Orbital Interaction and Electron Density Transfer in PdII([9]aneB2A)L2 Complexes: Theoretical Approaches
Abstract
:1. Introduction

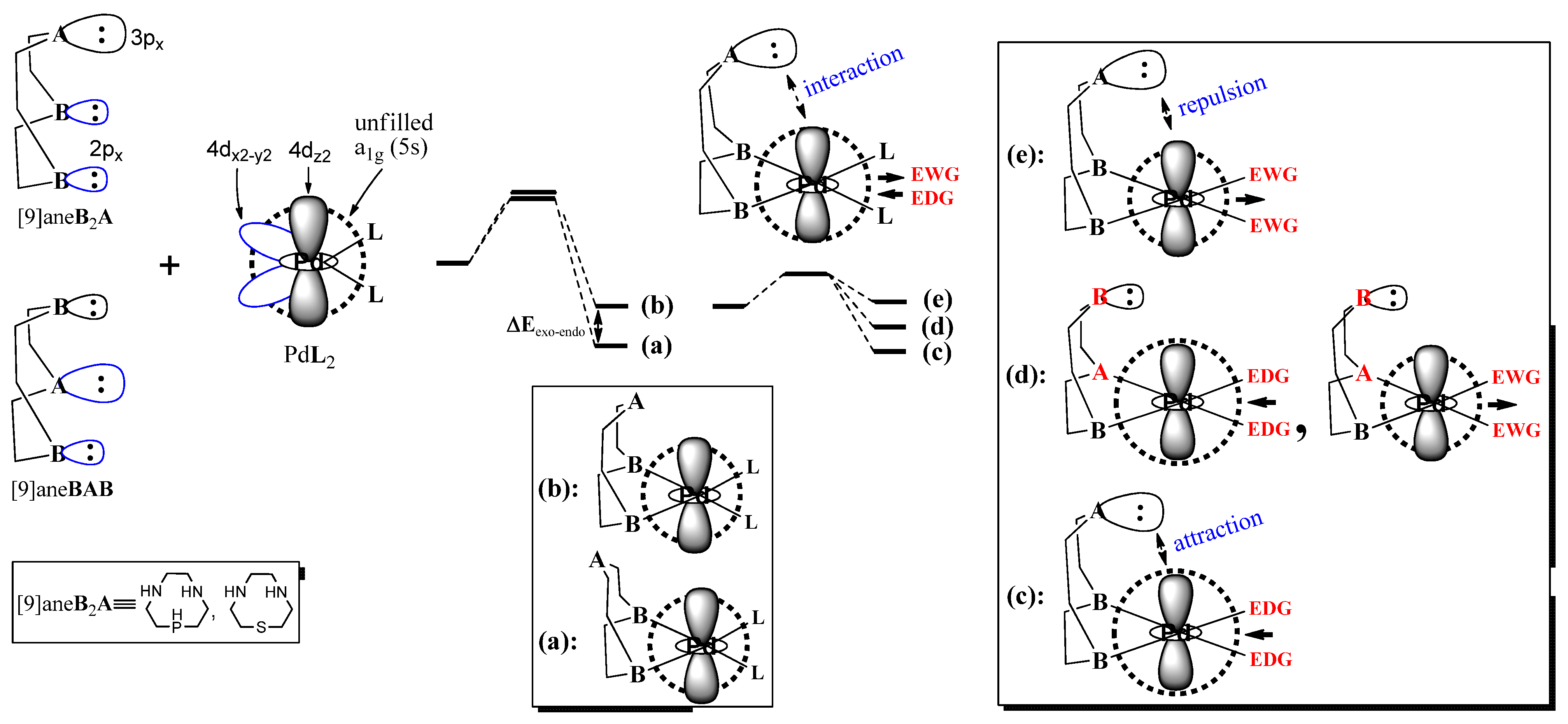
2. Computational Methods
3. Results and Discussion
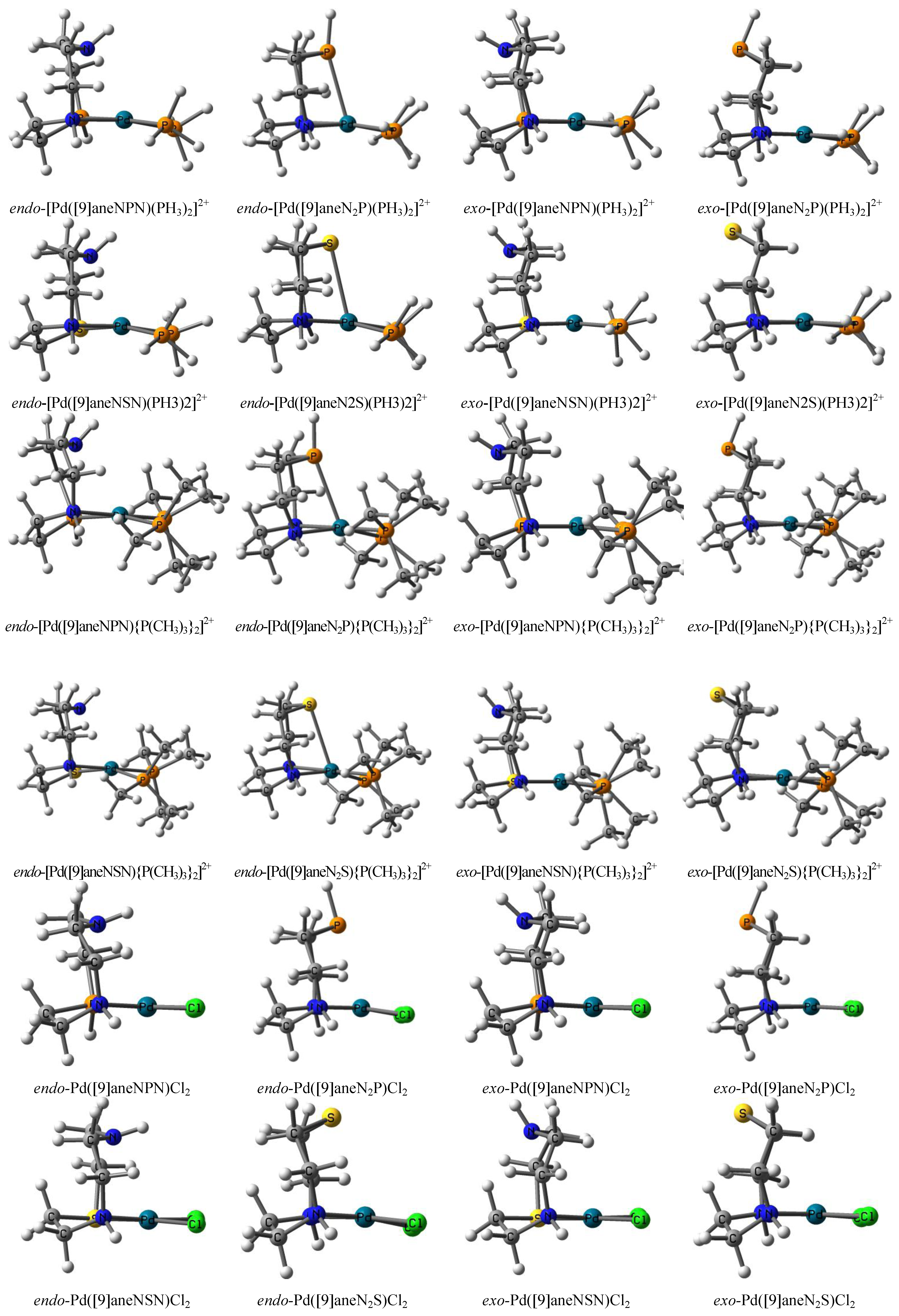
| Compound | Average distance | Average atomic charge | Relative energy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RaPd-N | RaPd-P | RbPd…N | RbPd…P | QcPd | QcPH3 | QdN | QdP | ΔEeH-L | ΔEfBAB-B2A | ΔEgexo-endo | |
| endo-[Pd([9]aneNPN)(PH3)2]2+ | 2.195 | 2.289 | 2.848 | 0.348 | 0.368 | −0.151 | 3.45 | 0.37 | 0.00 | ||
| exptl | 2.3214h | 2.298i2.3207i | |||||||||
| endo-[Pd([9]aneN2P)(PH3)2]2+ | 2.142 | 2.782 | 0.225 | 0.412 | −0.289 | 2.68 | 0.00 | 0.00 | |||
| theo | 2.340j | 0.598k | 0.150k | ||||||||
| exptl | 2.3337i | ||||||||||
| exo-[Pd([9]aneNPN)(PH3)2]2+ | 2.154 | 2.363 | 3.504 | 0.405 | 0.380 | −0.152 | 3.39 | −0.70 | −0.37 | ||
| exo-[Pd([9]aneN2P)(PH3)2]2+ | 2.129 | 3.963 | 0.263 | 0.449 | −0.018 | 2.74 | 0.00 | 0.70 | |||
| RaPd-N | RaPd-S | RbPd…N | RbPd…S | QcPd | QcPH3 | QdN | QdS | ||||
| endo-Pd([9]aneNSN)(PH3)2]2+ | 2.155 | 2.359 | 2.720 | 0.332 | 0.377 | −0.159 | 3.40 | 0.10 | 0.00 | ||
| endo-[Pd([9]aneN2S)(PH3)2]2+ | 2.138 | 2.877 | 0.294 | 0.447 | −0.269 | 2.67 | 0.00 | 0.00 | |||
| exo-[Pd([9]aneNSN)(PH3)2]2+ | 2.135 | 2.382 | 3.602 | 0.435 | 0.366 | −0.112 | 2.87 | −0.25 | −0.03 | ||
| exo-[Pd([9]aneN2S)(PH3)2]2+ | 2.127 | 3.954 | 0.343 | 0.444 | −0.033 | 2.42 | 0.00 | 0.32 | |||
| RaPd-N | RaPd-P | RbPd…N | RbPd…P | QcPd | QcP(CH3)3 | QdN | QdP | ΔEeH-L | ΔEfBAB-B2A | ΔEgexo-endo | |
| endo-[Pd([9]aneNPN){P(CH3)3}2]2+ | 2.214 | 2.324 | 3.076 | 0.464 | 0.425 | −0.133 | 4.01 | 0.35 | 0.00 | ||
| endo-[Pd([9]aneN2P){P(CH3)3}2]2+ | 2.195 | 2.843 | 0.380 | 0.389 | −0.416 | 3.51 | 0.00 | 0.00 | |||
| exo-[Pd([9]aneNPN){P(CH3)3}2]2+ | 2.188 | 2.368 | 3.678 | 0.575 | 0.439 | 0.048 | 3.85 | −0.51 | −0.28 | ||
| exo-[Pd([9]aneN2P){P(CH3)3}2]2+ | 2.178 | 4.049 | 0.414 | 0.372 | −0.061 | 3.52 | 0.00 | 0.58 | |||
| RaPd-N | RaPd-S | RbPd…N | RbPd…S | QcPd | QcP(CH3)3 | QdN | QdS | ||||
| endo-Pd([9]aneNSN){P(CH3)3}2]2+ | 2.185 | 2.402 | 2.920 | 0.409 | -0.392 | −0.118 | 3.88 | 0.08 | 0.00 | ||
| endo-[Pd([9]aneN2S){P(CH3)3}2]2+ | 2.194 | 2.935 | 0.353 | -0.396 | −0.300 | 3.50 | 0.00 | 0.00 | |||
| exo-[Pd([9]aneNSN){P(CH3)3}2]2+ | 2.179 | 2.420 | 3.705 | 0.434 | -0.352 | 0.129 | 3.55 | −0.20 | −0.05 | ||
| exo-[Pd([9]aneN2S){P(CH3)3}2]2+ | 2.176 | 4.037 | 0.458 | -0.412 | −0.107 | 3.19 | 0.00 | 0.23 | |||
| RaPd-N | RaPd-P | RbPd…N | RbPd…P | QcPd | QcCl | QdN | QdP | ΔEeH-L | ΔEfBAB-B2A | ΔEgexo-endo | |
| endo-Pd([9]aneNPN)Cl2 | 2.113 | 2.225 | 3.040 | 0.801 | −0.746 | −0.077 | 4.04 | −0.28 | 0.00 | ||
| exptl | 2.115l | 2.3337i | |||||||||
| 2.117l | |||||||||||
| endo-Pd([9]aneN2P)Cl2 | 2.088 | 3.033 | 0.720 | −0.740 | −0.357 | 3.84 | 0.00 | 0.00 | |||
| theo | 2.101k | 0.628k | −0.516k | ||||||||
| 2.049k | |||||||||||
| exptl | 2.0410i | ||||||||||
| 2.0354i | |||||||||||
| exo-Pd([9]aneNPN)Cl2 | 2.106 | 2.346 | 3.471 | 0.920 | −0.762 | −0.086 | 4.08 | −0.35 | −0.13 | ||
| exo-Pd([9]aneN2P)Cl2 | 2.095 | 3.868 | 0.695 | −0.708 | −0.152 | 4.03 | 0.00 | −0.06 | |||
| RaPd-N | RaPd-S | RbPd…N | RbPd…S | QcPd | QcCl | QdN | QdS | ||||
| endo-Pd([9]aneNSN)Cl2 | 2.094 | 2.324 | 2.923 | 0.771 | −0.747 | −0.073 | 3.73 | -0.18 | 0.00 | ||
| exptl | 2.124m | 2.2492m | 2.638m | ||||||||
| 2.332n | |||||||||||
| endo-Pd([9]aneN2S)Cl2 | 2.091 | 3.190 | 0.609 | −0.722 | −0.304 | 3.77 | 0.00 | 0.00 | |||
| exptl | 2.077m | 3.0865m 3.293o | |||||||||
| 2.117o | |||||||||||
| 2.144o | |||||||||||
| exo-Pd([9]aneNSN)Cl2 | 2.094 | 2.329 | 3.465 | 0.787 | −0.727 | 0.076 | 3.85 | −0.21 | −0.13 | ||
| exptl | 2.068p | 2.2685m | 3.499p | ||||||||
| 2.050p | 2.317q | ||||||||||
| exo-Pd([9]aneN2S)Cl2 | 2.094 | 3.854 | 0.774 | −0.724 | −0.178 | 4.04 | 0.00 | −0.09 | |||
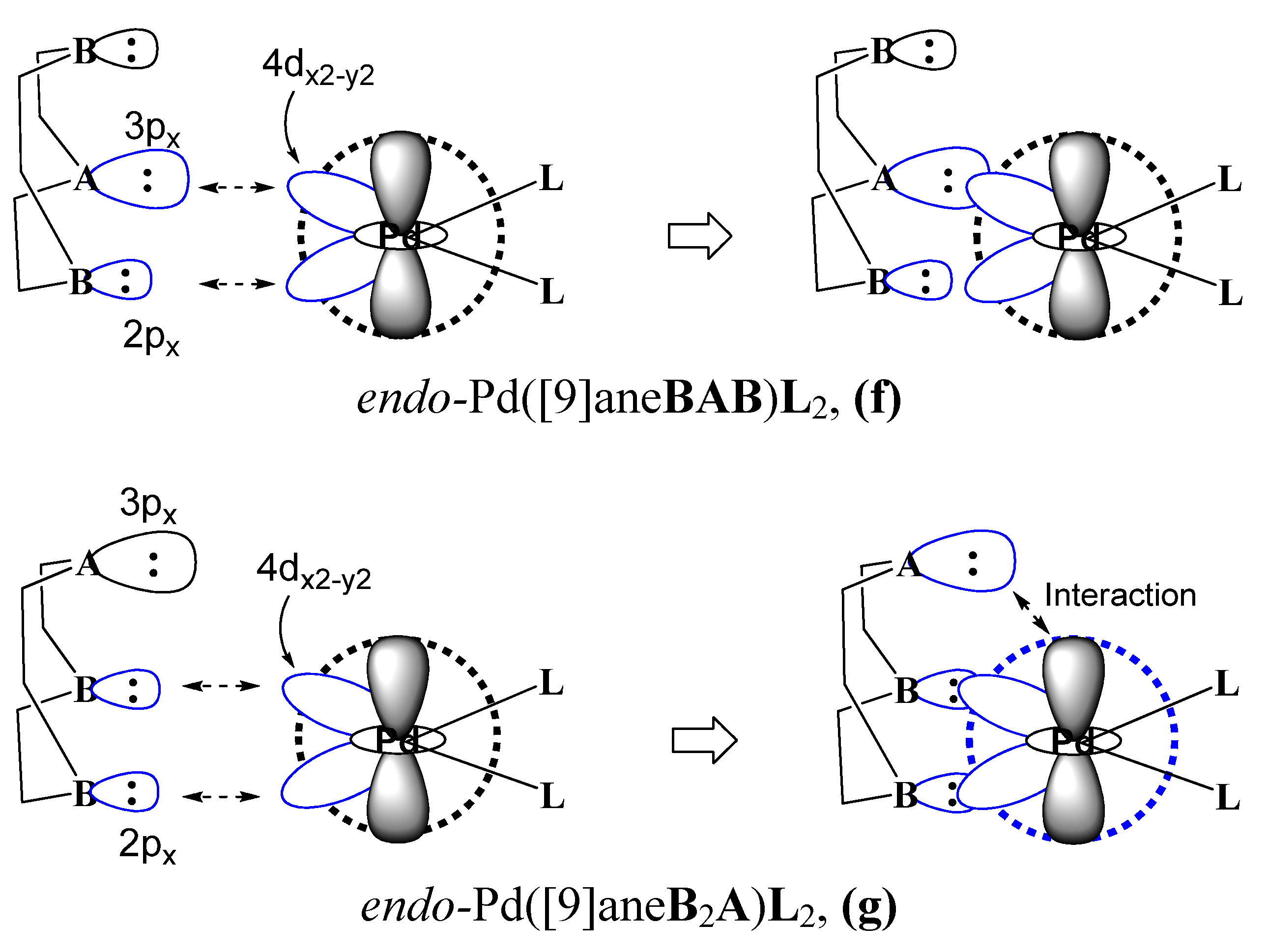

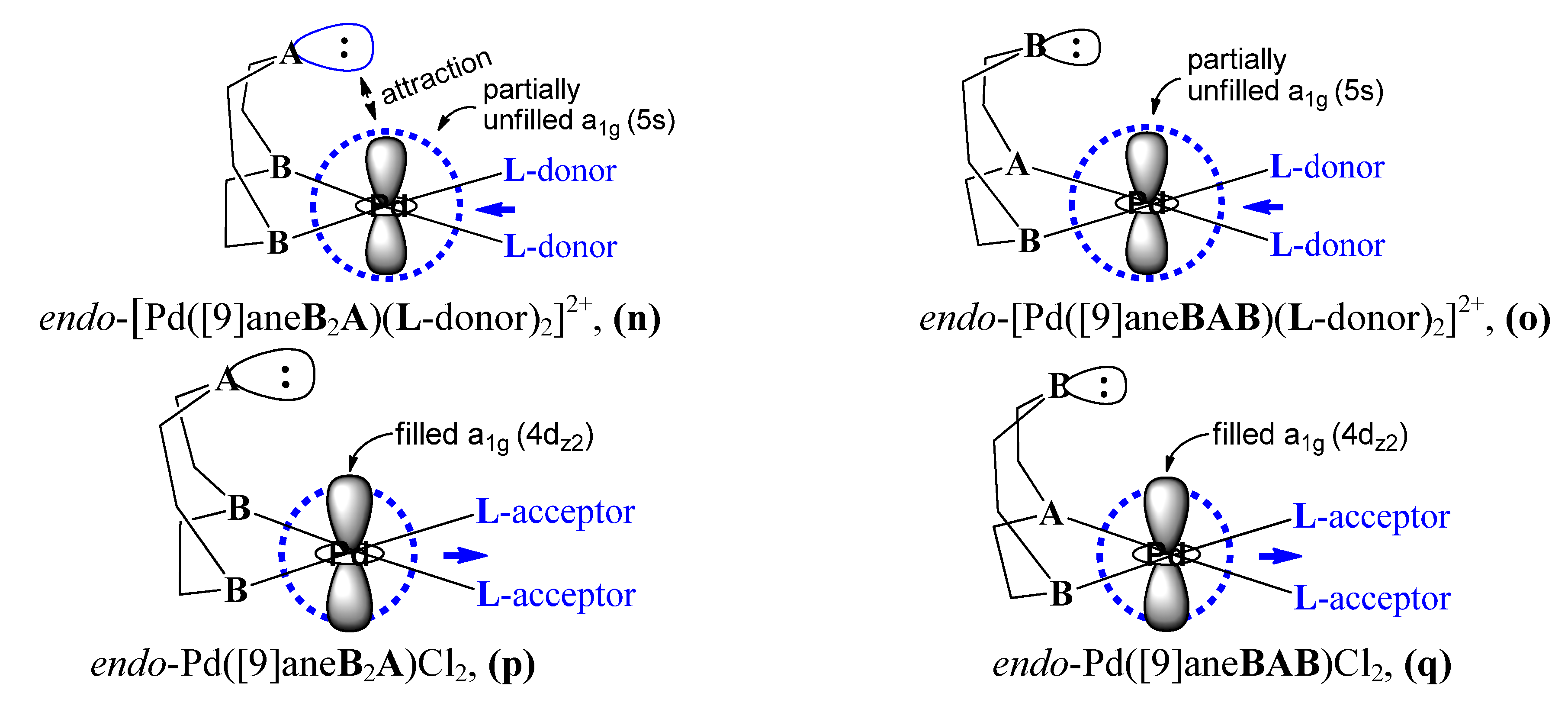
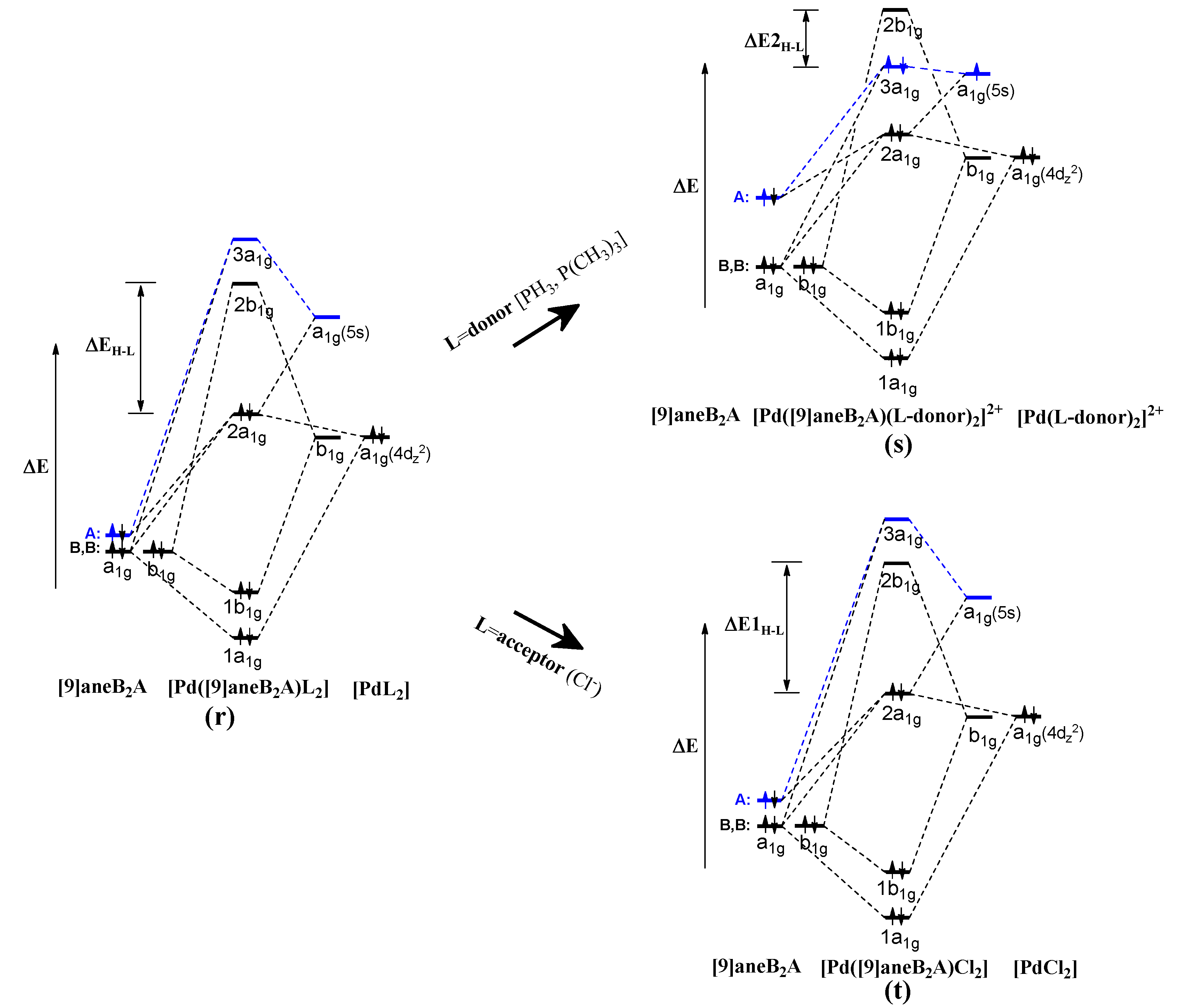

4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Amatore, C.; Jutand, A. Handbook of Organopalladium Chemistry for Organic Synthesis; John Wiley and Sons: New York, NY, USA, 2002; Volume 1, p. 943. [Google Scholar]
- De Meijere, A.; Diederich, F. Metal Catalyzed Cross Coupling Reactions, 2nd ed.; John Wiley and Sons: New York, NY, USA, 2004; Volumes1 and 2. [Google Scholar]
- Amatore, C.; Jutand, A. Anionic Pd(0) and Pd(II) Intermediates in Palladium-Catalyzed Heck and Cross-Coupling Reactions. Acc. Chem. Res. 2000, 33, 314–321. [Google Scholar] [CrossRef]
- Littke, A.F.; Fu, G.C. Palladium-catalyzed coupling reactions of aryl chlorides. Angew. Chem. Int. Ed. 2002, 41, 4176–4211. [Google Scholar] [CrossRef]
- Grushin, V.V. Mixed Phosphine-Phosphine Oxide Ligands. Chem. Rev. 2004, 104, 1629–1662. [Google Scholar] [CrossRef]
- Zapf, A.; Beller, M. The development of efficient catalysts for palladium-catalyzed coupling reactions of aryl halides. Chem. Commun. 2005, 431–440. [Google Scholar] [CrossRef]
- Christmann, U.; Vilar, R. Monoligated palladium species as catalysts in cross-coupling reactions. Angew. Chem. Int. Ed. 2005, 44, 366–374. [Google Scholar] [CrossRef]
- Trzeciak, A.M.; Ziolkowski, J.J. Structural and mechanistic studies of Pd-catalyzed C-C bond formation: The case of carbonylation and Heck reaction. Coor. Chem. Rev. 2005, 249, 2308–2322. [Google Scholar] [CrossRef]
- Matsubara, T.; Hirao, K. Density functional study of the σ and π bond activation at the Pd=X (X = Sn, Si, C) bonds of the (H2PC2H4PH2)Pd=XH2 complexes. is the bond cleavage homolytic or heterolytic? J. Am. Chem. Soc. 2002, 124, 679–689. [Google Scholar] [CrossRef]
- Albano, V.G.; Bandini, M.; Moorlag, C.; Piccinelli, F.; Pietrangelo, A.; Tommasi, S.; Umani-Ronchi, A.; Wolf, M.O. Electropolymerized Pd-containing thiophene polymer: A reusable supported catalyst for cross-coupling reactions. Organometallics 2007, 26, 4373–4375. [Google Scholar] [CrossRef]
- Braga, A.A.C.; Ujaque, G.; Maseras, F. A DFT Study of the full catalytic cycle of the suzuki-miyaura cross-coupling on a model system. Organometallics 2006, 25, 3647–3658. [Google Scholar] [CrossRef]
- Sicre, C.; Braga, A.A.C.; Maseras, F.; Cid, M.M. Mechanistic insights into the transmetalation step of a Suzuki-Miyaura reaction of 2(4)-bromopyridines: characterization of an intermediate. Tetrahedron 2008, 64, 7437–7443. [Google Scholar] [CrossRef]
- Meir, R.; Kozuch, S.; Uhe, A.; Shaik, S. How can theory predict the selectivity of palladium-catalyzed cross-coupling of pristine aromatic molecules? Chem. Eur. J. 2011, 17, 7623–7631. [Google Scholar] [CrossRef]
- Goossen, L.J.; Koley, D.; Hermann, H.L.; Thiel, W. The palladium-catalyzed cross-coupling reaction of carboxylic anhydrides with arylboronic acids: A DFT study. J. Am. Chem. Soc. 2005, 127, 11102–11114. [Google Scholar] [CrossRef]
- Goossen, L.J.; Koley, D.; Hermann, H.L.; Thiel, W. Palladium monophosphine intermediates in catalytic cross-coupling reactions: A DFT study. Organometallics 2006, 25, 54–57. [Google Scholar]
- Arooj, M.; Park, K.; Park, J.K. Effects for charge transfer in Pd(II) complexes along various terminal ligands. Bull. Korean Chem. Soc. 2010, 31, 3815–3817. [Google Scholar] [CrossRef]
- Ariafard, A.; Lin, Z.; Fairlamb, I.J.S. Effect of the leaving ligand X on transmetalation of organostannanes (vinylSnR3) with LnPd(Ar)(X) in stille cross-coupling reactions. A density functional theory study. Organometallics 2006, 25, 5788–5794. [Google Scholar] [CrossRef]
- Montoya, V.; Pons, J.; Garcia-Anton, J.; Solans, X.; Font-Bardia, M.; Ros, J. New (η3-Allyl)palladium complexes with pyridylpyrazole ligands: Synthesis, characterization, and study of the influence of N1 substituents on the apparent allyl rotation. Organometallics 2007, 26, 3183–3190. [Google Scholar] [CrossRef]
- Crociani, B.; Antonaroli, S.; Burattini, M.; Benetollo, F.; Scrivanti, A.; Bertoldini, M. Palladium complexes of 8-(di-tert-butylphosphinooxy) quinoline. J. Organomet. Chem. 2008, 693, 3932–3938. [Google Scholar] [CrossRef] [Green Version]
- Scrivanti, A.; Bertoldini, M.; Beghetto, V.; Matteoli, U.; Venzo, A. Protonation of palladium(II)-allyl and palladium(0)-olefin complexes containing 2-pyridyldiphenylphosphine. J. Organomet. Chem. 2009, 694, 131–136. [Google Scholar] [CrossRef]
- Park, S.W.; Chun, Y.; Cho, S.J.; Lee, S.; Kim, K.S. Design of carbene-based organocatalysts for nitrogen fixation: theoretical study. J. Chem. Theory Comput. 2012, 8, 1983–1988. [Google Scholar] [CrossRef]
- Faller, J.W.; Wilt, J.C. Palladium/BINAP(S)-catalyzed asymmetric allylic amination. Org. Lett. 2005, 7, 633–636. [Google Scholar] [CrossRef]
- Gladiali, S.; Taras, R.; Ceder, R.M.; Rocamora, M.; Muller, G.; Solans, X.; Font-Bardia, M. Asymmetric allylic alkylation catalyzed by Pd(II)-complexes with (S)-BINPO, a hemilabile axially chiral P,O-heterodonor inducer. Tetrahedron Asymmetry 2004, 15, 1477–1485. [Google Scholar] [CrossRef]
- Chernyshova, E.S.; Goddard, R.; Pörschke, K.-R. Mononuclear NHC-Pd-π-Allyl complexes containing weakly coordinating ligand. Organometallics 2007, 26, 3236–3251. [Google Scholar] [CrossRef]
- de Pater, J.J.M.; Tromp, D.S.; Tooke, D.M.; Spek, A.L.; Deelman, B.-J.; van Koten, G.; Elsevier, C.J. Palladium(0)-Alkene Bis(triarylphosphine) complexes as catalyst precursors for the methoxycarbonylation of styrene. Organometallics 2005, 24, 6411–6419. [Google Scholar] [CrossRef]
- Canovese, L.; Visentin, F.; Chessa, G.; Uguagliati, P.; Levi, C.; Dolmella, A.; Bandoli, G. Role of the ligand and of the size and flexibility of the palladium-ancillary ligand cycle on the reactivity of substituted alkynes toward Palladium(0) complexes bearing potentially terdentate nitrogen-sulfur-nitrogen or nitrogen-nitrogen-nitrogen ligands: Kinetic and structural study. Organometallics 2006, 25, 5355–5365. [Google Scholar]
- Douthwaite, R.E. Metal-mediated asymmetric alkylation using chiral N-heterocyclic carbenes derived from chiral amines. Coor. Chem. Rev. 2007, 251, 702–717. [Google Scholar] [CrossRef]
- Sivaramakrishna, A.; Clayton, H.S.; Mogorosi, M.M.; Moss, J.R. Hydrocarbon (π- and σ-) complexesof nickel, palladium and platinum: Synthesis, reactivity and applications. Coord. Chem. Rev. 2010, 254, 2904–2932. [Google Scholar] [CrossRef]
- Filipuzzi, S.; Pregosin, P.S.; Albinati, A.; Rizzato, S. Structure, bonding, and dynamics of several palladium η3-Allyl carbene complexes. Organometallics 2008, 27, 437–444. [Google Scholar] [CrossRef]
- Fairlamb, I.J.S.; Kapdi, A.R.; Lee, A.F.; McGlacken, G.P.; Weissburger, F.; de Vries, A.H.M.; Schmieder-van de Vondervoort, L. Exploiting Noninnocent (E,E)-Dibenzylideneacetone (dba) Effects in Palladium(0)-Mediated cross-coupling reactions: Modulation of the electronic properties of dba affects catalyst activity and stability in ligand and ligand-free reaction systems. Chem. Eur. J. 2006, 12, 8750–8761. [Google Scholar] [CrossRef]
- Macé, Y.; Kapdi, A.R.; Fairlamb, I.J.S.; Jutand, A. Influence of the dba substitution on the reactivity of Palladium(0) complexes generated from Pd02(dba-n,n'-Z)3 or Pd0(dba-n,n'-Z)2 and PPh3 in oxidative addition with iodobenzene. Organometallics 2006, 25, 1795–1800. [Google Scholar] [CrossRef]
- Köcher, S.; Walfort, B.; Mills, A.M.; Spek, A.L.; Klink, G.P.M.; van Koten, G.; Lang, H. Synthesis and reaction chemistry of 4-nitrile-substituted NCN-pincer palladium(II) and platinum(II) complexes (NCN=[N≡C-4-C6H2(CH2NMe2)2-2,6]−). J. Organomet. Chem. 2008, 693, 1991–1996. [Google Scholar] [CrossRef]
- Blum, K.; Chernyshova, E.S.; Goddard, R.; Jonas, K.; Pörschke, K.-R. 4,9-Diazadodeca-1,trans-6,11-trienes as ligands for Nickel(0), Palladium(0), and Platinum(0). Organometallics 2007, 26, 5174–5178. [Google Scholar] [CrossRef]
- Krause, J.; Cestaric, G.; Haack, K.-J.; Seevogel, K.; Storm, W.; Pörschke, K.-R. 1,6-Diene Complexes of Palladium(0) and Platinum(0): Highly reactive sources for the naked metals and [L-M0] Fragments. J. Am. Chem. Soc. 1999, 121, 9807–9823. [Google Scholar] [CrossRef]
- Pla-Quintana, A.; Torrent, A.; Dachs, A.; Roglans, A.; Pleixats, R.; Moreno-Manas, M.; Parella, T.; Benet-Buchholz, J. Chiral and stable Palladium(0) complexes of polyunsaturated Aza-macrocyclic Ligands: Synthesis and Structural analysis. Organometallics 2006, 25, 5612–5620. [Google Scholar] [CrossRef]
- Grundl, M.A.; Kennedy-Smith, J.J.; Trauner, D. Rational design of a chiral Palladium(0) olefin complex of unprecedented stability. Organometallics 2005, 24, 2831–2833. [Google Scholar] [CrossRef]
- Hunter, G.; McAuley, A.; Whitcombe, T.W. Crystal and Solution Structure of the Palladium(II) Bis(1,4,7-triazacyclononane) Ion: Evidence for rapid fluxional behavior in a macrocyclic complex. Inorg. Chem. 1988, 27, 2634–2639. [Google Scholar] [CrossRef]
- Scrivanti, A.; Beghetto, V.; Matteoli, U.; Antonaroli, S.; Marini, A.; Mandoj, F.; Paolesse, R.; Crociani, B. Iminophosphine-palladium(0) complexes as highly active catalysts in the Suzuki reaction. Synthesis of undecaaryl substituted corroles. Tetrahedron Lett. 2004, 45, 5861–5864. [Google Scholar] [CrossRef] [Green Version]
- Scrivanti, A.; Beghetto, V.; Matteoli, U.; Antonaroli, S.; Marini, A.; Crociani, B. Catalytic activity of η2-(olefin)palladium(0) complexes with iminophosphine ligands in the Suzuki-Miyaura reaction. Role of the olefin in the catalyst stabilization. Tetrahedron 2005, 61, 9752–9758. [Google Scholar] [CrossRef] [Green Version]
- Arca, M.; Blake, A.J.; Lippolis, V.; Montesu, D.R.; McMaster, J.; Tei, L.; Schroeder, M. Coordination Chemistry of Nitrile and Amino Pendant Arm Derivatives of [9]aneN2S and [9]aneNS2 with PdII and CuII. Eur. J. Inorg. Chem. 2003, 1232–1241. [Google Scholar]
- Albano, V.G.; Bandini, M.; Barbarella, G.; Melucci, M.; Monari, M.; Piccinelli, F.; Tommasi, S.; Umani-Ronchi, A. Controlling stereochemical outcomes of asymmetric processes by catalyst remote molecular functionalizations: Chiral Diamino-Oligothiophenes (DATs) as ligands in asymmetric catalysis. Chem. Eur. J. 2006, 12, 667–675. [Google Scholar] [CrossRef]
- Broering, M.; Brandt, C.D. A five coordinate PdII complex stable in solution and in the solid state. Chem. Commun. 2003, 2156–2157. [Google Scholar] [CrossRef]
- Park, J.K.; Cho, Y.G.; Lee, S.S.; Kim, B.G. Geometrical characteristics and atomic charge variations of Pd(II) complexes [Pd(L)Cl2] with an Axial (Pd…O) interaction. Bull. Korean Chem. Soc. 2004, 25, 85–89. [Google Scholar] [CrossRef]
- Yoo, J.S.; Ha, D.S.; Kim, J.S.; Kim, B.G.; Park, J.K. Geometrical characteristics and reactivities of tetracoordinated Pd complexes: Mono- and bidentate ligands and charged and uncharged ligands. Bull. Korean Chem. Soc. 2008, 29, 627–640. [Google Scholar] [CrossRef]
- Arooj, M.; Park, J.K. Geometrical Structures of the first solvation shell of the [PdCl4]2− core: charge-dipole vs. dipole-dipole interaction of (PdII...Solvent). Bull. Korean Chem. Soc. 2008, 29, 2295–2298. [Google Scholar] [CrossRef]
- Ayala, R.; Marcos, E.S.; Dǐaz-Moreno, S.; Solė, V.A.; Muňoz-Păez, A. Geometry and hydration structure of Pt(II) square planar complexes [Pt(H2O)4]2+ and [PtCl4]2− as studied by X-ray absorption spectroscopies and quantum-mechanical computations. J. Phys. Chem. B 2001, 105, 7588–7593. [Google Scholar]
- Burda, J.V.; Zeizinger, M.; Leszczynski, J. Activation barriers and rate constants for hydration of platinum and palladium square-planar complexes: An ab initio study. J. Chem. Phys. 2004, 120, 1253–1262. [Google Scholar] [CrossRef]
- Frish, M.J.; Trucks, G.W.; Head-Gordon, M.H.; Gill, P.M.W.; Wong, M.W.; Foresman, J.B.; Johnson, B.G.; Schlegel, H.B.; Robb, M.A.; Replogle, E.S.; et al. Gaussian 03; Gaussian Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
- Becke, A.D. The Challenge of d- and f-Electrons: Theory and Computation; ACS Symposium Series, No. 394; Salahub, D.R., Zerner, M.C., Eds.; American Chemical Society: Washington, DC, USA, 1989; p. 166. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. 1988, A38, 3098–3100. [Google Scholar]
- Perdew, J.P. Transport and relaxation of hot conduction electrons in an organic dielectric. Phys. Rev. 1986, B33, 8822–8824. [Google Scholar]
- Search Chemistry WebElements, WebElements Periodic Table of the Elements (the periodic table on the web). Available online: http://www.webelements.com (accessed on 10 July 2011).
- Blake, A.J.; Holder, A.J.; Hyde, T.I.; Roberts, Y.V.; Lavery, A.J.; Schroeder, M. Structural and electrochemical studies on trithia macrocyclic complexes of palladium. J. Organomet. Chem. 1987, 323, 261–270. [Google Scholar] [CrossRef]
- Lai, Y.-H.; Ma, L.; Mok, K.F. Synthesis and conformational and complexation studies of 2,17-dioxa-5,14-dithia[6.6] orthocyclophane. A ligand with monodentate and bidentate properties. New J. Chem. 1997, 21, 985–991. [Google Scholar]
- Pinkas, J.; Huffman, J.C.; Chisholm, M.H.; Caulton, K.G. Origin of different coordination polyhedra for Cu[CF3C(O)CHC(O)CF3]2L (L = H2O, NH3). Inorg. Chem. 1995, 34, 5314–5318. [Google Scholar]
- Kozelka, J.; Bergès, J.; Attias, R.; Fraitag, J. O-H…PtII: Hydrogen bond with a strong dispersion component. Angew. Chem. Int. Ed. 2000, 39, 198–201. [Google Scholar] [CrossRef]
- Miessler, G.L.; Tarr, D.A. Inorganic Chemistry, 3rd ed.; Pearson Prentice Hall, Pearson Education, Inc.: Upper Saddle River, NJ, USA, 2004; pp. 337–376. [Google Scholar]
- Albight, T.A.; Burdett, J.K.; Whangbo, M.-Y. Orbital Interactions in Chemistry; Wiley-Interscience: New York, NY, USA, 1985; pp. 295–296. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kwak, O.K.; Arooj, M.; Yoon, Y.-J.; Jeong, E.D.; Park, J.K. Orbital Interaction and Electron Density Transfer in PdII([9]aneB2A)L2 Complexes: Theoretical Approaches. Molecules 2013, 18, 12687-12706. https://doi.org/10.3390/molecules181012687
Kwak OK, Arooj M, Yoon Y-J, Jeong ED, Park JK. Orbital Interaction and Electron Density Transfer in PdII([9]aneB2A)L2 Complexes: Theoretical Approaches. Molecules. 2013; 18(10):12687-12706. https://doi.org/10.3390/molecules181012687
Chicago/Turabian StyleKwak, Ock Keum, Mahreen Arooj, Yong-Jin Yoon, Euh Duck Jeong, and Jong Keun Park. 2013. "Orbital Interaction and Electron Density Transfer in PdII([9]aneB2A)L2 Complexes: Theoretical Approaches" Molecules 18, no. 10: 12687-12706. https://doi.org/10.3390/molecules181012687




