Abstract
The purpose of this work was to synthesize and characterize the thiatetraaza macrocycle 1-thia-4,7,10,13-tetraazacyclopentadecane ([15]aneN4S). Its acid-base behaviour was studied by potentiometry at 25 °C and ionic strength 0.10 M in KNO3. The protonation sequence of this ligand was investigated by 1H-NMR titration that also allowed the determination of protonation constants in D2O. Binding studies of [15]aneN4S with Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, Hg2+ and Pb2+ metal ions were further performed under the same experimental conditions. The results demonstrated that this compound has a higher selectivity and thermodynamic stability for Hg2+ and Cu2+, followed by Ni2+. The UV-visible-near IR spectroscopies and magnetic moment data for the Co(II) and Ni(II) complexes indicated a tetragonal distorted coordination geometry for both metal centres. The value of magnetic moment and the X-band EPR spectra of the Cu(II) complex are consistent with a distorted square pyramidal geometry.
1. Introduction
Exposure to toxic metals is associated with the potential development of numerous health effects, including different types of cancer [1,2]. Humans are exposed to metals and their compounds through environmental and occupational scenarios and also by food consumption, being the adverse effects dependent on dose, period of exposure and metal bioavailability [3]. In fact, metals can disturb the normal function of several organ systems and generate different toxicological effects through their excess, lack or imbalance [4]. The mainstay treatment against metal toxicity is chelation therapy [5,6], where appropriate chelators are used to remove metals from the organism [7], and thereby reduce toxicity [8,9]. However, despite the available chelators, the search for new effective chelating compounds is still needed, since the ones currently used in clinic present a number of toxic side effects, low metal specificity and controversial efficacy [5,9,10].
Previously, we have explored the possible use of macrocyclic compounds for medical applications, namely in chelation therapy [7,11]. These compounds may exhibit important properties such as less toxicity, high kinetic and thermodynamic stabilities [12], rendering them very promising agents in this context. Macrocycles are also advantageous in terms of selectivity, since they have more rigid structures and can thus inflict specific coordination geometry to the metal ion, while open chain chelators can adapt more easily to the geometric requirements of the metal centre [7,13].
A large amount of data has been published on macrocyclic ligands containing only nitrogen, only sulphur or both as donor atoms, namely tetraaza- [13], thiadiaza- [14], thiatriaza- [15], dithiadiaza- [16,17,18,19,20], dithiatriaza- [20] or pentathia- macrocycles [15]. However, there is scarce information on thiatetraaza compounds. In fact, only one thiatetraaza macrocycle, 1-thia-4,7,11,14-tetraazacyclohexadecane, was studied [21]. In the present work, we investigate a 15-membered thiatetraaza macrocyclic compound, 1-thia-4,7,10,13-tetraazacyclopentadecane ([15]aneN4S, Figure 1). In this context, we address the synthesis and characterization of [15]aneN4S and assess its potential as a chelating agent. To accomplish this aim, the acid-base behaviour of this macrocycle was studied and its ability to specifically coordinate with several divalent ions was evaluated.
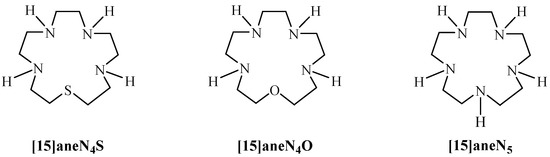
Figure 1.
[15]aneN4S, [15]aneN4O and [15]aneN5 macrocycles.
2. Results and Discussion
2.1. Synthesis and Characterization
The macrocycle 1-thia-4,7,10,13-tetraazacyclopentadecane ([15]aneN4S) was prepared according to the reactions depicted in Scheme 1. The first step involved the synthesis of the precursor diamide, 1-thia-4,7,10,13-tetraazacyclopentadecane-3,14-dione (dioxo-[15]aneN4S) by reaction of the dimethyl ester of thiodiglycolic acid, prepared according to a reported procedure [22], with triethylenetetramine in dry methanol at 40 °C, under N2 for nine days. The general procedure was similar to those reported in the literature by Tabushi et al. [23] and by Steenland et al. [24], who obtained this macrocycle with 15% to 17% yield. However, some modifications were introduced in the present work, such as controlled reaction temperature, dried solvent in a larger volume and nitrogen atmosphere, which led to the highly improved yield of 74% on dioxo-[15]aneN4S synthesis. The reduction of this cyclic diamide with borane, in refluxing dry THF under nitrogen for eight hours, afforded the [15]aneN4S. After purification by chromatography, the trihydrocloride salt was obtained by addition of 37% HCl until pH ≈ 2. A yield of 68% was obtained, which is within the range reported for similar compounds (60%–80%) [23].
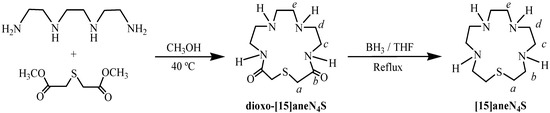
Scheme 1.
Schematic synthesis of [15]aneN4S.
The structure of the compound was confirmed by 1H and 13C-NMR spectroscopy (Table S1 and Figures S1–S2 in Supplementary Materials). NMR data for [15]aneN4S are consistent with a C2v symmetric structure in solution, according to the number of signals observed in the 1H and 13C-NMR spectra. Five resolved proton and carbon resonances were observed and a combination of the two-dimensional spectra HMBC, HMQC and COSY (Figures S3–S6 in Supplementary Materials), were used for the assignments. The singlet observed in the 1H-NMR spectrum at δ 3.34 was assigned to He. Cd at 43.65 ppm was identified through 3J correlation to He, which allowed the assignment of the coupled triplets Hd and Hc at 3.44 and 3.57 ppm, respectively. 3J C-H coupling of the more shielded CH2 group to its symmetric counterpart, bridged by the sulphur atom, allowed the assignment of the resonances at δ 29.55 and 3.17 to Ca and Ha, respectively. The HMBC spectrum also exhibits three-bond CH correlation of Hb, at δ 3.50, to Ca and two-bond coupling to Cd. The two pairs of triplets in the 1H-NMR spectrum display a nearly first-order A2X2 coupling pattern with some distortion. The resulting “roof effect” with a mirror image relationship is often observed for CH2-CH2 groups in an unsymmetrical environment with some strain. Leaning is more pronounced for the Hc/Hd pair which is closer in chemical shift (Δδ/ΔJ < 10), leading to slightly different coupling constants. For the Ha/Hb spin system, second order effects are negligible due to the higher chemical shift difference (Δδ/ΔJ > 20).
2.2. Acid-Base Behaviour
The acid-base behaviour of [15]aneN4S was studied by potentiometry in water at 25 °C and ionic strength 0.10 M in KNO3. This compound was also studied by 1H-NMR spectroscopy. The determined protonation constants are collected in Table 1 together with the values of the related [15]aneN4O and [15]aneN5 compounds (Figure 1) for comparison. The ligand has four basic centres. However only three constants could be accurately determined by potentiometry and the fourth one was obtained by 1H-NMR. The compound exhibits high and fairly high values respectively for the first two protonation constants corresponding to the protonation of nitrogen atoms in opposite positions, minimizing the electrostatic repulsion between positive charges of the ammonium groups formed. The third and fourth constants are much lower due to the stronger electrostatic repulsions as they correspond to protonation of nitrogen atoms at short distances from already protonated ones, and to the limited motion allowed in the ring backbone.
The overall basicity and all the stepwise protonation constants of [15]aneN4S (Table 1) are slightly lower than those of [15]aneN4O but even lower than those of [15]aneN5, as expected. Indeed sulphur and oxygen atoms have different electronic characteristics with respect to amino groups. Evidently, ethereal S and O cannot bind acidic protons, if not under particular conditions; they have different inductive effects on the adjacent aliphatic chains with respect to nitrogen and finally, they have a much lower tendency to form hydrogen bonds and are less solvated in aqueous solution than amino groups [25]. Considering these features, the introduction of S or O in the macrocyclic backbone may deeply influence the basicity of these molecules. The lower stabilization via intramolecular hydrogen bonds formation brought about by S and O in the mono and/or polyprotonated species gives rise to a consequent basicity decrease with respect to the corresponding unsubstituted polyazacycloalkanes [25].

Table 1.
Stepwise protonation constants (log 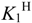 ) of [15]aneN4S and similar compounds.
) of [15]aneN4S and similar compounds.
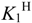 ) of [15]aneN4S and similar compounds.
) of [15]aneN4S and similar compounds.
| Reaction equilibrium | [15]aneN4S a,b | [15]aneN4O c | [15]aneN5 d |
|---|---|---|---|
L + H+  HL+ HL+ | 9.51(1) | 9.66 | 10.01 |
HL+ + H+  H2L2+ H2L2+ | 8.56(2) | 8.77 | 9.28 |
H2L2+ + H+  H3L3+ H3L3+ | 4.47(4) | 5.30 | 5.87 |
H3L3+ + H+  H4L4+ H4L4+ | 0.8(2) e | 1.2 | 1.84 |
L + 4 H+  H4L4+ H4L4+ | 23.34 | 24.93 | 27.00 |
a Values in parentheses are standard deviations on the last significant figure. b Present work T = 25.0 °C; I = 0.10 M in KNO3. c T = 25.0 °C; I = 0.10 M in KNO3; ref. [26]. d T = 25.0 °C; I = 0.10 M in KCl; ref. [27]. e Determined in this work by 1H-NMR spectroscopy, using the calculated value of pKD4 and the equation pKD = 0.11 + 1.10 × pKH; ref. [28].
1H-NMR spectroscopic titration of [15]aneN4S was carried out in order to understand its protonation sequence and to determine the lower protonation constant. The 1H-NMR spectrum of the compound at pD 1.72 (Figure S1) and the titration curves are shown in Figure 2a,b, respectively. In the 0.72 to 4.80 pD region, five resonances were observed in the 1H-NMR spectra. The Hc and Hb resonances overlap for pD values above 5.09 until 12.17. The same behaviour was observed for the He and Hd resonances between 6.40 and 9.42. The triplets at 3.57, 3.50, 3.44 and 3.17 ppm were assigned to protons Hc, Hb, Hd and Ha, respectively, and the singlet at 3.34 ppm corresponds to He. (Figure 2a).
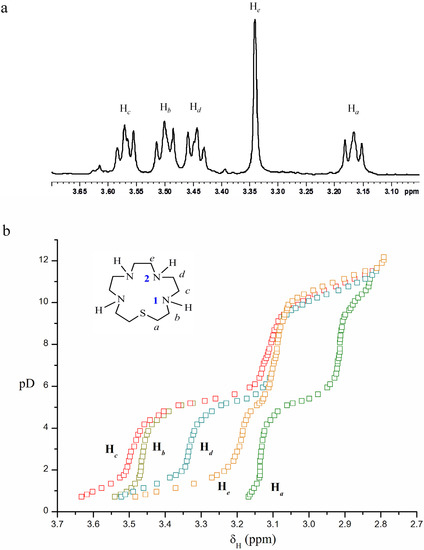
Figure 2.
(a) 1H-NMR spectrum of [15]aneN4S in D2O, pD 1.72; (b) 1H-NMR titration curves for [15]aneN4S, chemical shift δH (ppm) in function of pD.
The 1H-NMR titration curves (Figure 2b) show the effect of successive protonation of the basic centres of the molecule on the chemical shifts of the protons. The first two equivalents of acid added to the basic form of the ligand (pD 11.65–9.31) affected the four N–atoms of the compound, as all the resonances shift downfield in this pD region. The first equivalent protonates mainly N2–centres, since the He resonance undergoes a larger downfield shift, followed by Hd, Hc, Hb and Ha, meaning a small degree of protonation of N1–atoms. The second equivalent of acid protonates mainly N1–centres, as Ha, Hb and Hc resonances move downfield, although simultaneous protonation of N2–centres is also evident, since Hd and He resonances show downfield shifts as well.
The third equivalent of acid (pD 5.94–3.69) continues protonating the N1–centres, since Ha, Hb and Hc signals show a significant shift downfield. However, a smaller downfield shift of Hd and He resonances is also observed, revealing some degree of protonation on N2–centres still occurring. Addition of one more equivalent of acid (pD < 2.15) causes protonation on N2 centres at a larger extent, according to the larger downfield shift observed for the He and Hd resonances. The last protonation occurs in a centre at a very short distance from others already protonated, and the strong repulsions then aroused in the molecule, where the motion is limited by the skeleton of the ring, renders the protonation difficult.
The 1H-NMR titration also allowed the determination of the protonation constants in D2O for [15]aneN4S: pKD1 = 10.90(7), pKD2 = 9.4(l), pKD3 = 5.3(1) and pKD4 = 1.0(1). These values are in agreement with the equation for the correlation between the protonation constants determined in H2O and in D2O for similar compounds: pKD = 0.11 + 1.10 × pKH [28].
2.3. Thermodynamic Stability of Metal Complexes
The stability constants of [15]aneN4S with Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+, Cd2+, Hg2+ and Pb2+, determined by potentiometric titrations at 25 °C and ionic strength 0.1 M in KNO3, are collected in Table 2 together with the corresponding constants of complexes of the related macrocycles [15]aneN4O [26,29] and [15]aneN5 [30,31,32,33] taken from the literature for comparison. Only mononuclear ML, M(HL) and ML(OH) complexes were found. In the case of Hg2+ and Mn2+, the protonated species were not formed under our experimental conditions. For Co2+ and Ni2+, the determination of the stability constants for hydroxocomplexes was precluded since precipitation occurred (Table 2). In all cases the proposed model was accepted by the HYPERQUAD program [34] using all data points from all titration curves, with good statistical parameters.

Table 2.
Stepwise stability constants (log units) of the complexes of [15]aneN4S, [15]aneN4O and [15]aneN5 with different metal ions.
| Reaction equilibrium | [15]aneN4S a,b | [15]aneN4O c | [15]aneN5 d |
|---|---|---|---|
Mn2+ + L  MnL2+ MnL2+ | 6.65(2) | 8.53 c | 10.85 e |
MnL2+ + H+  MnHL3+ MnHL3+ | - | - | 5.04 e |
MnL(OH)+ + H+  MnL2+ MnL2+ | 9.68(4) | - | 11.22 e |
Fe2+ + L  FeL2+ FeL2+ | 10.08(1) | 10.34 c | - |
FeL2+ + H+  FeHL3+ FeHL3+ | 4.83(6) | - | - |
FeL(OH)+ + H+  FeL2+ FeL2+ | 8.25(7) | pp. | - |
Co2+ + L  CoL2+ CoL2+ | 13.62(6) | 12.72 c | 16.76 f |
CoL2+ + H+  CoHL3+ CoHL3+ | 5.20(7) | - | - |
Ni2+ + L  NiL2+ NiL2+ | 17.95(5) | 14.76 c | 18.1 g |
NiL2+ + H+  NiHL3+ NiHL3+ | 4.00(7) | - | - |
NiL(OH)+ + H+  NiL2+ NiL2+ | - | 8.38 c | - |
Cu2+ + L  CuL2+ CuL2+ | 22.31(2) | 20.34 c | 28.0 h |
CuL2+ + H+  CuHL3+ CuHL3+ | 2.49(5) | - | - |
CuL(OH)+ + H+  CuL2+ CuL2+ | 9.8(1) | 10.4 c | - |
Zn2+ + L  ZnL2+ ZnL2+ | 13.472(8) | 13.21 c | 19.1 i |
ZnL2+ + H+  ZnHL3+ ZnHL3+ | 4.06(3) | - | 3.1 i |
ZnL(OH)+ + H+  ZnL2+ ZnL2+ | 7.16(4) | - | - |
Cd2+ + L  CdL2+ CdL2+ | 13.61(2) | 13.41 d | 19.2 i |
CdL2+ + H+  CdHL3+ CdHL3+ | 3.97(4) | - | 3.4 i |
CdL(OH)+ + H+  CdL2+ CdL2+ | 9.25(7) | - | - |
Hg2+ + L  HgL2+ HgL2+ | 23.74(5) | - | 28.5 j |
HgL(OH)+ + H+  HgL2+ HgL2+ | 10.3(1) | - | - |
Pb2+ + L  PbL2+ PbL2+ | 12.44(2) | 12.28 d | 17.3 i |
PbL2+ + H+  PbHL3+ PbHL3+ | 4.44(4) | - | 3.8 i |
PbL(OH)+ + H+  PbL2+ PbL2+ | 7.76(7) | - | - |
a Values in parentheses are standard deviations on the last significant figure. b Present work T = 25.0 °C; I = 0.10 M in KNO3. c T = 25.0 °C; I = 0.10 M in KNO3; ref. [26]. d T = 25.0 °C; I = 0.10 M in NaNO3; ref. [29]. e T= 25.0 °C; I = 0.10 M in NaClO4; ref. [30]. f T= 35.0 °C; I = 0.20 M in NaClO4; ref. [31]. g T = 35.0 °C; ref: [32]. h T = 25.0 °C; I = 0.2 M; polarographic method; ref. [35]. i T = 25.0 °C; I = 0.2 M; ref. [33]. j T = 25.0 °C; I = 0.2 M; polarographic method; ref. [33].
Potentiometric studies with [15]aneN4S and Ca(NO3)2 showed that both titration curves (protonation and complexation at a molar ratio 1:1) overlapped, suggesting that Ca2+ does not bind to the ligand. This hypothesis was supported by NMR data, as no changes were observed in the 1H-NMR spectrum of the ligand in presence of Ca2+.
Direct determinations of the stability constants of Cu[15]aneN4S2+ and Hg[15]aneN4S2+ were not possible as ML2+ was almost completely or completely formed in the beginning of the titration (pH ≈ 2.2) (Figures S9 and S11 in Supplementary Materials, respectively). Consequently, reliable values for the constants were obtained through a competition with a reference ligand, for which the protonation and stability constants are accurately known [36]. Among the various ligands tried, H4EDTA was chosen as the best reference ligand. Figure 3 shows the species distribution diagram of the ligand-ligand competition reaction between [15]aneN4S, Hg2+ and ethylenediaminetetraacetic acid (EDTA), obtained with the HySS program [37]. In the Supplementary Materials the species distribution curves for Cu(II) competition titration are shown (Figure S10).
[15]aneN4S is very selective, exhibiting a very high stability constant for Cu2+ (log KML = 22.31), and a fairly high value for Ni2+ (log KML = 17.95). On the other hand, the stability constants for the complexes of the remaining first-row transition metal ions decrease sharply. The Cd2+ and Pb2+ complexes have low stabilities. Hg2+ has the highest stability constant (log KML = 23.74) of all the metal ions studied.
In spite of the slightly lower overall basicity of [15]aneN4S, this ligand forms ML2+ complexes more stable than those of the oxatetraaza macrocycle [15]aneN4O, except in the case of the Mn2+and Fe2+complexes (variations of 1.88 and 0.26 log units, respectively). [15]aneN4S is particularly advantageous over [15]aneN4O for Ni2+ and Cu2+complexes, being the stability constants much higher (an increase of 3.19 and 1.97 log units, respectively, was found). Comparing with the pentaaza macrocycle [15]aneN5, the stability constants (log values) of the ML2+ metal complexes of [15]aneN4S are smaller except in the case of the Ni2+ complexes which have almost the same value (Table 2).
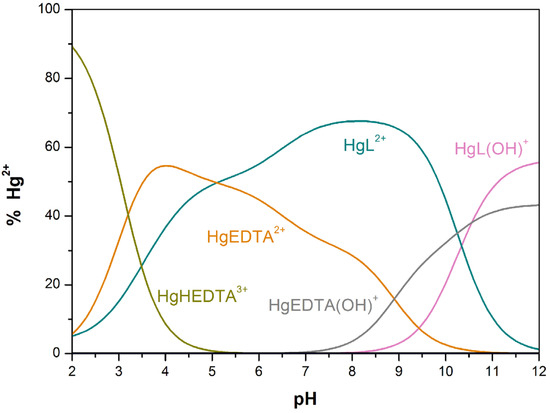
Figure 3.
Species distribution curves calculated for an aqueous solution containing [15]aneN4S (L), Hg2+ and EDTA at 0.75:1:1 molar ratio. Percentages are given relative to Hg2+ at an initial value of 1.73 × 10−3 M.
However, stability constants do not provide directly comparable basis for the measuring of total ion sequestering abilities of the ligands at physiological conditions (pH 7.4) and therefore they were used to calculate the pM values, defined as −log [M2+] (Table 3). The advantage of comparing pM values rather than stability constants is that the pM values reflect the influence of ligand basicity and metal chelate protonation.

Table 3.
pM values calculated for metal complexes of [15]aneN4S, [15]aneN4O and [15]aneN5 at pH 7.4 a.
| Metal ion | [15]aneN4S | [15]aneN4O | [15]aneN5 |
|---|---|---|---|
| Mn2+ | 5.02 | 5.31 | 6.38 |
| Fe2+ | 6.85 | 5.74 | - |
| Co2+ | 10.32 | 9.07 | 12.25 |
| Ni2+ | 14.65 | 11.15 | 13.59 |
| Cu2+ | 19.01 | 16.69 | 23.49 |
| Zn2+ | 10.61 | 9.56 | 14.59 |
| Cd2+ | 10.32 | 9.76 | 14.69 |
| Hg2+ | 20.44 | - | 23.99 |
| Pb2+ | 9.3 | 8.63 | 12.79 |
If the pM values of [15]aneN4S are compared with those of [15]aneN4O (Table 3) we conclude that, with the exception of Mn2+, all the values are higher, especially for nickel(II) and copper(II) complexes (differences, in log units, are 3.5 and 2.32, respectively). On the other hand, the comparison of pM values of the metal complexes of [15]aneN4S with [15]aneN5 (Table 3) revealed that the former complexes present lower values, except in the case of the Ni2+ complex, which has a higher value (difference, in log units, is 1.06). The selectivity and the high pM values for mercury(II) and copper(II) complexes of [15]aneN4S render this ligand of possible interest in chelation therapy.
2.4. Spectroscopic Studies
UV-visible-near IR spectroscopic studies of the Co(II), Ni(II) and Cu(II) complexes of [15]aneN4S in water solution were performed and the magnetic moments determined by the Evans method [38]. The results are collected in Table 4.
The electronic spectrum of the pink Co[15]aneN4S2+ exhibits two principal bands, at 1075 and 504 nm, and four shoulders (Table 4), consistent with a high-spin octahedral array tetragonally distorted around the Co(II) ion [39] with one water molecule or a metal counter-ion nitrate occupying a coordination site. The octahedral field splitting parameter 10Dq [39] of 11450 cm−1 is in the expected range for relatively weak ligands. The band at 504 nm is more intense than expected for forbidden transitions in Oh complexes, which can be explained by the loss of symmetry caused by distortion. The magnetic moment of 4.9 MB is within the range for high-spin six coordinate Co(II) complexes [38]. Although five coordinate Co(II) species would also have a similar value, the absence of a weak absorption in the visible region between 830 and 670 nm, characteristic of five coordinate Co(II) complexes [39,40,41],supports the proposed six coordination for this metal ion.

Table 4.
Spectroscopic UV-visible-near IR data and magnetic moments (µ) for the Co(II), Ni(II) and Cu(II) complexes of [15]aneN4S.
| Complex; (colour) | pH | UV-visible-near IR a λmax/nm (ε, M−1 cm−1) | µ (MB) |
|---|---|---|---|
| Co[15]aneN4S2+ (pink) | 6.99 | 1075 (4.2), 970 (sh., 4.9), 504 (67.6), 491 (sh., 99.0), 325 (sh., 2.61 × 103), 270 (sh., 2.61 × 103). | 4.9 |
| Ni[15]aneN4S2+ (yellow) | 6.98 | 1040 (2.12), 945 (23.0), 847 (sh., 27.2), 813 (sh., 26.0), 528 (19.3), 310 (sh., 1.49 × 103), 264 (1.91 × 104). | 3.1 |
| Cu[15]aneN4S2+ (purple) | 7.08 | 977 (23.8), 748 (sh., 16.3), 562 (45.6), 273 (1.17 × 103). | 1.8 |
a sh. = shoulder.
The electronic spectrum of the yellow Ni[15]aneN4S2+ shows three absorption bands of low intensity in the visible-near IR regions and a charge transfer band at 264 nm (Table 4).
Taking into account the work of Busch et al. [42], we assigned the two bands in the visible-near IR regions to transitions 3B1g → 3B2g, (directly related to 10Dqxy) and 3B1g → 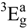 (equal to the difference between 10Dqxy and 35/4Dt). The octahedral field splitting parameter 10Dq and the values of the equatorial (Dqxy) and axial (Dqz) ligand field were calculated according to those assignments: 10Dq = 18975 cm−1, Dqxy = 1898 cm−1 and Dqz = 219 cm−1. These values, together with the ratio ν1/ν2 of 1.79 (ν1 and ν2 being the near IR and the visible absorption bands, respectively) and the magnetic moment of 3.1 MB, are characteristic of six coordinate nickel(II) environments, indicating a tetragonal (D4h) distorted octahedral geometry for this complex [43].
(equal to the difference between 10Dqxy and 35/4Dt). The octahedral field splitting parameter 10Dq and the values of the equatorial (Dqxy) and axial (Dqz) ligand field were calculated according to those assignments: 10Dq = 18975 cm−1, Dqxy = 1898 cm−1 and Dqz = 219 cm−1. These values, together with the ratio ν1/ν2 of 1.79 (ν1 and ν2 being the near IR and the visible absorption bands, respectively) and the magnetic moment of 3.1 MB, are characteristic of six coordinate nickel(II) environments, indicating a tetragonal (D4h) distorted octahedral geometry for this complex [43].
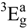 (equal to the difference between 10Dqxy and 35/4Dt). The octahedral field splitting parameter 10Dq and the values of the equatorial (Dqxy) and axial (Dqz) ligand field were calculated according to those assignments: 10Dq = 18975 cm−1, Dqxy = 1898 cm−1 and Dqz = 219 cm−1. These values, together with the ratio ν1/ν2 of 1.79 (ν1 and ν2 being the near IR and the visible absorption bands, respectively) and the magnetic moment of 3.1 MB, are characteristic of six coordinate nickel(II) environments, indicating a tetragonal (D4h) distorted octahedral geometry for this complex [43].
(equal to the difference between 10Dqxy and 35/4Dt). The octahedral field splitting parameter 10Dq and the values of the equatorial (Dqxy) and axial (Dqz) ligand field were calculated according to those assignments: 10Dq = 18975 cm−1, Dqxy = 1898 cm−1 and Dqz = 219 cm−1. These values, together with the ratio ν1/ν2 of 1.79 (ν1 and ν2 being the near IR and the visible absorption bands, respectively) and the magnetic moment of 3.1 MB, are characteristic of six coordinate nickel(II) environments, indicating a tetragonal (D4h) distorted octahedral geometry for this complex [43].The purple Cu[15]aneN4S2+ complex exhibits a broad band in the visible region with the maximum centred at 565 nm assigned to the copper d-d transition, an intense band in the UV region and a small broad band in the near IR region (Table 4).
The X-band EPR spectra of Cu[15]aneN4S2+ at the 1:1 ratio in water solution are shown in Figure 4 at three pH values. The spectra are very similar and suggest the presence of only one species. Spectroscopic visible data (λmax), the hyperfine coupling constants Ai and g values, determined by the simulation of the spectra [44] (Figure 4) are compiled in Table 5 together with those of the related complex Cu[15]aneN4O2+, from the literature [45].
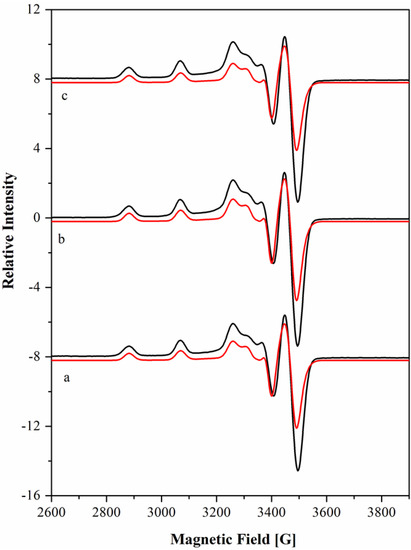
Figure 4.
EPR X-band spectra of Cu(II) complex of [15]aneN4S in 1:1 ratio at pH 4.10 (a), 7.40 (b) and 9.00 (c) in 1.0 M of NaClO4. The spectra were recorded at 104 K, microwave power of 2.0 mW and modulation amplitude of 1.0 mT. The frequency (ν) was of 9.67 GHz. The simulated spectra (red lines) are below of the experimental ones (black lines).

Table 5.
Spectroscopic X-band EPR data for the Cu(II) complexes of [15]aneN4S a and the related ligand [15]aneN4O.
| Complex | pH | Visible band λmax/nm (εmolar, M−1 cm−1) | EPR parameters (Ai × 104 cm−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| gx | gy | gz | Ax | Ay | Az | ref. | |||
| 9.00 | 2.043 | 2.046 | 2.186 | 30.6 | 28.7 | 192.5 | - | ||
| Cu[15]aneN4S2+ | 7.40 | 565 (46) | 2.042 | 2.045 | 2.182 | 30.3 | 28.0 | 191.1 | - |
| 4.10 | 2.041 | 2.042 | 2.185 | 30.4 | 28.0 | 191.3 | - | ||
| Cu[15]aneN4O2+ | 7.41 | 580 (141) | 2.042 | 2.046 | 2.192 | 30.0 | 27.9 | 199.4 | [45] |
a This work.
The parameters obtained for Cu[15]aneN4S2+ are characteristic of mononuclear copper(II) complexes in rhombic geometry with elongation of the axial bonds and a dx2−y2 ground state, consistent with distorted octahedral (with the sixth coordination site occupied by one water molecule or a metal counter-ion nitrate) or square pyramidal environment. Trigonal bipyramidal or tetragonal geometries involving compression of axial bonds should be excluded [46,47,48].
The hyperfine constants Ai and g values are related to the electronic transitions by the factors derived from the ligand field theory [49,50,51]: the gz values increase and the Az values decrease when the planar ligand field becomes weaker or the axial ligand field becomes stronger, and this occurs with the simultaneous red-shift of the d-d absorption bands in the electronic spectra. This sequence parallels the degree of distortion from square planar to square pyramidal (C4v) and then to octahedral (Oh) or tetragonal (D4h) geometries [47].
The EPR parameters and the electronic spectra data for the complex Cu[15]aneN4S2+ are similar to those obtained for Cu[15]aneN4O2+ (Table 5), for which a square pyramidal geometry around the copper ion was proposed, with the four nitrogen atoms in the equatorial plane and the oxygen atom occupying the apical position [45]. The Cu[15]aneN4S2+ complex presents slightly lower gz values and a small blue-shift of the absorption band, consistent with a slightly stronger equatorial ligand field. Although the coordination geometry of the complex cannot be unequivocally established without an X-ray structure, the similar results for both compounds suggest for Cu[15]aneN4S2+ a distorted square pyramidal environment around the Cu(II) centre, with the sulphur atom in the apical position and the four nitrogen atoms in the equatorial plane.
3. Experimental
3.1. General
FT-IR spectra were recorded in a Nicolet 6700 FT-IR spectrophotometer (Thermo Electron Corporation, Runcord, UK) using KBr pellets. Electrospray ionization-mass spectrometry (ESI-MS) was carried out with a Micromass Quattro Micro triple quadrupole instrument (Waters, Milford, MA, USA) and MassLynx software (version 4.1) was used for data analysis. The ionization of the compound was performed by an electrospray source in positive mode (ESI+). UV-visible spectra were recorded with a UNICAM UV-4 (Thermo Scientific, Waltham, MA, USA) and UV-visible-near IR spectra were collected with a Shimadzu UV-1603 (Tokyo, Japan). EPR spectroscopic measurements were recorded with a Bruker ESP EMX 300 spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany) equipped with continuous-flow cryostats for liquid nitrogen, operating at X-band. The compounds were characterized by 1H (400.13 MHz) and 13C-NMR (100.62 MHz) spectra recorded on a Bruker Avance 400 MHz spectrometer (Bruker BioSpin GmbH, Rheinstetten, Germany) at ≈ 20 °C probe temperature. The internal reference used for the 1H-NMR measurements in CDCl3 was tetramethylsilane (TMS) and in D2O was 3-(trimethylsilyl)propionic acid-d4-sodium salt (DSS). Chemical shifts (δ) were given in ppm and coupling constants (J) in Hz. Resonance assignments are based on chemical shift, peak integration and multiplicity for 1H-NMR spectra and on 2D experiments (COSY, HMQC and HMBC) for 13C-NMR spectra. FIDs were processed using the TopSpin software version 3.2 from Bruker.
3.1.1. Reagents
The dimethylester of tiodiglycolic acid (Scheme 1) was prepared according to a reported procedure [22]. Triethylenetetramine, tiodiglycolic acid and borane tetrahydrofuran complex solution 1 M in THF were purchased from Aldrich (Sigma-Aldrich, Madrid, Spain). All the commercially available chemicals were of reagent analytical grade and used as supplied without further purification. Organic solvents were purified or dried by standard methods [52].
3.2. Synthesis of 1-Thia-4,7,10,13-tetraazacyclopentadecane ([15]aneN4S)
A solution of dimethyl thiodiglycolate (3.03 g, 17 mmol) in dry methanol (100 mL) was added to a solution of triethylenetetramine (2.94 g, 20 mmol) in the same solvent (400 mL), at rt. This mixture was stirred under nitrogen at 40 °C for 9 days. The solvent was then removed under reduced pressure. The remaining oil was dissolved in a minimum amount of chloroform and purified through a silica-gel column (2.5 × 30 cm) using a mixture of CHCl3–MeOH (20:80 v/v) as eluent. The pure compound was dissolved in methanol and 37% hydrochloric acid was added until pH ≈ 3. The cyclic diamide precipitated as an off-yellow salt (2.94 g, 74%). IR (KBr, cm−1): ν 3427 (N–H), 1652 (C=O). 1H-NMR (400.13 MHz; D2O; DSS; pD = 3.4): δ 3.26 (t, 4H, (triplet), Hd) 3.35 (s, 4H, (singlet), Ha), 3.53 (t, 4H, Hc), 3.57 (s, 4H, He) ppm. 13C-NMR (100.61 MHz; D2O; dioxane; pD = 3.4): δ 35.3 (Cc), δ 37.5 (Ca), 42.8 (Ce), 48.2 (Cd), 175.0 (C2) ppm.
The cyclic diamide (1.10 g, 4,2 mmol) was reduced with a large excess of borane in refluxing dry THF (100 mL) under nitrogen, for eight hours. After reduction, the obtained oil was dissolved in chloroform and purified by silica-gel chromatography. The trihydrocloride salt was obtained by addition of 37% HCl until pH ≈ 2 (0.98 g, 68%). IR (KBr, cm−1): ν 3426 (N–H). 1H-NMR (400.13 MHz; D2O; DSS; pD = 1.72): δ 3.17 (t, 4H, 3J = 6, Ha), 3.34 (s, 4H, 3J = 6, He), 3.44 (t, 4H, Hd), δ 3.50 (t, 4H, 3J = 6, Hb), 3.57 (t, 4H, 3J = 6, Hc) ppm. 13C-NMR (100.61 MHz; D2O; dioxane; pD = 1.72): δ 29.55 (Ca), 43.64 (Cd), 45.00 (Cc), 45.37 (Ce), 46.94 (Cb). m/z (ESI-MS; methanol; positive ion mode) 233.20 [M + H]+ (Figures S7–S8 in Supplementary Materials).
3.3. Potentiometric Studies
3.3.1. Reagents and Solutions
All solutions were prepared with demineralized water obtained by a Millipore/Milli-Q system. Solutions of the ligand were prepared at ca. 2.50 × 10−3 M and their exact concentrations were obtained by titration with the standardised solution of KOH.
Metal ion solutions were prepared at about 0.050 M from nitrate salts (analytical grade), except in the case of mercury nitrate (ca. 0.01 M) that was kept in excess of nitric acid to prevent precipitation. The solutions were standardised by titration with Na2H2EDTA [53].
Carbonate-free solutions of the titrant KOH were freshly prepared by dilution of a commercial ampoule of Fixanal (Fluka, Sigma-Aldrich, Madrid, Spain) at ca. 0.10 M, under a stream of pure argon gas. Solutions were discarded every time carbonate concentration was about 0.5% of the total amount of base. A 0.100 M standard solution of HNO3 (prepared from a Merck ampoule) was used for the back titrations. The titrant solutions were standardised (tested by Gran's method) [54]. For the competition titrations a standard K2H2EDTA aqueous solution was used.
The equipment used has been described before [7]. The temperature was controlled at 25.0 ± 0.1 °C. CO2 was excluded from the titration cell during experiments by passing argon across the experimental solution. The ionic strength of the solutions was kept at 0.10 ± 0.01 M with KNO3.
3.3.2. Measurements
The [H+] of the solutions was determined by the measurement of the electromotive force (emf) of the cell, E = E'o + Q log[H+] + Ej. E'o and Q were determined by titration of a solution of known hydrogen-ion concentration at the same ionic strength, using the acid pH range of the titration. Ej was found to be negligible under our experimental conditions. The value of Kw was obtained from data acquired in the alkaline range of the titration, considering E'o and Q valid for the entire pH range and found to be equal to 10−13.80 M2 in our experimental conditions. The electromotive force data were determined after additions of 0.050 mL increments of standardised KOH solution, and after stabilization in this direction, equilibrium was then approached from the other direction by adding standard 0.100 M nitric acid (back titration). Before and after each determination, a calibration of the system was performed by titration of a 2.00 × 10−3 M HNO3 solution.
The potentiometric equilibrium measurements were carried out using 20.00 mL of ≈ 2.50 × 10−3 M ligand solutions diluted to a final volume of 30.00 mL, in the absence of metal ions and in the presence of each metal ion for which the CM:CL ratio was 1:1. A minimum of two replicate measurements was made. For the reactions of Cu2+ and Hg2+, ligand-ligand competition titrations were performed to determine the stability constants. K2H2EDTA was used as the second ligand in ratios CL1: CL: CM 1:0.75:1, being L1, K2H2EDTA [55]. EDTA protonation and copper(II) stability constants values were determined before, under the same experimental conditions: log K1H = 10.22, log K2H = 6.16, log K3H = 2.71, log K4H = 2.0, log KCuEDTA = 19.23, log KCuHEDTA = 3.06, log KCuEDTAOH = 11.33 [55]; for the Hg(II) complexes the following values were selected from the literature: log KHgEDTA = 21.50, log KHgHEDTA = 3.20, log KHgEDTAOH = 8.90 [56].
3.3.3. Calculation of Equilibrium Constants
Overall equilibrium constants 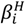 and
and 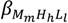 (being
(being 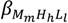 = [MmHhLl]/[M]m [H]h [L]l) were calculated by fitting the potentiometric data from protonation or complexation titrations with the HYPERQUAD program [34]. Species distribution diagrams were plotted from the calculated constants with the HySS program [37]. Only mononuclear species, ML, MHL and MH-1L were found for the metal complexes of [15]aneN4S (being βMH-1L = βMLOH × Kw). The hydrolysis constants of the metal ions were taken from literature and kept constant for the calculations. Differences, in log units, between the values of βMHL (or βMH-1L) and βML constants, provide the stepwise reaction constants. The species considered in a particular model were those that could be justified by the principles of coordination chemistry. The errors quoted are the standard deviations of the overall stability constants given directly by the program for the input data, which include all the experimental points of all titration curves, and were determined by the normal propagation rules for the stepwise constants.
= [MmHhLl]/[M]m [H]h [L]l) were calculated by fitting the potentiometric data from protonation or complexation titrations with the HYPERQUAD program [34]. Species distribution diagrams were plotted from the calculated constants with the HySS program [37]. Only mononuclear species, ML, MHL and MH-1L were found for the metal complexes of [15]aneN4S (being βMH-1L = βMLOH × Kw). The hydrolysis constants of the metal ions were taken from literature and kept constant for the calculations. Differences, in log units, between the values of βMHL (or βMH-1L) and βML constants, provide the stepwise reaction constants. The species considered in a particular model were those that could be justified by the principles of coordination chemistry. The errors quoted are the standard deviations of the overall stability constants given directly by the program for the input data, which include all the experimental points of all titration curves, and were determined by the normal propagation rules for the stepwise constants.
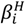 and
and 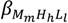 (being
(being 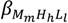 = [MmHhLl]/[M]m [H]h [L]l) were calculated by fitting the potentiometric data from protonation or complexation titrations with the HYPERQUAD program [34]. Species distribution diagrams were plotted from the calculated constants with the HySS program [37]. Only mononuclear species, ML, MHL and MH-1L were found for the metal complexes of [15]aneN4S (being βMH-1L = βMLOH × Kw). The hydrolysis constants of the metal ions were taken from literature and kept constant for the calculations. Differences, in log units, between the values of βMHL (or βMH-1L) and βML constants, provide the stepwise reaction constants. The species considered in a particular model were those that could be justified by the principles of coordination chemistry. The errors quoted are the standard deviations of the overall stability constants given directly by the program for the input data, which include all the experimental points of all titration curves, and were determined by the normal propagation rules for the stepwise constants.
= [MmHhLl]/[M]m [H]h [L]l) were calculated by fitting the potentiometric data from protonation or complexation titrations with the HYPERQUAD program [34]. Species distribution diagrams were plotted from the calculated constants with the HySS program [37]. Only mononuclear species, ML, MHL and MH-1L were found for the metal complexes of [15]aneN4S (being βMH-1L = βMLOH × Kw). The hydrolysis constants of the metal ions were taken from literature and kept constant for the calculations. Differences, in log units, between the values of βMHL (or βMH-1L) and βML constants, provide the stepwise reaction constants. The species considered in a particular model were those that could be justified by the principles of coordination chemistry. The errors quoted are the standard deviations of the overall stability constants given directly by the program for the input data, which include all the experimental points of all titration curves, and were determined by the normal propagation rules for the stepwise constants.3.4. Spectroscopic Studies
1H-NMR titration was performed with a 4.80 × 10−2 M solution of [15]aneN4S prepared in D2O. The pD values were adjusted by adding DCl or CO2-free KOD solutions. Following each addition, the −log [H*] was measured after equilibration, directly in the NMR tube with an Orion 3 Star pH meter equipped with a combined glass Ag-AgCl microelectrode U402-M3-S7/200, Mettler-Toledo (Barcelona, Spain).
The electrode was previously calibrated with standard aqueous buffers solutions and the pD values were calculated according to the equation pD = pH* + (0.40 ± 0.02), where pH* corresponds to the reading of the pH meter [28].
The dissociation constants in D2O (pKD) were determined from the 1H-NMR titration by using the HypNMR program [57]. These pKD values were converted to pKH values obtained in water by the equation pKD = 0.11 + 1.10 × pKH [28].
Magnetic moments were measured at 293.6 K using aqueous solutions of Co[15]aneN4S2+ (1.99 × 10−3 M, pH 7.02), Ni[15]aneN4S2+ (2.39 × 10−3 M, pH 7.11) and Cu[15]aneN4S2+ (3.98 × 10−3 M, pH 7.09). The 1H-NMR spectra of the solutions with DSS as internal reference, were acquired in a tube containing an internal capillary filled with D2O and DSS, and the corresponding magnetic moments calculated from the shift (Δδ) between both reference signals, according to Evans method [38].
Electronic spectra were recorded using aqueous solutions of Co2+, Ni2+ and Cu2+ complexes for visible-near IR regions of 1.21 × 10−2 M, 1.23 × 10−2 M and 1.29 × 10−2 M at pHs 6.99, 6.98, 7.08, respectively; for UV region 1.02 × 10−3 M, 2.04 × 10−3 M and 2.02 × 10−3 M at pHs 6.92, 6.87 and 7.08, respectively.
EPR spectroscopy measurements of copper(II) complexes of [15]aneN4S were performed at 104 K. The complexes were prepared in 1.25 × 10−3 M, at pH 4.10, 7.40 and 9.00, in 1 M NaClO4 aqueous solutions.
4. Conclusions
A thiatetraaza macrocycle [15]aneN4S having four nitrogen and one sulphur as donor atoms has been synthesised. Potentiometric studies have shown that this compound has a high selectivity towards Hg(II) and Cu(II) over the other divalent metal ions under study. The UV-visible-near IR spectroscopies and magnetic moment data for the Co(II) and Ni(II) complexes indicated a tetragonal distorted coordination geometry for both metal centres. The value of magnetic moment and the X-band EPR spectra of the Cu(II) complex are consistent with a distorted square pyramidal geometry.
This work suggest that [15]aneN4S should be evaluated as a potential ligand to be used in chelation therapy on disorders triggered by these metal ions. Further studies would be performed in order to evaluate the efficacy of this chelator in the biological milieu.
Supplementary Materials
Supplementary materials can be acceded at: http://www.mdpi.com/1420-3049/19/1/550/s1.
Acknowledgments
The authors acknowledge Rita Delgado and the Instituto de Tecnologia Química e Biológica, Universidade Nova de Lisboa, Portugal, for the UV-visible and EPR spectroscopies. The authors also thank the Fundação para a Ciência e a Tecnologia (FCT) for the project REDE/1518/REM/2005 for ESI-MS/MS experiments at LCLEM, Faculdade de Farmácia, Universidade de Lisboa, Portugal.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hayes, R.B. The carcinogenicity of metals in humans. Cancer Cause. Control 1997, 8, 371–385. [Google Scholar] [CrossRef]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Caussy, D.; Gochfeld, M.; Gurzau, E.; Neagu, C.; Ruedel, H. Lessons from case studies of metals: Investigating exposure, Bioavailability, and risk. Ecotoxicol. Environ. Saf. 2003, 56, 45–51. [Google Scholar] [CrossRef]
- Blanuša, M.; Varnai, V.M.; Piasek, M.; Kostial, K. Chelators as antidotes of metal toxicity: Therapeutic and experimental aspects. Curr. Med. Chem. 2005, 12, 2771–2794. [Google Scholar] [CrossRef]
- Flora, S.J.S.; Pachauri, V. Chelation in metal intoxication. Int. J. Environ. Res. Public Health 2010, 7, 2745–2788. [Google Scholar] [CrossRef]
- Sears, M.E. Chelation: Harnessing and enhancing heavy metal detoxification—A review. Sci. World J. 2013, 2013, 1–13. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Cabral, M.F.; Costa, J.; Castro, M.; Delgado, R.; Drew, M.G.; Felix, V. Two macrocyclic pentaaza compounds containing pyridine evaluated as novel chelating agents in copper(II) and nickel(II) overload. J. Inorg. Biochem. 2011, 105, 410–419. [Google Scholar] [CrossRef]
- Andersen, O.; Aaseth, J. Molecular mechanisms of in vivo metal chelation: Implications for clinical treatment of metal intoxications. Environ. Health. Perspect. 2002, 110, 887–890. [Google Scholar] [CrossRef]
- Andersen, O. Chemical and biological considerations in the treatment of metal intoxications by chelating agents. Mini Rev. Med. Chem. 2004, 4, 11–21. [Google Scholar] [CrossRef]
- Aposhian, H.V.; Maiorino, R.M.; Gonzalez-Ramirez, D.; Zuniga-Charles, M.; Xu, Z.; Hurlbut, K.M.; Junco-Munoz, P.; Dart, R.C.; Aposhian, M.M. Mobilization of heavy metals by newer, Therapeutically useful chelating agents. Toxicology 1995, 97, 23–38. [Google Scholar] [CrossRef]
- Gonçalves, S.; Fernandes, A.S.; Oliveira, N.G.; Marques, J.; Costa, J.; Cabral, M.F.; Miranda, J.; Cipriano, M.; Guerreiro, P.S.; Castro, M. Cytotoxic effects of cadmium in mammary epithelial cells: Protective role of the macrocycle [15]pyN5. Food Chem. Toxicol. 2012, 50, 2180–2187. [Google Scholar] [CrossRef]
- Mewis, R.E.; Archibald, S. Biomedical applications of macrocyclic ligand complexes. Coord. Chem. Rev. 2010, 254, 1686–1712. [Google Scholar] [CrossRef]
- Hancock, R.D.; Martell, A.E. Ligand design for selective complexation of metal ions in aqueous solution. Chem. Rev. 1989, 89, 1875–1914. [Google Scholar] [CrossRef]
- Hancock, R.D.; Dobson, S.M.; Boeyens, J.C.A. Metal ion size selectivity of 1-Thia-4, 7-diazacyclononane (9-aneN2S), and other tridentate macrocycles. A study by molecular mechanics calculation, structure determination, and formation constant determination of complexes of 9-aneN2S. Inorganica Chim. Acta 1987, 133, 221–231. [Google Scholar] [CrossRef]
- Westerby, B.C.; Juntunen, K.L.; Leggett, G.H.; Pett, V.B.; Koenigbauer, M.J.; Purgett, M.D.; Taschner, M.J.; Ochrymowycz, L.A.; Rorabacher, D.B. Macrocyclic polyamino polythiaether ligands with NxS4-x and NxS5-x donor sets: Protonation constants, stability constants, and kinetics of complex formation with the aquocopper(II) ion. Inorg. Chem. 1991, 30, 2109–2120. [Google Scholar] [CrossRef]
- Arnaud-Neu, F.; Schwing-Weill, M.J.; Louis, R.; Weiss, R. Thermodynamic and spectroscopic properties in aqueous solutions of pentadentate macrocyclic complexes. Inorg. Chem. 1979, 18, 2956–2961. [Google Scholar] [CrossRef]
- Balakrishnan, K.P.; Kaden, T.A.; Siegfried, L.; Zuberbühler, A.D. Stabilities and redox properties of Cu(I) and Cu(II) complexes with macrocyclic ligands containing the N2S2 donor set. Helv. Chim. Acta 1984, 67, 1060–1069. [Google Scholar] [CrossRef]
- Walker, T.L.; Malasi, W.; Bhide, S.; Parker, T.; Zhang, D.; Freedman, A.; Modarelli, J.M.; Engle, J.T.; Ziegler, C.J.; Custer, P.; et al. Synthesis and characterization of 1,8-dithia-4,11-diazacyclotetradecane. Tetrahedron Lett. 2012, 53, 6548–6551. [Google Scholar] [CrossRef]
- Papini, G.; Alidori, S.; Lewis, J.S.; Reichert, D.E.; Pellei, M.; Lobbia, G.G.; Biddlecombe, G.B.; Anderson, C.J.; Santini, C. Synthesis and characterization of the copper(II) complexes of new N2S2-donor macrocyclic ligands: Synthesis and in vivo evaluation of the 64Cu complexes. Dalton Trans. 2009, 7, 177–184. [Google Scholar]
- Aquilanti, G.; Giorgetti, M.; Minicucci, M.; Papini, G.; Pellei, M.; Tegoni, M.; Trasatti, A.; Santini, C. A study on the coordinative versatility of new N,S-donor macrocyclic ligands: XAFS, and Cu2+ complexation thermodynamics in solution. Dalton Trans. 2011, 40, 2764–2777. [Google Scholar] [CrossRef]
- Kodama, M.; Koike, T.; Hoshiga, N.; Machida, R.; Kimura, E. Metal chelates of sulphur-containing polyamine macrocycles and oxygenation of the corresponding cobalt(II) complexes. J. Chem. Soc. Dalton Trans. 1984, 673–678. [Google Scholar]
- Vollhardt, K.P.C.; Schore, N.E. Organic Chemistry, 6th ed.; Freeman and Co.: New York, NY, USA, 2011. [Google Scholar]
- Tabushi, I.; Okino, H.; Kuroda, Y. Convenient synthesis of macrocyclic-compounds containing two of nitrogen, Oxygen or sulphur atoms. Tetrahedron Lett. 1976, 17, 4339–4342. [Google Scholar] [CrossRef]
- Steenland, M.W.A.; Westbroek, P.; Dierck, I.; Herman, G.G.; Lippens, W.; Temmerman, E.; Goeminne, A.M. Aqueous solution study of Cu(II) and Ni(II) complexes of macrocyclic oxa- and thia- containing trans-dioxo-tetraamines. Polyhedron 1999, 18, 3417–3424. [Google Scholar]
- Bencini, A.; Bianchi, A.; Garcia-España, E.; Micheloni, M.; Ramirez, J.A. Proton coordination by polyamine compounds in aqueous solution. Coord. Chem. Rev. 1999, 188, 97–156. [Google Scholar] [CrossRef]
- Cabral, M.F.; Delgado, R. Metal complexes of pentadentate macrocyclic ligands containing oxygen and nitrogen as donor atoms. Helv. Chim. Acta 1994, 77, 515–524. [Google Scholar] [CrossRef]
- Motekaitis, R.J.; Rogers, B.E.; Reichert, D.E.; Martell, A.E.; Welch, M.J. Stability and structure of activated macrocycles. Ligands with biological applications. Inorg. Chem. 1996, 35, 3821–3827. [Google Scholar] [CrossRef]
- Delgado, R.; Fraústo da Silva, J.J.R.; Amorim, M.T.S.; Cabral, M.F.; Chaves, S.; Costa, J. Dissociation constants of Brønsted acids in D2O and H2O: Studies on polyaza and polyoxa-polyaza macrocycles and a general correlation. Anal. Chim. Acta 1991, 245, 271–282. [Google Scholar]
- Hancock, R.D.; Wade, P.W.; Ngwenya, M.P.; de Sousa, A.S.; Damu, K.V. Ligand design for complexation in aqueous solution. 2. Chelate ring size as a basis for control of size-based selectivity for metal ions. Inorg. Chem. 1990, 29, 1968–1974. [Google Scholar] [CrossRef]
- Riley, D.P.; Henke, S.L.; Lennon, P.J.; Weiss, R.H.; Neumann, W.L.; Rivers, W.J., Jr.; Aston, K.W.; Sample, K.R.; Rahman, H.; Ling, C.S.; et al. Synthesis, characterization, and stability of manganese(II) C-substituted 1,4,7,10,13-pentaazacyclopentadecane complexes exhibiting superoxide dismutase activity. Inorg. Chem. 1996, 35, 5213–5231. [Google Scholar] [CrossRef]
- Kodama, M.; Kimura, E. Effects of axial ligation on molecular oxygen binding by donor atoms built in saturated macrocycles. Equilibrium and kinetic study with cobalt(II) complexes of macrocyclic pentaamines and oxatetraamine. Inorg. Chem. 1980, 19, 1871–1875. [Google Scholar] [CrossRef]
- Kodama, M.; Kimura, E.; Yamaguchi, S. Complexation of the macrocyclic hexa-amine ligand 1,4,7,10,13,16-hexa-azacyclo-octadecane(“18-azacrown-6”). J. Chem. Soc. Dalton Trans. 1980, 2536–2538. [Google Scholar] [CrossRef]
- Kodama, M.; Kimura, E. Equilibria of complex formation between several bivalent metal ions and macrocyclic tri- and penta-amines. J. Chem. Soc. Dalton Trans. 1978, 1081–1085. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Kodama, M.; Kimura, E. Effects of cyclization and ring size on complex formation between penta-amine ligands and copper(II). J. Chem. Soc. Dalton Trans. 1978, 104–110. [Google Scholar] [CrossRef]
- Costa, J.; Delgado, R.; Drew, M.G.B.; Félix, V. Design of selective macrocyclic ligands for the divalent first-row transition-metal ions. J. Chem. Soc. Dalton Trans. 1998, 1063–1071. [Google Scholar]
- Alderighi, L.; Gans, P.; Ienco, A.; Peters, D.; Sabatini, A.; Vacca, A. Hyperquad simulation and speciation (HySS): A utility program for the investigation of equilibria involving soluble and partially soluble species. Coord. Chem. Rev. 1999, 184, 311–318. [Google Scholar] [CrossRef]
- Evans, D.F. The determination of the paramagnetic susceptibility of substances in solution by nuclear magnetic resonance. J. Chem. Soc. 1959, 2003–2005. [Google Scholar] [CrossRef]
- Lever, A.B.P. Inorganic Electronic Spectroscopy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Banci, L.; Bencini, A.; Benelli, C.; Gatteschi, D.; Zanchini, C. Spectral-structural correlations in high-spin cobalt(II) complexes. Sruct. Bond. 1982, 52, 37–86. [Google Scholar]
- Bertini, I.; Luchinat, C. High spin cobalt(II) as a probe for the investigation of metalloproteins. Adv. Inorg. Biochem. 1984, 6, 71–111. [Google Scholar]
- Martin, L.Y.; Sperati, C.R.; Busch, D.H. The spectrochemical properties of tetragonal complexes of high spin nickel(II) containing macrocyclic ligands. J. Am. Chem. Soc. 1977, 99, 2968–2981. [Google Scholar] [CrossRef]
- Sacconi, L.; Mani, F.; Bencini, A. Nickel. In Comprehensive Coordination Chemistry; Wilkinson, G., Gillard, R.D., McCleverty, J.A., Eds.; Pergamon Press: Oxford, UK, 1987; pp. 1–137. [Google Scholar]
- Neese, F. Electronic Structure and Spectroscopy of Novel Copper Chromophores in Biology. Diploma Thesis, University of Konstanz, Konstanz, Germany, June 1993. [Google Scholar]
- Fernandes, A.S.; Gaspar, J.; Cabral, M.F.; Caneiras, C.; Guedes, R.; Rueff, J.; Castro, M.; Costa, J.; Oliveira, N.G. Macrocyclic copper(II) complexes: Superoxide scavenging activity, Structural studies and cytotoxicity evaluation. J. Inorg. Biochem. 2007, 101, 849–858. [Google Scholar] [CrossRef]
- Li, Y. X-Ray structures and spectroscopic studies of diaqua- and dichlorocopper(II) complexes of 15 crown 5 and 4' substituted benzo-15-Crown-5 with a 3dx(x=z2) ground state doublet. Bull. Chem. Soc. Jpn. 1996, 69, 2513–2523. [Google Scholar] [CrossRef]
- Barbaro, P.; Bianchini, C.; Capannesi, G.; di Luca, L.; Laschi, F.; Petroni, D.; Salvadori, P.A.; Vacca, A.; Vizza, F. Synthesis and characterization of the tetraazamacrocycle 4,10-dimethyl-1,4,7,10-tetraazacyclododecane-1,7-diacetic acid (H2Me2DO2A) and of its neutral copper(II) complex [Cu(Me2DO2A)]. A new 64Cu-labeled macrocyclic complex for positron emission tomography imaging. J. Chem. Soc. Dalton Trans. 2000, 2393–2401. [Google Scholar]
- Hathaway, B.J. Copper. Coord. Chem. Rev. 1983, 52, 87–169. [Google Scholar] [CrossRef]
- Gersmann, H.R.; Swalen, J.D. Electron paramagnetic resonance spectra of copper complexes. J. Chem. Phys. 1962, 36, 3221–3233. [Google Scholar] [CrossRef]
- Yokoi, H.; Sai, M.; Isobe, T.; Ohsawa, S. ESR studies of the copper(II) complexes of amino acids. Bull. Chem. Soc. Jpn. 1972, 45, 2189–2195. [Google Scholar] [CrossRef]
- Lau, P.W.; Lin, W.C. Electron spin resonance and electronic structure of some metalloporphyrins. J. Inorg. Nucl. Chem. 1975, 37, 2389–2398. [Google Scholar]
- Perrin, D.D.; Armarego, W.L.F. Purification of Laboratory Chemicals, 3rd ed.; Pergamon Press: Oxford, UK, 1988. [Google Scholar]
- Schwarzenbach, G.; Flaschka, W. Complexometric Titrations, 2nd ed.; Methuen & Co: London, UK, 1969. [Google Scholar]
- Rossotti, F.J.C.; Rossotti, H. Potentiometric titrations using Gran plots: A textbook omission. J. Chem. Educ. 1965, 42, 375–378. [Google Scholar] [CrossRef]
- Delgado, R.; do Carmo Figueira, M.; Quintino, S. Redox method for the determination of stability constants of some trivalent metal complexes. Talanta 1997, 45, 451–462. [Google Scholar] [CrossRef]
- Pettit, L.D.; Powell, H.K.J. IUPAC Stability Constants Database; Academic Software: Timble, Otley, Yorks, UK, 2003. [Google Scholar]
- Frassineti, C.; Ghelli, S.; Gans, P.; Sabatini, A.; Moruzzi, M.S.; Vacca, A. Nuclear magnetic resonance as a tool for determining protonation constants of natural polyprotic bases in solution. Anal. Biochem. 1995, 231, 374–382. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).