of 4.4 nN.
Figure 5.
A nanoshaved area and nanografted pattern of ODT placed side-by-side within a BMPHA bilayer. (a) Topography image; (b) lateral force frame; (c) cursor profile for a; (d) proposed height model.
Figure 5.
A nanoshaved area and nanografted pattern of ODT placed side-by-side within a BMPHA bilayer. (a) Topography image; (b) lateral force frame; (c) cursor profile for a; (d) proposed height model.
2.4. Nanofabrication Experiments with BMPHA Using Immersion Particle Lithography
Using immersion particle lithography, periodic arrangements of nanostructures were produced through surface self-assembly of BMPHA and ODT on gold. Solution self-assembly of thiol-based molecules on gold substrates enables construction of thin films with well-defined dimensions and composition. Particle lithography enables exquisite design of interfacial chemistry by defining the surface coverage of component molecules [
30,
32]. For protocols with BMPHA, a mask of mesospheres was placed on template-stripped gold (TSG). Two immersion steps were used to prepare islands of ODT within a matrix monolayer of BMPHA. In the first immersion step, the masked TSG substrate was submerged in an ethanolic solution of BMPHA to prepare nanoholes. When the mesosphere mask was removed, a periodic arrangement of uncovered areas of substrate is disclosed (
Figure 6a–c). The nanoholes were filled in by a second immersion step with ODT (
Figure 6d–f). This protocol produced circular islands of methyl-terminated ODT surrounded by a matrix monolayer of acid-functionalized BMPHA.
Figure 6.
Nanopatterns prepared with ODT and BMPHA using immersion particle lithography. (a) Nanoholes within BMPHA; (a') lateral force image; (b) successive zoom-in topograph; (b') lateral force image; (c) cursor profile for the line shown in b; (c') structural model of BMPHA nanostructures. (d) Nanoholes filled with ODT; (d') corresponding lateral force frame; (e) zoom-in view of ODT nanopatterns; (e') lateral force image; (f) cursor profile for the line in e; (f') chemical model of backfilled ODT within BMPHA.
Figure 6.
Nanopatterns prepared with ODT and BMPHA using immersion particle lithography. (a) Nanoholes within BMPHA; (a') lateral force image; (b) successive zoom-in topograph; (b') lateral force image; (c) cursor profile for the line shown in b; (c') structural model of BMPHA nanostructures. (d) Nanoholes filled with ODT; (d') corresponding lateral force frame; (e) zoom-in view of ODT nanopatterns; (e') lateral force image; (f) cursor profile for the line in e; (f') chemical model of backfilled ODT within BMPHA.
Representative AFM images of samples prepared with the two immersion steps are presented in
Figure 6; the top panels are views of the nanoholes within BMPHA produced by particle lithography, and the bottom panels were acquired for the same sample after backfilling with ODT. A periodic arrangement of nanoholes is shown in
Figure 6a; the dark circles of the topography frame are areas of uncovered substrate and can be more clearly distinguished in the lateral force frame (
Figure 6a'). There are approximately 25 nanoholes within the 3 × 3 µm
2 area, which would scale to an approximate surface density of 10
8 nanostructures/cm
2. The spacing between nanopatterns measures 500 nm, matching the diameter of the silica mesospheres used for patterning. With immersion particle lithography, the sizes of the nanoholes are exquisitely small because the areas where the beads make physical contact are much smaller than the periodicity of the mesosphere surface mask. The shape of the nanoholes is more clearly revealed in zoom-in views of
Figure 6b,b'. Six sites of nanoholes are shown within the 1.2 × 1.2 µm
2 frame, defining the areas where the mesospheres were displaced. The topography frames also resolve the shapes of the edges of steps and terraces of the gold substrate beneath the BMPHA film. The depth of the nanoholes measures 2.1 ± 0.2 nm, shown with a representative cursor profile in
Figure 6c. A side-view model for a monolayer of BMPHA with areas of uncovered gold is shown in
Figure 6c'.
The nanoholes were backfilled with ODT by immersing the sample in a solution of 1 mM ODT in ethanol for 24 h. Changes in surface morphology are readily apparent after backfilling, the locations of the circular holes are no longer visible in the topography frames of
Figure 6d,e. Since ODT and BMPHA are similar in height, there is no clear height difference revealed in the topographs, as shown with an example cursor profile in
Figure 6f. However, the simultaneously acquired lateral force frames disclose distinct differences in color contrast for the –COOH groups of BMPHA compared to methyl-terminated ODT. Lateral force images enable visualization of the locations and sizes of the ODT nanopatterns in
Figure 6d',e'. Using the lateral force frames, the surface coverage of methyl and acid headgroups was determined to be ~5%.
Immersion particle lithography was similarly applied for making islands of ODT within a bilayer film of BMPHA (
Figure 7). The bilayer was produced by using higher concentration of 4 mM BMPHA immersed for 30 h. Approximately 60 nanoholes of uncovered TSG are visible within a BMPHA bilayer for a 5 × 5 µm
2 area, as shown in
Figure 7a,a'. The diameter of the nanoholes measures 110 ± 10 nm, with a surface coverage of 5%. A close-up view of three nanoholes is shown in
Figure 7b,b', revealing a somewhat rougher texture at the surface of the BMPHA bilayer. The reference features of the underlying gold substrate cannot be distinguished in
Figure 7b as was apparent for the monolayer film of BMPHA (
Figure 6a,b). Self-assembly of a monolayer of BMPHA is mediated by binding through S-Au chemisorption to the gold substrate, whereas for the bilayer, the second layer is formed through electrostatic head-to-head interactions between molecules. One might predict that a bilayer film would be less densely packed than a monolayer, and the surface morphology revealed in
Figure 7b indicates that the packing density of the BMPHA bilayer has changed in comparison to a monolayer film. The depth of nanoholes formed within the BMPHA was measured to be 5.0 ± 1.2 nm (
Figure 7c). This distance is slightly longer than expected for a double layer of BMPHA, possibly attributable to adsorbates on the surface of the bilayer. A model of the side-view of the BMPHA bilayer is presented in
Figure 7c', showing head-to-head interactions between carboxylic acid groups that associate to form the double layer structure.
After backfilling the nanoholes with ODT, the patterns still appear as holes within the matrix film of the BMPHA bilayer (
Figure 7d,e). Images shown in the lower half of
Figure 7 were acquired after 24 h immersion in 1 mM ODT in ethanol. The rougher texture of the BMPHA bilayer appears to be smoother because of additional steps of rinsing and sonication to remove loose adsorbates.
Figure 7.
Nanopatterns prepared in a bilayer of BMPHA using immersion particle lithography. (a) Nanoholes exposing TSG within a BMPHA bilayer; (a') corresponding lateral force image; (b) zoom-in view; (b') lateral force image; (c) cursor profile for the line shown in b; (c') chemical model of BMPHA bilayer. (d) Nanoholes filled in with ODT; (d') corresponding lateral force frame; (e) zoom-in view of ODT backfilled nanopatterns; (e') lateral force image; (f) cursor profile for the line in e; (f') chemical model of backfilled ODT within a bilayer of BMPHA.
Figure 7.
Nanopatterns prepared in a bilayer of BMPHA using immersion particle lithography. (a) Nanoholes exposing TSG within a BMPHA bilayer; (a') corresponding lateral force image; (b) zoom-in view; (b') lateral force image; (c) cursor profile for the line shown in b; (c') chemical model of BMPHA bilayer. (d) Nanoholes filled in with ODT; (d') corresponding lateral force frame; (e) zoom-in view of ODT backfilled nanopatterns; (e') lateral force image; (f) cursor profile for the line in e; (f') chemical model of backfilled ODT within a bilayer of BMPHA.
Features of the underlying gold substrate such as step edges can be vaguely resolved for areas of the BMPHA bilayer in the zoom-in view of
Figure 7e. Chemical maps of the areas of nanopatterns with methyl headgroups are provided by the lateral force images (
Figure 7d',e'). The depth of backfilled nanoholes within BMPHA multilayer was measured to be 1.8 ± 0.2 nm (
Figure 7f), which closely matches the expected difference in thickness between a BMPHA bilayer and ODT. The measurement of nanopattern depths extrapolates to a local thickness of 3.9 ± 0.2 nm for a BMPHA bilayer. The proposed model for the height of the double layer pattern is shown in
Figure 7f'.