The Emerging Role of Ferumoxytol-Enhanced MRI in the Management of Cerebrovascular Lesions
Abstract
:1. Introduction
2. Inflammation and Macrophages in IAs and AVMs
3. Ferumoxytol-Enhanced MRI
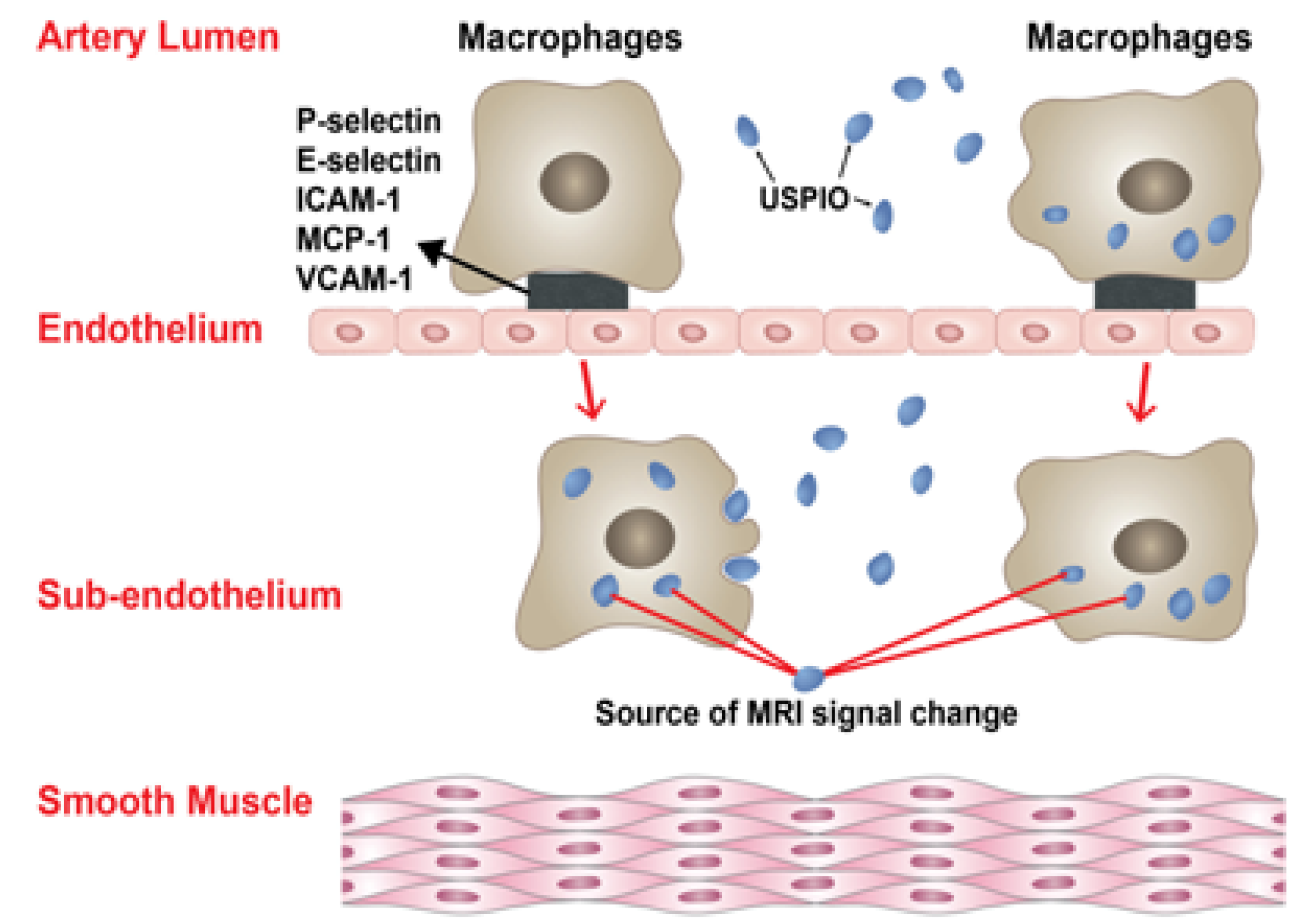
4. Ferumoxytol-Enhanced MRI in IAs
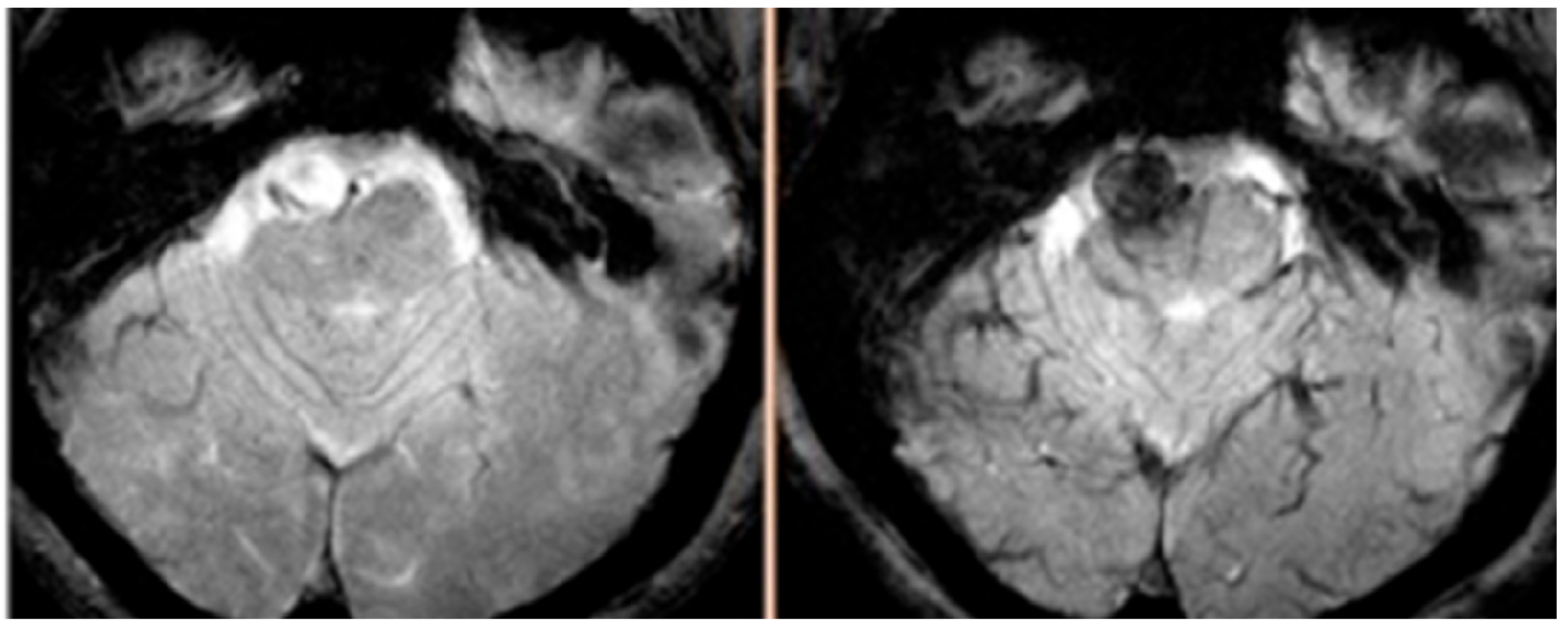
5. Ferumoxytol-Enhanced MRI in AVMs
6. Future Perspectives
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Chalouhi, N.; Dumont, A.S.; Randazzo, C.; Tjoumakaris, S.; Gonzalez, L.F.; Rosenwasser, R.; Jabbour, P. Management of incidentally discovered intracranial vascular abnormalities. Neurosurg. Focus 2011, 31, E1. [Google Scholar]
- Al-Shahi, R.; Warlow, C. A systematic review of the frequency and prognosis of arteriovenous malformations of the brain in adults. Brain 2001, 124, 1900–1926. [Google Scholar] [CrossRef]
- van Gijn, J.; Kerr, R.S.; Rinkel, G.J. Subarachnoid haemorrhage. Lancet 2007, 369, 306–318. [Google Scholar] [CrossRef]
- Nieuwkamp, D.J.; Setz, L.E.; Algra, A.; Linn, F.H.; de Rooij, N.K.; Rinkel, G.J. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, Sex, And region: a meta-analysis. Lancet Neurol. 2009, 8, 635–642. [Google Scholar] [CrossRef]
- Wiebers, D.O.; Whisnant, J.P.; Huston, J., 3rd; Meissner, I.; Brown, R.D., Jr.; Piepgras, D.G.; Forbes, G.S.; Thielen, K.; Nichols, D.; O’Fallon, W.M.; et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003, 362, 103–110. [Google Scholar] [CrossRef]
- Chalouhi, N.; Jabbour, P.; Singhal, S.; Drueding, R.; Starke, R.M.; Dalyai, R.T.; Tjoumakaris, S.; Gonzalez, L.F.; Dumont, A.S.; Rosenwasser, R.; et al. Stent-assisted coiling of intracranial aneurysms: predictors of complications, Recanalization, And outcome in 508 cases. Stroke 2013, 44, 1348–1353. [Google Scholar] [CrossRef]
- Chalouhi, N.; Tjoumakaris, S.; Starke, R.M.; Gonzalez, L.F.; Randazzo, C.; Hasan, D.; McMahon, J.F.; Singhal, S.; Moukarzel, L.A.; Dumont, A.S.; et al. Comparison of Flow Diversion and Coiling in Large Unruptured Intracranial Saccular Aneurysms. Stroke 2013, 44, 2150–2154. [Google Scholar] [CrossRef]
- Jabbour, P.; Chalouhi, N.; Tjoumakaris, S.; Gonzalez, L.F.; Dumont, A.S.; Randazzo, C.; Starke, R.M.; Hasan, D.; Chitale, R.; Singhal, S.; Moukarzel, L.A.; et al. The Pipeline Embolization Device: Learning Curve and Predictors of Complications and Aneurysm Obliteration. Neurosurgery 2013, 73, 113–120. [Google Scholar] [CrossRef]
- Chalouhi, N.; Jabbour, P.; Ibrahim, I.; Starke, R.M.; Younes, P.; El Hage, G.; Samaha, E. Surgical treatment of ruptured anterior circulation aneurysms: Comparison of pterional and supraorbital keyhole approaches. Neurosurgery 2013, 72, 437–441; discussion 441–442. [Google Scholar] [CrossRef]
- Chalouhi, N.; Jabbour, P.; Gonzalez, L.F.; Dumont, A.S.; Rosenwasser, R.; Starke, R.M.; Gordon, D.; Hann, S.; Tjoumakaris, S. Safety and efficacy of endovascular treatment of basilar tip aneurysms by coiling with and without stent assistance: a review of 235 cases. Neurosurgery 2012, 71, 785–794. [Google Scholar] [CrossRef]
- Chalouhi, N.; Jabbour, P.; Kung, D.; Hasan, D. Safety and efficacy of tirofiban in stent-assisted coil embolization of intracranial aneurysms. Neurosurgery 2012, 71, 710–714; discussion 714. [Google Scholar] [CrossRef]
- Lawton, M.T.; Du, R.; Tran, M.N.; Achrol, A.S.; McCulloch, C.E.; Johnston, S.C.; Quinnine, N.J.; Young, W.L. Effect of presenting hemorrhage on outcome after microsurgical resection of brain arteriovenous malformations. Neurosurgery 2005, 56, 485–493; discussion 485–493. [Google Scholar] [CrossRef]
- Stapf, C.; Mohr, J.P. Unruptured brain arteriovenous malformations should be treated conservatively: yes. Stroke 2007, 38, 3308–3309. [Google Scholar] [CrossRef]
- Laakso, A.; Dashti, R.; Seppanen, J.; Juvela, S.; Vaart, K.; Niemela, M.; Sankila, R.; Hernesniemi, J.A. Long-term excess mortality in 623 patients with brain arteriovenous malformations. Neurosurgery 2008, 63, 244–253; discussion 253–255. [Google Scholar]
- Chalouhi, N.; Ali, M.S.; Jabbour, P.M.; Tjoumakaris, S.I.; Gonzalez, L.F.; Rosenwasser, R.H.; Koch, W.J.; Dumont, A.S. Biology of intracranial aneurysms: Role of inflammation. J. Cereb. Blood Flow Metab. 2012, 32, 1659–1676. [Google Scholar] [CrossRef]
- Chalouhi, N.; Ali, M.S.; Starke, R.M.; Jabbour, P.M.; Tjoumakaris, S.I.; Gonzalez, L.F.; Rosenwasser, R.H.; Koch, W.J.; Dumont, A.S. Cigarette smoke and inflammation: Role in cerebral aneurysm formation and rupture. Mediators Inflamm. 2012, 271582. [Google Scholar]
- Starke, R.M.; Chalouhi, N.; Ali, M.S.; Jabbour, P.M.; Tjoumakaris, S.I.; Gonzalez, L.F.; Rosenwasser, R.H.; Koch, W.J.; Dumont, A.S. The Role of Oxidative Stress in Cerebral Aneurysm Formation and Rupture. Curr. Neurovasc. Res. 2013, 10, 247–255. [Google Scholar] [CrossRef]
- Hasan, D.; Hashimoto, T.; Kung, D.; Macdonald, R.L.; Winn, H.R.; Heistad, D. Upregulation of Cyclooxygenase-2 (COX-2) and Microsomal Prostaglandin E2 Synthase-1 (mPGES-1) in Wall of Ruptured Human Cerebral Aneurysms: Preliminary Results. Stroke 2012, 43, 1964–1967. [Google Scholar] [CrossRef]
- Chalouhi, N.; Points, L.; Pierce, G.L.; Ballas, Z.; Jabbour, P.; Hasan, D. Localized increase of chemokines in the lumen of human cerebral aneurysms. Stroke 2013. [CrossRef]
- Starke, R.M.; Chalouhi, N.; Ali, M.S.; Jabbour, P.; Tjoumakaris, S.I.; Gonzalez, L.F.; Rosenwasser, R.H.; Owens, G.; Greig, N.H.; Dumont, A.S. 190 Critical role of TNF-a in cerebral aneurysm formation and rupture. Neurosurgery 2013. [Google Scholar] [CrossRef]
- Sho, E.; Sho, M.; Singh, T.M.; Nanjo, H.; Komatsu, M.; Xu, C.; Masuda, H.; Zarins, C.K. Arterial enlargement in response to high flow requires early expression of matrix metalloproteinases to degrade extracellular matrix. Exp. Mol. Pathol. 2002, 73, 142–153. [Google Scholar] [CrossRef]
- Jamous, M.A.; Nagahiro, S.; Kitazato, K.T.; Tamura, T.; Aziz, H.A.; Shono, M.; Satoh, K. Endothelial injury and inflammatory response induced by hemodynamic changes preceding intracranial aneurysm formation: experimental study in rats. J. Neurosurg. 2007, 107, 405–411. [Google Scholar] [CrossRef]
- Jamous, M.A.; Nagahiro, S.; Kitazato, K.T.; Satoh, K.; Satomi, J. Vascular corrosion casts mirroring early morphological changes that lead to the formation of saccular cerebral aneurysm: an experimental study in rats. J. Neurosurg. 2005, 102, 532–535. [Google Scholar] [CrossRef]
- Tada, Y.; Yagi, K.; Kitazato, K.T.; Tamura, T.; Kinouchi, T.; Shimada, K.; Matsushita, N.; Nakajima, N.; Satomi, J.; Kageji, T.; et al. Reduction of endothelial tight junction proteins is related to cerebral aneurysm formation in rats. J. Hypertens. 2010, 28, 1883–1891. [Google Scholar] [CrossRef]
- Chien, S. Effects of disturbed flow on endothelial cells. Ann. Biomed. Eng. 2008, 36, 554–562. [Google Scholar] [CrossRef]
- Aoki, T.; Kataoka, H.; Shimamura, M.; Nakagami, H.; Wakayama, K.; Moriwaki, T.; Ishibashi, R.; Nozaki, K.; Morishita, R.; Hashimoto, N. NF-kappaB is a key mediator of cerebral aneurysm formation. Circulation 2007, 116, 2830–2840. [Google Scholar] [CrossRef]
- Aoki, T.; Kataoka, H.; Ishibashi, R.; Nozaki, K.; Egashira, K.; Hashimoto, N. Impact of monocyte chemoattractant protein-1 deficiency on cerebral aneurysm formation. Stroke 2009, 40, 942–951. [Google Scholar] [CrossRef]
- Krischek, B.; Kasuya, H.; Tajima, A.; Akagawa, H.; Sasaki, T.; Yoneyama, T.; Ujiie, H.; Kubo, O.; Bonin, M.; Takakura, K.; et al. Network-based gene expression analysis of intracranial aneurysm tissue reveals role of antigen presenting cells. Neuroscience 2008, 154, 1398–1407. [Google Scholar] [CrossRef]
- Kosierkiewicz, T.A.; Factor, S.M.; Dickson, D.W. Immunocytochemical studies of atherosclerotic lesions of cerebral berry aneurysms. J. Neuropathol. Exp. Neurol. 1994, 53, 399–406. [Google Scholar] [CrossRef]
- Shi, C.; Awad, I.A.; Jafari, N.; Lin, S.; Du, P.; Hage, Z.A.; Shenkar, R.; Getch, C.C.; Bredel, M.; Batjer, H.H.; Bendok, B.R. Genomics of human intracranial aneurysm wall. Stroke 2009, 40, 1252–1261. [Google Scholar]
- Chitale, R.; Gonzalez, L.F.; Randazzo, C.; Dumont, A.S.; Tjoumakaris, S.; Rosenwasser, R.; Chalouhi, N.; Gordon, D.; Jabbour, P. Single center experience with pipeline stent: Feasibility, technique, and complications. Neurosurgery 2012, 71, 679–691; discussion 691. [Google Scholar] [CrossRef]
- Kanematsu, Y.; Kanematsu, M.; Kurihara, C.; Tada, Y.; Tsou, T.L.; van Rooijen, N.; Lawton, M.T.; Young, W.L.; Liang, E.I.; Nuki, Y.; et al. Critical roles of macrophages in the formation of intracranial aneurysm. Stroke 2011, 42, 173–178. [Google Scholar] [CrossRef]
- Aoki, T.; Kataoka, H.; Moriwaki, T.; Nozaki, K.; Hashimoto, N. Role of TIMP-1 and TIMP-2 in the progression of cerebral aneurysms. Stroke 2007, 38, 2337–2345. [Google Scholar] [CrossRef]
- Ishibashi, R.; Aoki, T.; Nishimura, M.; Hashimoto, N.; Miyamoto, S. Contribution of mast cells to cerebral aneurysm formation. Curr. Neurovasc. Res. 2010, 7, 113–124. [Google Scholar] [CrossRef]
- Kolega, J.; Gao, L.; Mandelbaum, M.; Mocco, J.; Siddiqui, A.H.; Natarajan, S.K.; Meng, H. Cellular and molecular responses of the basilar terminus to hemodynamics during intracranial aneurysm initiation in a rabbit model. J. Vasc. Res. 2011, 48, 429–442. [Google Scholar] [CrossRef]
- Aoki, T.; Kataoka, H.; Morimoto, M.; Nozaki, K.; Hashimoto, N. Macrophage-derived matrix metalloproteinase-2 and -9 promote the progression of cerebral aneurysms in rats. Stroke 2007, 38, 162–169. [Google Scholar] [CrossRef]
- Tronc, F.; Mallat, Z.; Lehoux, S.; Wassef, M.; Esposito, B.; Tedgui, A. Role of matrix metalloproteinases in blood flow-induced arterial enlargement: interaction with NO. Arterioscler. Thromb. Vasc. Biol. 2000, 20, E120–E126. [Google Scholar] [CrossRef]
- Jin, D.; Sheng, J.; Yang, X.; Gao, B. Matrix metalloproteinases and tissue inhibitors of metalloproteinases expression in human cerebral ruptured and unruptured aneurysm. Surg. Neurol. 2007, 68, S6–S11; discussion S16. [Google Scholar] [CrossRef]
- Hasan, D.; Chalouhi, N.; Jabbour, P.; Hashimoto, T. Macrophage imbalance (M1 vs. M2) and upregulation of mast cells in wall of ruptured human cerebral aneurysms: Preliminary results. J. Neuroinflammation 2012, 9, 222. [Google Scholar] [CrossRef]
- Chen, Y.; Fan, Y.; Poon, K.Y.; Achrol, A.S.; Lawton, M.T.; Zhu, Y.; McCulloch, C.E.; Hashimoto, T.; Lee, C.; Barbaro, N.M.; et al. MMP-9 expression is associated with leukocytic but not endothelial markers in brain arteriovenous malformations. Front. Biosci. 2006, 11, 3121–3128. [Google Scholar] [CrossRef]
- Chen, Y.; Pawlikowska, L.; Yao, J.S.; Shen, F.; Zhai, W.; Achrol, A.S.; Lawton, M.T.; Kwok, P.Y.; Yang, G.Y.; Young, W.L. Interleukin-6 involvement in brain arteriovenous malformations. Ann. Neurol. 2006, 59, 72–80. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, W.; Bollen, A.W.; Lawton, M.T.; Barbaro, N.M.; Dowd, C.F.; Hashimoto, T.; Yang, G.Y.; Young, W.L. Evidence of inflammatory cell involvement in brain arteriovenous malformations. Neurosurgery 2008, 62, 1340–1349; discussion 1349–1350. [Google Scholar] [CrossRef]
- Chen, G.; Zheng, M.; Shu, H.; Zhan, S.; Wang, H.; Zhou, D.; Zeng, S.; Tang, K.; Feng, L. Macrophage migration inhibitory factor reduces apoptosis in cerebral arteriovenous malformations. Neurosci. Lett. 2012, 508, 84–88. [Google Scholar] [CrossRef]
- Aziz, M.M.; Takagi, Y.; Hashimoto, N.; Miyamoto, S. Activation of nuclear factor kappaB in cerebral arteriovenous malformations. Neurosurgery 2010, 67, 1669–1679; discussion 1679–1680. [Google Scholar] [CrossRef]
- Pawlikowska, L.; Tran, M.N.; Achrol, A.S.; McCulloch, C.E.; Ha, C.; Lind, D.L.; Hashimoto, T.; Zaroff, J.; Lawton, M.T.; Marchuk, D.A.; et al. Polymorphisms in genes involved in inflammatory and angiogenic pathways and the risk of hemorrhagic presentation of brain arteriovenous malformations. Stroke 2004, 35, 2294–2300. [Google Scholar] [CrossRef]
- Achrol, A.S.; Pawlikowska, L.; McCulloch, C.E.; Poon, K.Y.; Ha, C.; Zaroff, J.G.; Johnston, S.C.; Lee, C.; Lawton, M.T.; et al. Tumor necrosis factor-alpha-238G>A promoter polymorphism is associated with increased risk of new hemorrhage in the natural course of patients with brain arteriovenous malformations. Stroke 2006, 37, 231–234. [Google Scholar] [CrossRef]
- Kim, H.; Hysi, P.G.; Pawlikowska, L.; Poon, A.; Burchard, E.G.; Zaroff, J.G.; Sidney, S.; Ko, N.U.; Achrol, A.S.; Lawton, M.T.; et al. Common variants in interleukin-1-Beta gene are associated with intracranial hemorrhage and susceptibility to brain arteriovenous malformation. Cerebrovasc. Dis. 2009, 27, 176–182. [Google Scholar] [CrossRef]
- Lu, M.; Cohen, M.H.; Rieves, D.; Pazdur, R. FDA report: Ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. Am. J. Hematol. 2010, 85, 315–319. [Google Scholar]
- Spinowitz, B.S.; Kausz, A.T.; Baptista, J.; Noble, S.D.; Sothinathan, R.; Bernardo, M.V.; Brenner, L.; Pereira, B.J. Ferumoxytol for treating iron deficiency anemia in CKD. J. Am. Soc. Nephrol. 2008, 19, 1599–1605. [Google Scholar] [CrossRef]
- Stabi, K.L.; Bendz, L.M. Ferumoxytol use as an intravenous contrast agent for magnetic resonance angiography. Ann. Pharmacother. 2011, 45, 1571–1575. [Google Scholar] [CrossRef]
- Bailie, G.R. Comparison of rates of reported adverse events associated with i.v. iron products in the United States. Am. J. Health Syst. Pharm. 2012, 69, 310–320. [Google Scholar] [CrossRef]
- Li, W.; Tutton, S.; Vu, A.T.; Pierchala, L.; Li, B.S.; Lewis, J.M.; Prasad, P.V.; Edelman, R.R. First-pass contrast-enhanced magnetic resonance angiography in humans using ferumoxytol, a novel ultrasmall superparamagnetic iron oxide (USPIO)-based blood pool agent. J. Magn. Reson. Imaging 2005, 21, 46–52. [Google Scholar] [CrossRef]
- Prince, M.R.; Zhang, H.L.; Chabra, S.G.; Jacobs, P.; Wang, Y. A pilot investigation of new superparamagnetic iron oxide (ferumoxytol) as a contrast agent for cardiovascular MRI. J. Xray Sci. Technol. 2003, 11, 231–240. [Google Scholar]
- Ersoy, H.; Jacobs, P.; Kent, C.K.; Prince, M.R. Blood pool MR angiography of aortic stent-graft endoleak. AJR Am. J. Roentgenol. 2004, 182, 1181–1186. [Google Scholar] [CrossRef]
- Li, W.; Salanitri, J.; Tutton, S.; Dunkle, E.E.; Schneider, J.R.; Caprini, J.A.; Pierchala, L.N.; Jacobs, P.M.; Edelman, R.R. Lower extremity deep venous thrombosis: Evaluation with ferumoxytol-enhanced MR imaging and dual-contrast mechanism--preliminary experience. Radiology 2007, 242, 873–881. [Google Scholar] [CrossRef]
- Neuwelt, E.A.; Varallyay, C.G.; Manninger, S.; Solymosi, D.; Haluska, M.; Hunt, M.A.; Nesbit, G.; Stevens, A.; Jerosch-Herold, M.; Jacobs, P.M.; et al. The potential of ferumoxytol nanoparticle magnetic resonance imaging, perfusion, and angiography in central nervous system malignancy: a pilot study. Neurosurgery 2007, 60, 601–611; discussion 611–612. [Google Scholar]
- Gahramanov, S.; Raslan, A.M.; Muldoon, L.L.; Hamilton, B.E.; Rooney, W.D.; Varallyay, C.G.; Njus, J.M.; Haluska, M.; Neuwelt, E.A. Potential for differentiation of pseudoprogression from true tumor progression with dynamic susceptibility-weighted contrast-enhanced magnetic resonance imaging using ferumoxytol vs. gadoteridol: A pilot study. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 514–523. [Google Scholar] [CrossRef]
- Dosa, E.; Guillaume, D.J.; Haluska, M.; Lacy, C.A.; Hamilton, B.E.; Njus, J.M.; Rooney, W.D.; Kraemer, D.F.; Muldoon, L.L.; et al. Magnetic resonance imaging of intracranial tumors: Intra-patient comparison of gadoteridol and ferumoxytol. Neuro. Oncol. 2011, 13, 251–260. [Google Scholar] [CrossRef]
- Dosa, E.; Tuladhar, S.; Muldoon, L.L.; Hamilton, B.E.; Rooney, W.D.; Neuwelt, E.A. MRI using ferumoxytol improves the visualization of central nervous system vascular malformations. Stroke 2011, 42, 1581–1588. [Google Scholar] [CrossRef]
- Harisinghani, M.; Ross, R.W.; Guimaraes, A.R.; Weissleder, R. Utility of a new bolus-injectable nanoparticle for clinical cancer staging. Neoplasia 2007, 9, 1160–1165. [Google Scholar] [CrossRef]
- Herborn, C.U.; Vogt, F.M.; Lauenstein, T.C.; Dirsch, O.; Corot, C.; Robert, P.; Ruehm, S.G. Magnetic resonance imaging of experimental atherosclerotic plaque: Comparison of two ultrasmall superparamagnetic particles of iron oxide. J. Magn. Reson. Imaging 2006, 24, 388–393. [Google Scholar] [CrossRef]
- Hyafil, F.; Laissy, J.P.; Mazighi, M.; Tchetche, D.; Louedec, L.; Adle-Biassette, H.; Chillon, S.; Henin, D.; Jacob, M.P.; Letourneur, D.; et al. Ferumoxtran-10-enhanced MRI of the hypercholesterolemic rabbit aorta: Relationship between signal loss and macrophage infiltration. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 176–181. [Google Scholar] [CrossRef]
- Ruehm, S.G.; Corot, C.; Vogt, P.; Kolb, S.; Debatin, J.F. Magnetic resonance imaging of atherosclerotic plaque with ultrasmall superparamagnetic particles of iron oxide in hyperlipidemic rabbits. Circulation 2001, 103, 415–422. [Google Scholar] [CrossRef]
- Trivedi, R.A.; JM, U.K.-I.; Graves, M.J.; Cross, J.J.; Horsley, J.; Goddard, M.J.; Skepper, J.N.; Quartey, G.; Warburton, E.; Joubert, I.; et al. In vivo detection of macrophages in human carotid atheroma: temporal dependence of ultrasmall superparamagnetic particles of iron oxide-enhanced MRI. Stroke 2004, 35, 1631–1635. [Google Scholar] [CrossRef]
- Trivedi, R.A.; JM, U.K.-I.; Graves, M.J.; Kirkpatrick, P.J.; Gillard, J.H. Noninvasive imaging of carotid plaque inflammation. Neurology 2004, 63, 187–188. [Google Scholar] [CrossRef]
- Weinstein, J.S.; Varallyay, C.G.; Dosa, E.; Gahramanov, S.; Hamilton, B.; Rooney, W.D.; Muldoon, L.L.; Neuwelt, E.A. Superparamagnetic iron oxide nanoparticles: Diagnostic magnetic resonance imaging and potential therapeutic applications in neurooncology and central nervous system inflammatory pathologies, a review. J. Cereb. Blood Flow Metab. 2010, 30, 15–35. [Google Scholar] [CrossRef]
- Yancy, A.D.; Olzinski, A.R.; Hu, T.C.; Lenhard, S.C.; Aravindhan, K.; Gruver, S.M.; Jacobs, P.M.; Willette, R.N.; Jucker, B.M. Differential uptake of ferumoxtran-10 and ferumoxytol, ultrasmall superparamagnetic iron oxide contrast agents in rabbit: Critical determinants of atherosclerotic plaque labeling. J. Magn. Reson. Imaging 2005, 21, 432–442. [Google Scholar] [CrossRef]
- Hasan, D.M.; Mahaney, K.B.; Magnotta, V.A.; Kung, D.K.; Lawton, M.T.; Hashimoto, T.; Winn, H.R.; Saloner, D.; Martin, A.; Gahramanov, S.; et al. Macrophage imaging within human cerebral aneurysms wall using ferumoxytol-enhanced MRI: a pilot study. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1032–1038. [Google Scholar] [CrossRef]
- Hasan, D.; Chalouhi, N.; Jabbour, P.; Dumont, A.S.; Kung, D.K.; Magnotta, V.A.; Young, W.L.; Hashimoto, T.; Winn, H.R.; Heistad, D. Early change in ferumoxytol-enhanced magnetic resonance imaging signal suggests unstable human cerebral aneurysm: a pilot study. Stroke 2012, 43, 3258–3265. [Google Scholar] [CrossRef]
- Hasan, D.M.; Chalouhi, N.; Jabbour, P.; Magnotta, V.A.; Kung, D.K.; Young, W.L. Imaging aspirin effect on macrophages in the wall of human cerebral aneurysms using ferumoxytol-enhanced MRI: Preliminary results. J. Neuroradiol. 2013, 40, 187–191. [Google Scholar] [CrossRef]
- Hasan, D.M.; Mahaney, K.B.; Brown, R.D., Jr.; Meissner, I.; Piepgras, D.G.; Huston, J.; Capuano, A.W.; Torner, J.C. Aspirin as a promising agent for decreasing incidence of cerebral aneurysm rupture. Stroke 2011, 42, 3156–3162. [Google Scholar] [CrossRef]
- Hasan, D.M.; Chalouhi, N.; Jabbour, P.; Dumont, A.S.; Kung, D.K.; Magnotta, V.A.; Young, W.L.; Hashimoto, T.; Richard Winn, H.; Heistad, D. Evidence that acetylsalicylic acid attenuates inflammation in the walls of human cerebral aneurysms: Preliminary results. J. Am. Heart Assoc. 2013, 2, e000019. [Google Scholar] [CrossRef]
- Chalouhi, N.; Witte, S.; Penn, D.L.; Soni, P.; Starke, R.M.; Jabbour, P.; Gonzalez, L.F.; Dumont, A.S.; Rosenwasser, R.; Tjoumakaris, S. Diagnostic yield of cerebral angiography in patients with ct-negative, lumbar puncture-positive subarachnoid hemorrhage. Neurosurgery 2013, 73, 282–288. [Google Scholar] [CrossRef]
- Matouk, C.C.; Mandell, D.M.; Gunel, M.; Bulsara, K.R.; Malhotra, A.; Hebert, R.; Johnson, M.H.; Mikulis, D.J.; Minja, F.J. Vessel wall magnetic resonance imaging identifies the site of rupture in patients with multiple intracranial aneurysms: Proof of principle. Neurosurgery 2013, 72, 492–496; discussion 496. [Google Scholar] [CrossRef]
- Dumont, A.S.; Dumont, R.J.; Chow, M.M.; Lin, C.L.; Calisaneller, T.; Ley, K.F.; Kassell, N.F.; Lee, K.S. Cerebral vasospasm after subarachnoid hemorrhage: Putative role of inflammation. Neurosurgery 2003, 53, 123–133; discussion 133–135. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chalouhi, N.; Jabbour, P.; Magnotta, V.; Hasan, D. The Emerging Role of Ferumoxytol-Enhanced MRI in the Management of Cerebrovascular Lesions. Molecules 2013, 18, 9670-9683. https://doi.org/10.3390/molecules18089670
Chalouhi N, Jabbour P, Magnotta V, Hasan D. The Emerging Role of Ferumoxytol-Enhanced MRI in the Management of Cerebrovascular Lesions. Molecules. 2013; 18(8):9670-9683. https://doi.org/10.3390/molecules18089670
Chicago/Turabian StyleChalouhi, Nohra, Pascal Jabbour, Vincent Magnotta, and David Hasan. 2013. "The Emerging Role of Ferumoxytol-Enhanced MRI in the Management of Cerebrovascular Lesions" Molecules 18, no. 8: 9670-9683. https://doi.org/10.3390/molecules18089670



