Structures of New Phenolics Isolated from Licorice, and the Effectiveness of Licorice Phenolics on Vancomycin-Resistant Enterococci
Abstract
:1. Introduction
2. Results and Discussion
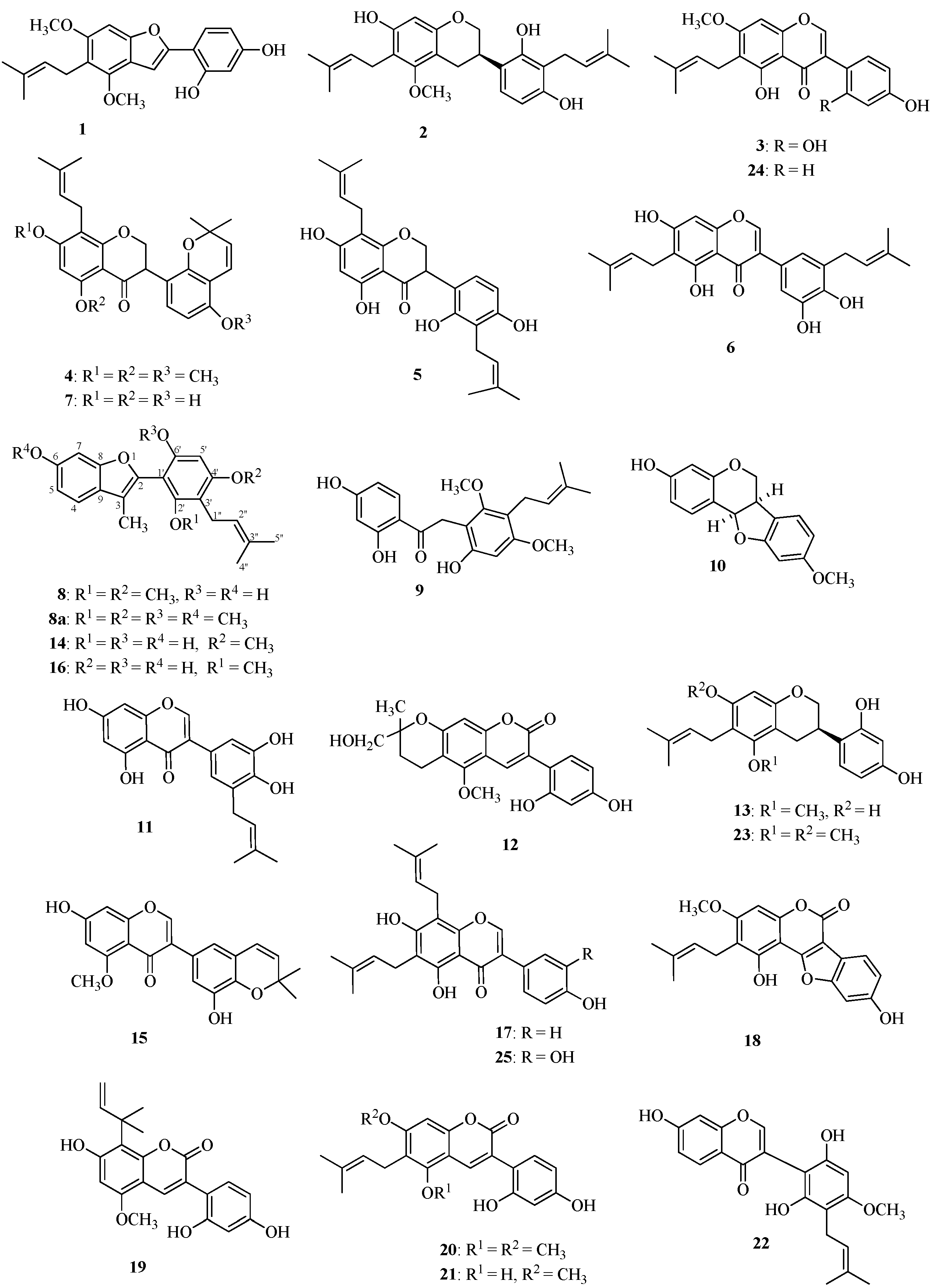
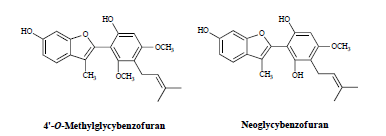
2.1. Structures of the New Compounds
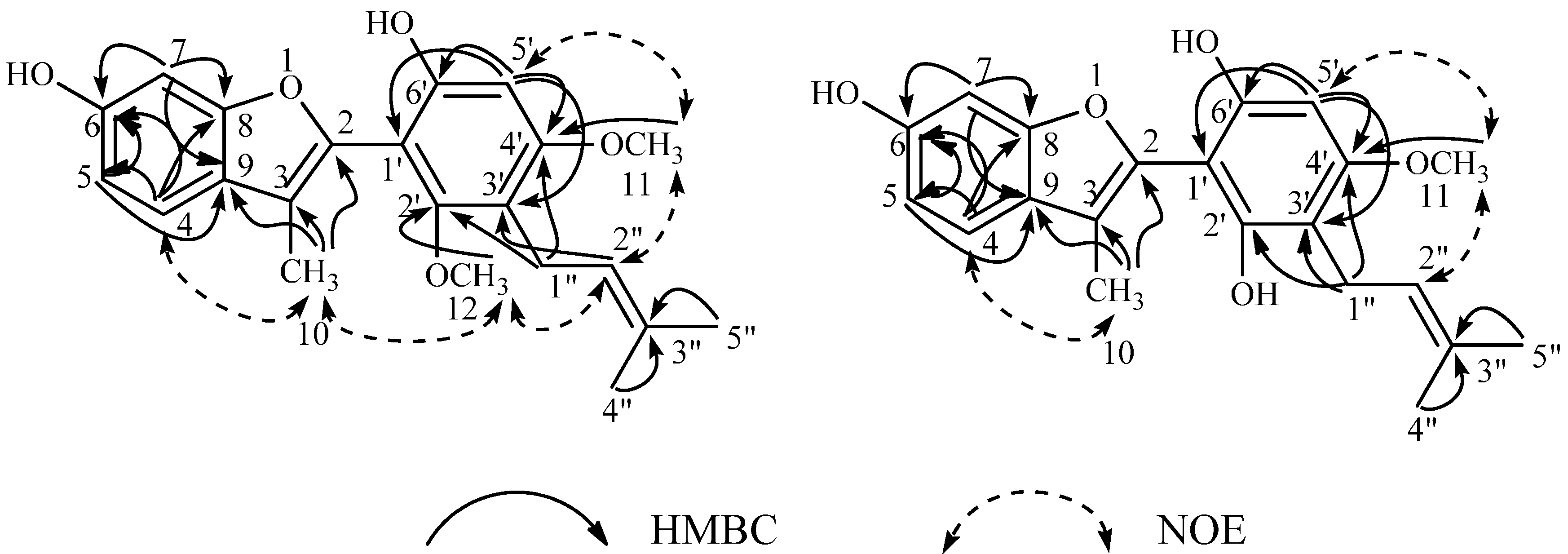
| Position | 4'-O-Methylglycybenzofuran (8) | Neoglycybenzofuran (14) | ||||||
|---|---|---|---|---|---|---|---|---|
| δC | HSQC a | δH ( J in Hz) | HMBC b | δC | HSQC a | δH ( J in Hz) | HMBC b | |
| C-2 | 145.7 | C | H-10 | 145.5 | C | H-10 | ||
| C-3 | 114.2 | C | H-10 | 114.4 | C | H-10 | ||
| C-4 | 119.6 | CH | 7.30, d (8.4) | 119.3 | CH | 7.23, d (9.0) | ||
| C-5 | 111.5 | CH | 6.77, dd (2.4, 8.4) | H-4 | 111.4 | CH | 6.71, dd (2.4, 9.0) | |
| C-6 | 155.8 | C | H-5, 7 | 155.7 | C | H-4, 5, 7 | ||
| C-7 | 97.9 | CH | 6.87, d (2.4) | 97.8 | CH | 6.82, d (2.4) | ||
| C-8 | 156.2 | C | H-4, 7 | 156.2 | CH | H-4, 7 | ||
| C-9 | 123.0 | C | H-5, 7, 10 | 123.8 | CH | H-5, 7, 10 | ||
| C-10 | 7.9 | CH3 | 1.89 s | 8.7 | CH3 | 1.97 s | ||
| C-1' | 102.1 | C | H-5' | 103.5 | C | H-5' | ||
| C-2' | 158.9 | C | H-1" | 159.1 | C | H-1" | ||
| C-3' | 114.2 | C | H-5', H-2" | 112.9 | C | H-5', 1", 2" | ||
| C-4' | 160.0 | C | H-5', H-1" | 160.0 | C | H-5', 1" | ||
| C-5' | 96.6 | CH | 6.41 s | 98.7 | CH | 6.28 s | ||
| C-6' | 153.1 | C | H-5' | 155.7 | C | H-5' | ||
| C-1" | 22.5 | CH2 | 3.32, d (6.6) | 22.9 | CH2 | 3.13, d (6.6) | ||
| C-2" | 124.3 | CH | 5.16, t (6.6) | 124.6 | CH | 5.12, t (6.6) | ||
| C-3" | 129.9 | C | H-4", H-5" | 129.7 | C | H-4", 5" | ||
| C-4" | 17.2 | CH3 | 1.60 s | 17.8 | CH3 | 1.69 s | ||
| C-5" | 25.3 | CH3 | 1.70 s | 25.5 | CH3 | 1.63 s | ||
| -OCH3 | 60.7 | CH3 | 3.82 s | H-11 | 60.6 | CH3 | 3.28 s | H-11 |
| -OCH3 | 55.0 | CH3 | 3.35 s | H-12 | ||||
2.2. Antibacterial Effects of Licorice Phenolics on VRE
| Compounds | Number of -OH Groups | Number of Prenyl Groups | MIC (10−5 M) | ||
|---|---|---|---|---|---|
| Enterococcus faecium FN-1 | Enterococcus faecalis NCTC12201 | ||||
| Isoflavones | |||||
| 7-O-Methylluteone (3) | 3 | 1 | 8.7 | 8.7 | |
| Isoangustone A (6) | 4 | 2 | 3.8 | 3.8 | |
| Glycyrrhisofavone (11) | 4 | 1 | 9.0 | 9.0 | |
| Glycyrrhiza-isoflavone B (15) | 2 | 0 | 35 | 35 | |
| 8-(γ,γ-Dimethylallyl)-wighteone (17) | 3 | 2 | 1.9 | 3.8 | |
| Glicoricone (22) a | 3 | 1 | >35 | >35 | |
| 6,8-Diprenylorobol (25) a | 4 | 2 | 30 | 30 | |
| Isoflavans | |||||
| Licoricidin (2) | 3 | 2 | 1.9 | 1.9 | |
| Glyasperin C (13) | 3 | 1 | 4.5 | 4.5 | |
| Isoflavanones | |||||
| Glyasperin J trimethyl ether (4) | 0 | 1 | 14 | 14 | |
| 3'-(γ,γ-Dimethylallyl)-kievitone (5) | 4 | 2 | 3.8 | 3.8 | |
| Glyasperin J (7) | 3 | 1 | 7.5 | 7.5 | |
| 3-Arylcoumarins | |||||
| Licopyranocoumarin (12) | 2 | 0 | >33 | 33 | |
| Glycyrin (20) a | 2 | 1 | 4.2 | 8.4 | |
| Glycycoumarin (21) a | 3 | 1 | 4.3 | 4.3 | |
| Coumestans | |||||
| Glycyrol (18) a | 2 | 1 | 35 | >35 | |
| Pterocarpans | |||||
| Demethylhomopterocarpan (10) | 1 | 0 | 12 | 12 | |
| 2-Aryl-3-methylbenzofurans | |||||
| Gancaonin I (1) a | 2 | 1 | 4.5 | 4.5 | |
| 4'-O-Methylglycybenfuran (8) | 2 | 1 | 8.7 | 8.7 | |
| Noeglycybenzofuran (14) | 3 | 1 | 4.5 | 4.5 | |
| Glycybenzofuran (16) | 3 | 1 | 18 | 18 | |
| Benzylphenylketones | |||||
| Licoriphenone (9) | 3 | 1 | >34 | 34 | |
| Standard antibacterial agents | |||||
| Vancomycin a | >6.9 | >6.9 | |||
| Linezolid a | 0.74 | 0.74 | |||
| EtOAc extract from Tohoku licorice | 16 µg/mL | 32 µg/mL | |||
2.3. HPLC Analyses of Anti-VRE Phenolics for the Evaluation of EtOAc Extract from G. uralensis as a Source of Antibacterial Agent

| Compound | Content (% w/w) a |
|---|---|
| Glycyrol (18) | 0.54 ± 0.036 |
| Gancaonin I (1) | 0.49 ± 0.025 |
| Isoangustone A (6) | 0.34 ± 0.031 |
| Glycyrin (20) | 0.26 ± 0.015 |
| Glycycoumarin (21) | 0.24 ± 0.010 |
| Glicoricone (22) | 0.18 ± 0.023 |
| 6,8-Diprenylorobol (25) | 0.094 ± 0.013 |
| Licoriphenone (9) | 0.082 ± 0.017 |
3. Experimental Section
3.1. General Information
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectral Data
3.5. Methylation of Compounds A and B, and Glycybenzofuran
3.6. Antibacterial Assay
3.7. Simultaneous HPLC Analysis of Phenolic Constituents in the EtOAc Extract of Licorice
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Horiuchi, K.; Shiota, S.; Hatano, T.; Yoshida, T.; Kuroda, T.; Tsuchiya, T. Antimicrobial activity of oleanolic acid from Salvia officinalis and related compounds on vancomycin-resistant Enterococci (VRE). Biol. Pharm. Bull. 2007, 30, 1147–1149. [Google Scholar] [CrossRef]
- Orsi, G.B.; Ciorba, V. Vancomycin resistant Enterococci healthcare associated infections. Ann. Ig. 2013, 25, 485–492. [Google Scholar]
- Rice, L.B. Emergence of vancomycin-resistant Enterococci. Emerg. Infect. Dis. 2001, 7, 183–187. [Google Scholar] [CrossRef]
- McNeil, S.A.; Clark, N.M.; Chandrasekar, P.H.; Kauffman, C.A. Successful treatment of vancomycin-resistant Enterococcus faecium bacteremia with linezolid after failure of treatment with synercid (quinupristin/dalfopristin). Clin. Infect. Dis. 2000, 30, 403–404. [Google Scholar] [CrossRef]
- Isbrucker, R.A.; Burdock, G.A. Risk and safety assessment on the consumption of licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul. Toxicol. Pharmacol. 2006, 46, 167–192. [Google Scholar]
- Shen, X.-P.; Xiao, P.-G.; Liu, C.-X. Research and application of Radix Glycyrrhizae. Asian J. Pharmacodyn. Pharmacokinet. 2007, 7, 181–200. [Google Scholar]
- Asl, M.N.; Hosseinzadeh, H. Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother. Res. 2008, 22, 709–724. [Google Scholar] [CrossRef]
- Messier, C.; Epifano, F.; Genovese, S.; Grenier, D. Licorice and its potential beneficial effects in common oro-dental diseases. Oral Dis. 2012, 18, 32–39. [Google Scholar] [CrossRef]
- Villinski, J.R.; Bergeron, C.; Cannistra, J.C.; Gloer, J.B.; Coleman, C.M.; Ferreira, D.; Gafner, S. Pyrano-isoflavans from Glycyrrhiza uralensis with antibacterial activity against Streptococcus mutans and Porphyromonas gingivalis. J. Nat. Prod. 2014, 77, 521–526. [Google Scholar] [CrossRef]
- Gafner, S.; Bergeron, C.; Villinski, J.R.; Godejohann, M.; Kessler, P.; Cardellina, J.H.; Grenier, D. Isoflavonoids and coumarins from Glycyrrhiza uralensis: Antibacterial activity against oral pathogens and conversion of isoflavans into isoflavan-quinones during purification. J. Nat. Prod. 2011, 74, 2514–2519. [Google Scholar] [CrossRef]
- He, J.; Chen, L.; Heber, D.; Shi, W.; Lu, Q.Y. Antibacterial compounds from Glycyrrhiza uralensis. J. Nat. Prod. 2006, 69, 121–124. [Google Scholar] [CrossRef]
- Fukai, T.; Marumo, A.; Kaitou, K.; Kanda, T.; Terada, S.; Nomura, T. Anti-Helicobacter pylori flavonoids from licorice extract. Life Sci. 2002, 71, 1449–1463. [Google Scholar] [CrossRef]
- Irani, M.; Sarmadi, M.; Bernard, F. Leaves antimicrobial activity of Glycyrrhiza glabra. Iran. J. Pharm. Res. 2010, 9, 425–428. [Google Scholar]
- Badr, A.E.; Omar, N.; Badria, F.A.A. Laboratory evaluation of the antibacterial and cytotoxic effect of liquorice when used as root canal medicament. Int. Endod. J. 2011, 44, 51–58. [Google Scholar] [CrossRef]
- Fukai, T.; Oku, Y.; Hano, Y.; Terada, S. Antimicrobial activities of hydrophobic 2-arylbenzofurans and an isoflavone against vancomycin-resistant Enterococci and methicillin-resistant Staphylococcus aureus. Planta Med. 2004, 70, 685–687. [Google Scholar] [CrossRef]
- Hatano, T.; Shintani, Y.; Aga, Y.; Shiota, S.; Tsuchiya, T.; Yoshida, T. Phenolic constituents of licorice. VIII. Structures of glicophenone and glicoisoflavanone, and effects of licorice phenolics on methicillin-resistant Staphylococcus aureus. Chem. Pharm. Bull. 2000, 48, 1286–1292. [Google Scholar] [CrossRef]
- Eerdunbayaer; Orabi, M.A.; Aoyama, H.; Kuroda, T.; Hatano, T. Structures of two new flavonoids and effects of licorice phenolics on vancomycin-resistant Enterococcus species. Molecules 2014, 19, 3883–3897. [Google Scholar] [CrossRef]
- Fukai, T.; Toyono, M.; Nomura, T. On the structure of licoricidin. Heterocycles 1988, 27, 2309–2313. [Google Scholar] [CrossRef]
- Tahara, S.; Ingham, J.L.; Mizutani, J. Metabolites of 7-O-methylluteone from Botrytis cinerea. Nippon Nogeikagaku Kagaku Kaishi 1989, 63, 999–1007. [Google Scholar] [CrossRef]
- Zeng, L.; Fukai, T.; Nomura, T.; Zhang, R.Y.; Lou, Z.C.; Fukai, T.; Nomura, T. Five new isoprenoid-substituted flavonoids, glyasperins F, G, H, I, and J from the roots of Glycyrrhiza aspera. Heterocycles 1992, 34, 1813–1828. [Google Scholar] [CrossRef]
- O’Neill, M.J.; Adesanya, S.A.; Roberts, M.F.; Inez, R.P. Inducible isoflavonoids from the lima bean, Phaseolus lunatus. Phytochemistry 1986, 25, 1315–1322. [Google Scholar] [CrossRef]
- Sil Lee, Y.; Ha Kim, S.; Kyu Kim, J.; Shin, H.K.; Kang, Y.H.; Park, Y.; Lim, S.S. Rapid identification and preparative isolation of antioxidant components in licorice. J. Sept. Sci. 2010, 33, 664–671. [Google Scholar] [CrossRef]
- Kiuchi, F.; Chen, X.; Tsuda, Y. Four new phenolic constituents from licorice (root of Glycyrrhiza sp.). Heterocycles 1990, 31, 629–636. [Google Scholar] [CrossRef]
- Sasaki, H.; Kashiwada, Y.; Shibatav, H.; Takaishi, Y. Prenylated flavonoids from the roots of Desmodium caudatum and evaluation of their antifungal activity. Planta Med. 2012, 78, 1851–1856. [Google Scholar] [CrossRef]
- Hatano, T.; Kagawa, H.; Yasuhara, T.; Okuda, T. Two new flavonoids and other constituents in licorice root: Their relative astringency and radical scavenging effects. Chem. Pharm. Bull. 1988, 36, 2090–2097. [Google Scholar] [CrossRef]
- Hatano, T.; Yasuhara, T.; Fukuda, T.; Noro, T.; Okuda, T. Phenolic constituents of licorice. II. Structures of licopyranocoumarin, licoarylcoumarin and glisoflavone, and glisoflavone, and inhibitory effects of licorice phenolics on xanthine oxidase. Chem. Pharm. Bull. 1989, 37, 3005–3009. [Google Scholar] [CrossRef]
- Kwon, H.J.; Kim, H.H.; Ryu, Y.B.; Kim, J.H.; Jeong, H.J.; Lee, S.W.; Lee, W.S. In vitro anti-rotavirus activity of polyphenol compounds isolated from the roots of Glycyrrhiza uralensis. Bioorg. Med. Chem. 2010, 18, 7668–7674. [Google Scholar]
- Hatano, T.; Takagi, M.; Ito, H.; Yoshida, T. Phenolic constituents of liquorice. VII. A new chalcone with a potent radical scavenging activity and accompanying phenolics from liquorice. Chem. Pharm. Bull. 1997, 45, 1485–1492. [Google Scholar] [CrossRef]
- Li, S.; Li, W.; Wang, Y.; Asada, Y.; Koike, K. Prenyl flavonoids from Glycyrrhiza uralensis and their protein tyrosine phosphatase-1B inhibitory activities. Bioorg. Med. Chem. Lett. 2010, 20, 5398–5401. [Google Scholar] [CrossRef]
- Singhal, A.K.; Sharma, R.P.; Thyagarajan, G.; Herz, W.; Govindan, S.V. New prenylated isoflavones and a prenylated dihydroflavonol from Millettia pachycarpa. Phytochemistry 1980, 9, 929–934. [Google Scholar]
- Zhang, Q.; Ye, M. Chemical analysis of the Chinese herbal medicine Gan-Cao (licorice). J. Chromatogr. A 2009, 1216, 1954–1969. [Google Scholar] [CrossRef]
- Chen, X.J.; Zhao, J.; Meng, Q.; Li, S.P.; Wang, Y.T. Simultaneous determination of five flavonoids in licorice using pressurized liquid extraction and capillary electrochromatography coupled with peak suppression diode array detection. J. Chromatogr. A 2009, 1216, 7329–7335. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Zhang, X.; Dai, W.; Li, H.; Hu, L.; Zhang, W. Qualitative and quantitative analysis of traditional Chinese medicine Niu Huang Jie Du Pill using ultra performance liquid chromatography coupled with tunable UV detector and rapid resolution liquid chromatography coupled with time-of-flight tandem mass spectrometry. J. Pharm. Biomed. Anal. 2010, 51, 565–571. [Google Scholar] [CrossRef]
- Seo, C.S.; Lee, J.A.; Jung, D.; Lee, H.Y.; Lee, J.K.; Ha, H.; Shin, H.K. Simultaneous determination of liquiritin, hesperidin, and glycyrrhizin by HPLC-photodiode array detection and the anti-inflammatory effect of Pyungwi-san. Arch. Pharm. Res. 2011, 34, 203–210. [Google Scholar] [CrossRef]
- Wen, J.; Qiao, Y.; Yang, J.; Liu, X.; Song, Y.; Liu, Z.; Li, F. UPLC-MS/MS determination of paeoniflorin, naringin, naringenin and glycyrrhetinic acid in rat plasma and its application to a pharmacokinetic study after oral administration of SiNiSan decoction. J. Pharm. Biomed. Anal. 2012, 66, 271–277. [Google Scholar]
- Zhou, S.; Cao, J.; Qiu, F.; Kong, W.; Yang, S.; Yang, M. Simultaneous determination of five bioactive components in radix glycyrrhizae by pressurised liquid extraction combined with UPLC-PDA and UPLC/ESI-QTOF-MS confirmation. Phytochem. Anal. 2013, 24, 527–533. [Google Scholar] [CrossRef]
- Sample Availability: Samples of all of the compounds are unavailable.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Eerdunbayaer; Orabi, M.A.A.; Aoyama, H.; Kuroda, T.; Hatano, T. Structures of New Phenolics Isolated from Licorice, and the Effectiveness of Licorice Phenolics on Vancomycin-Resistant Enterococci. Molecules 2014, 19, 13027-13041. https://doi.org/10.3390/molecules190913027
Eerdunbayaer, Orabi MAA, Aoyama H, Kuroda T, Hatano T. Structures of New Phenolics Isolated from Licorice, and the Effectiveness of Licorice Phenolics on Vancomycin-Resistant Enterococci. Molecules. 2014; 19(9):13027-13041. https://doi.org/10.3390/molecules190913027
Chicago/Turabian StyleEerdunbayaer, Mohamed A. A. Orabi, Hiroe Aoyama, Teruo Kuroda, and Tsutomu Hatano. 2014. "Structures of New Phenolics Isolated from Licorice, and the Effectiveness of Licorice Phenolics on Vancomycin-Resistant Enterococci" Molecules 19, no. 9: 13027-13041. https://doi.org/10.3390/molecules190913027