Liposomal Encapsulation Enhances In Vivo Near Infrared Imaging of Exposed Phosphatidylserine in a Mouse Glioma Model
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characteristics of PGN-L-800CW
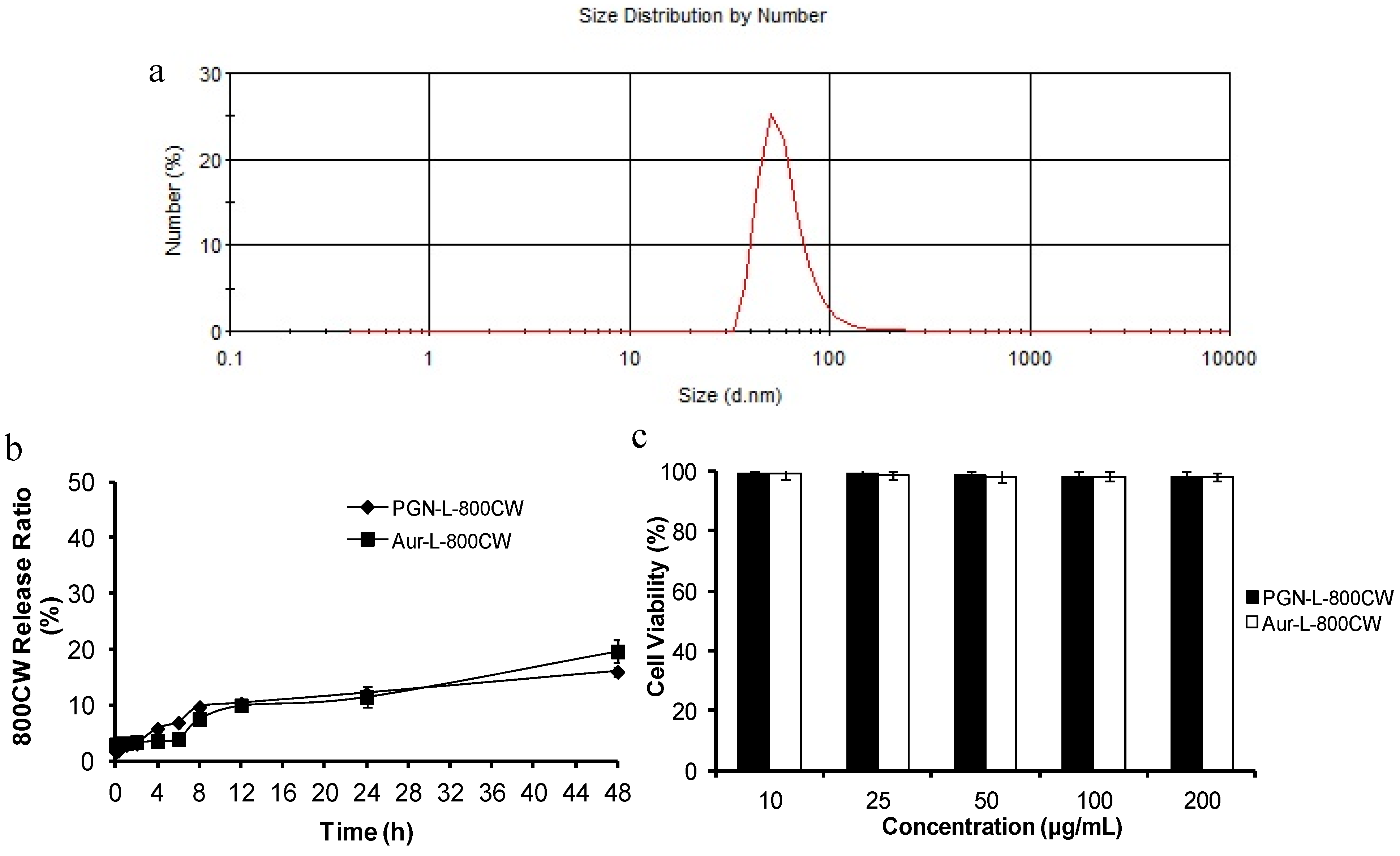
| a. Encapsulation efficiency of 800CW in the liposomes | ||
| Liposomes | 800CW (%) | |
| PGN-L-800CW | 55.61 ± 2.21 | |
| Aur-L-800CW | 51.16 ± 3.39 | |
| b. Modifying efficiency of antibody on the liposomes | ||
| Liposomes | Modifying rate (%) | |
| PGN-L-800CW | 82.66 ± 9.98 | |
| Aur-L-800CW | 80.33 ± 10.01 | |
| c. Size and electrical potential | ||
| Liposomes | Mean size (nm) | Zeta potential (mV) |
| PGN-L-800CW | 65.59 ± 3.98 | −3.52 ± 0.46 |
| Aur-L-800CW | 67.71 ± 4.06 | −3.49 ± 0.87 |
2.2. In Vitro PS-Targeting Specificity of PGN-L-800CW
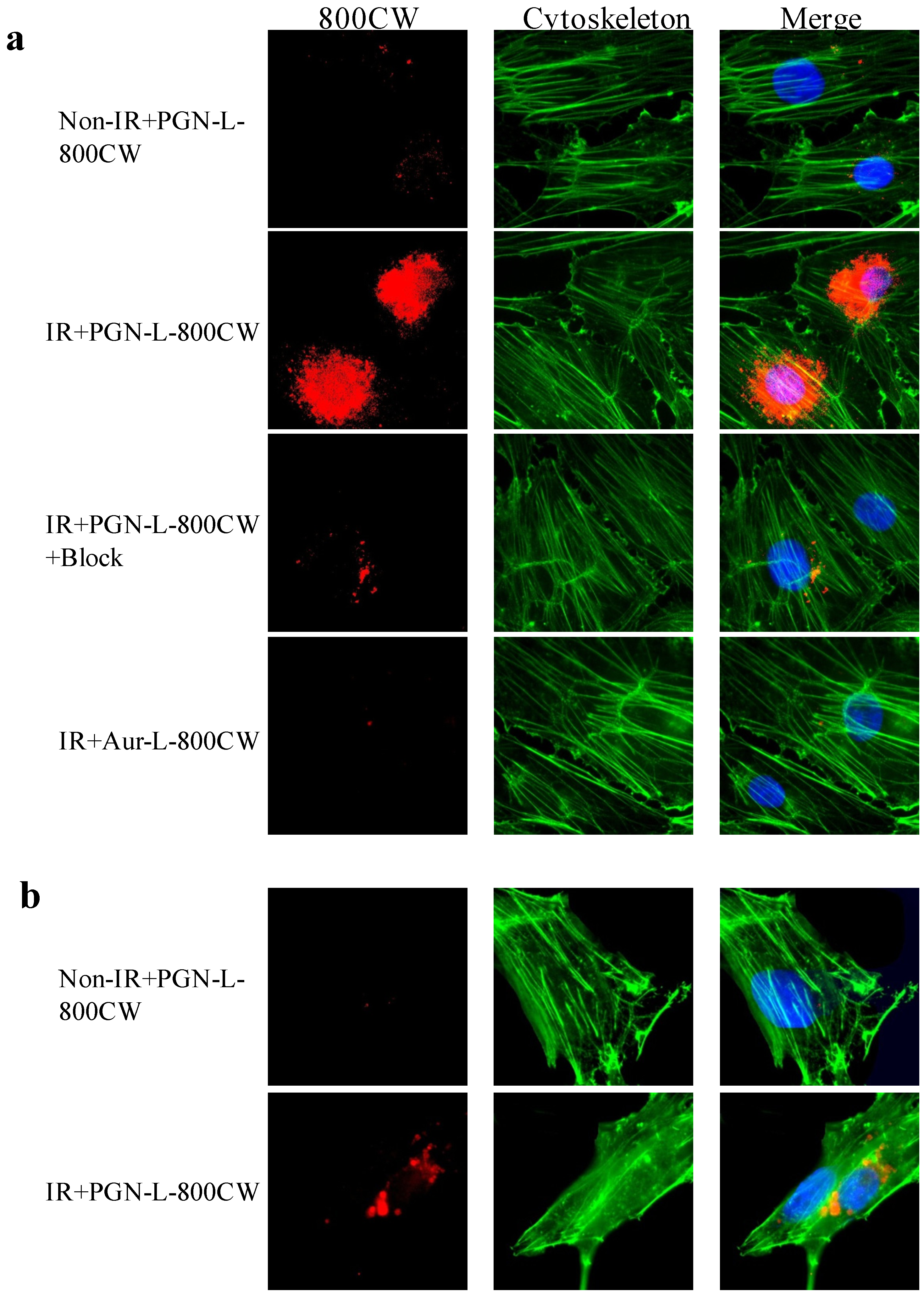
2.3.In vivo Longitudinal NIR Optical Imaging of PGN-L-800CW in U87 Glioma Xenografts
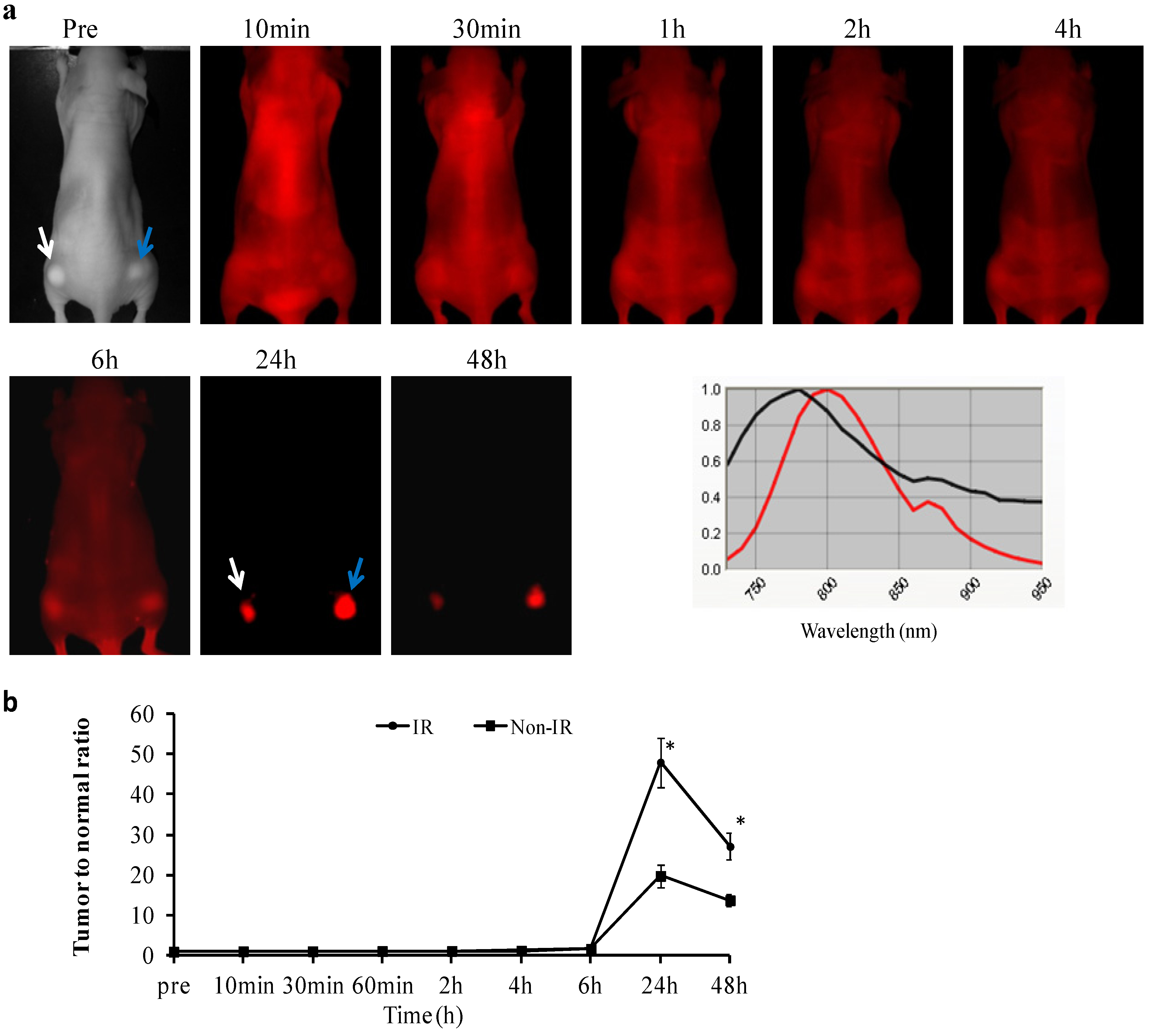
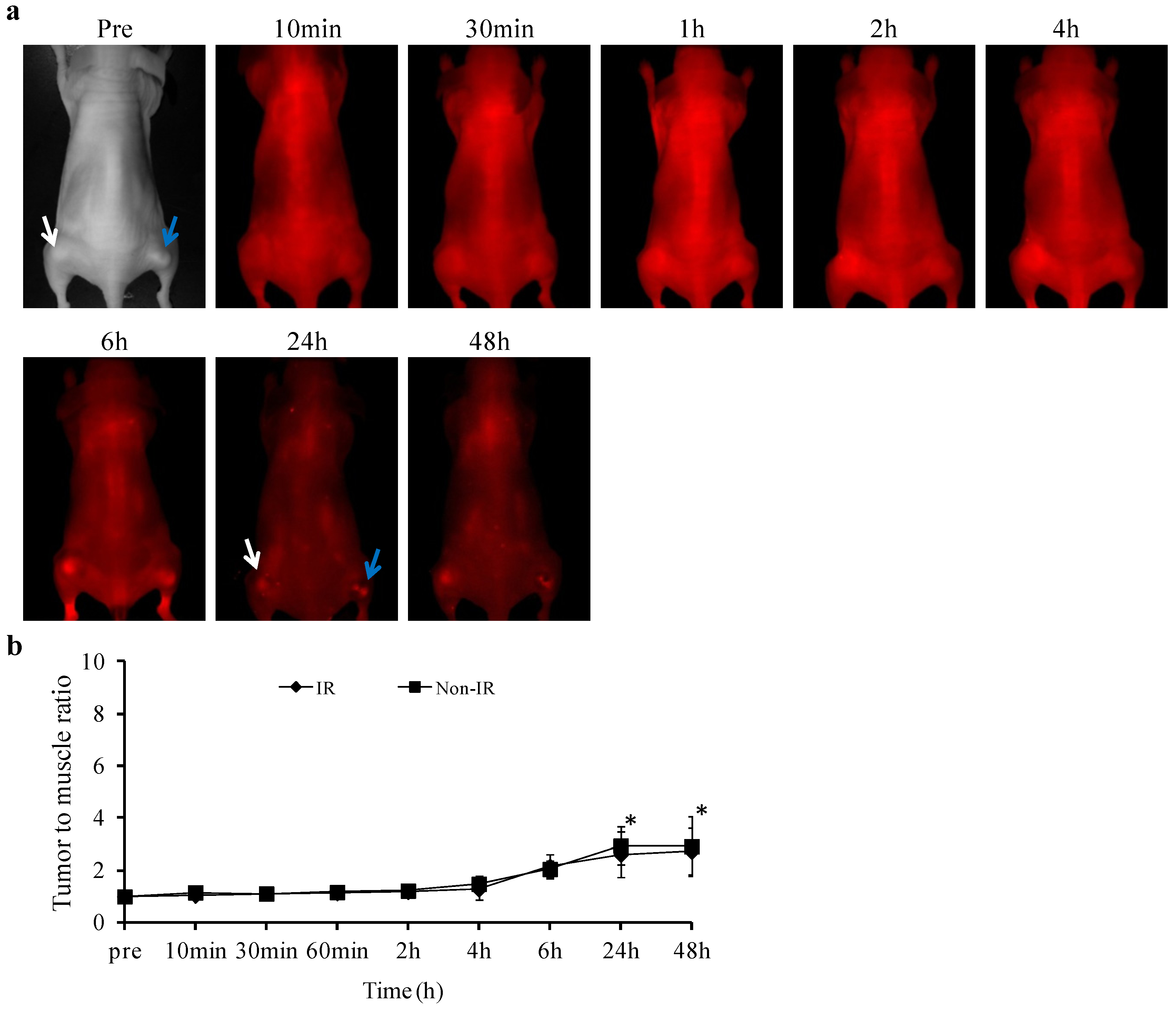
2.4. Ex vivo Imaging of Biodistribution of PGN-L-800CW
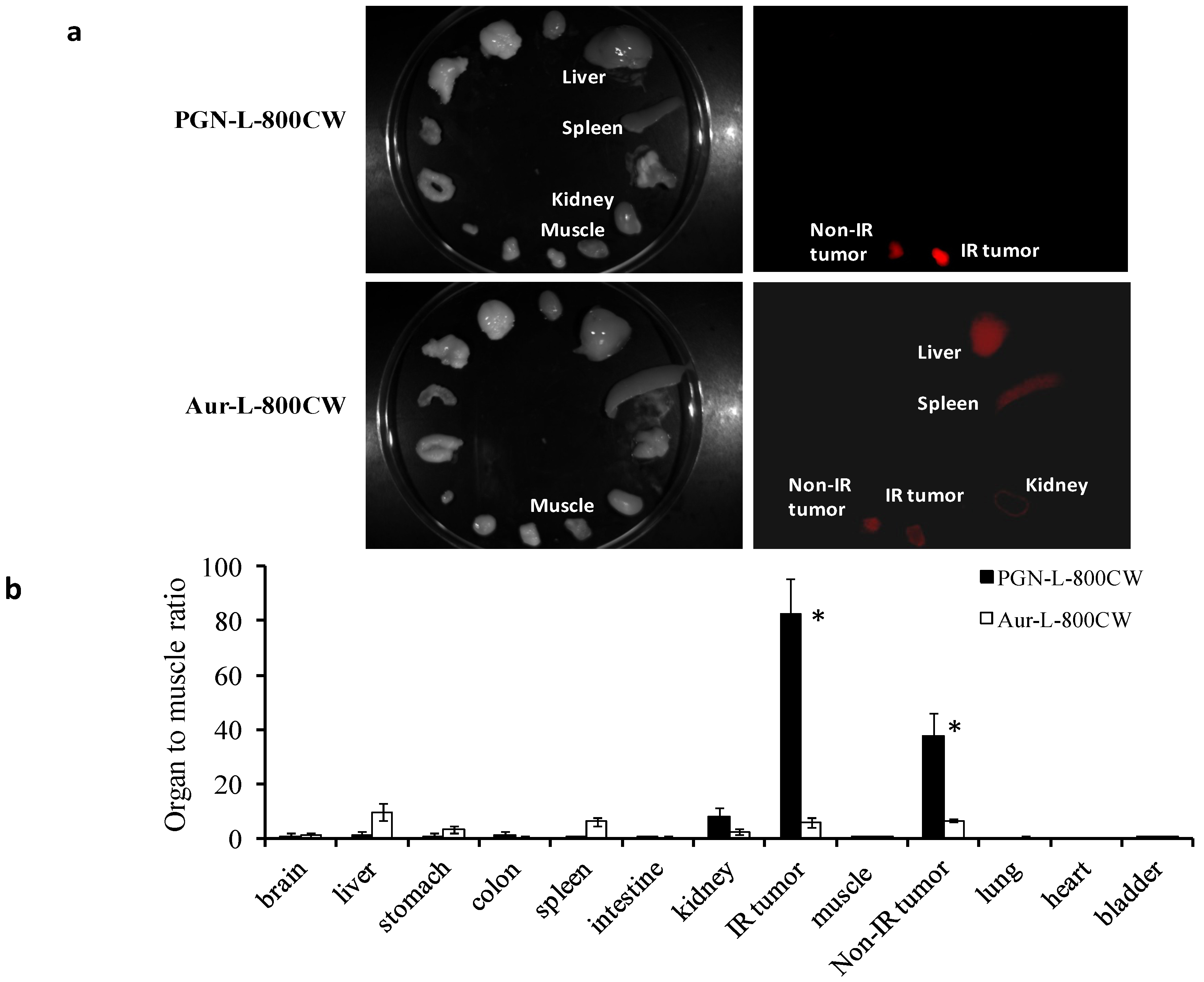
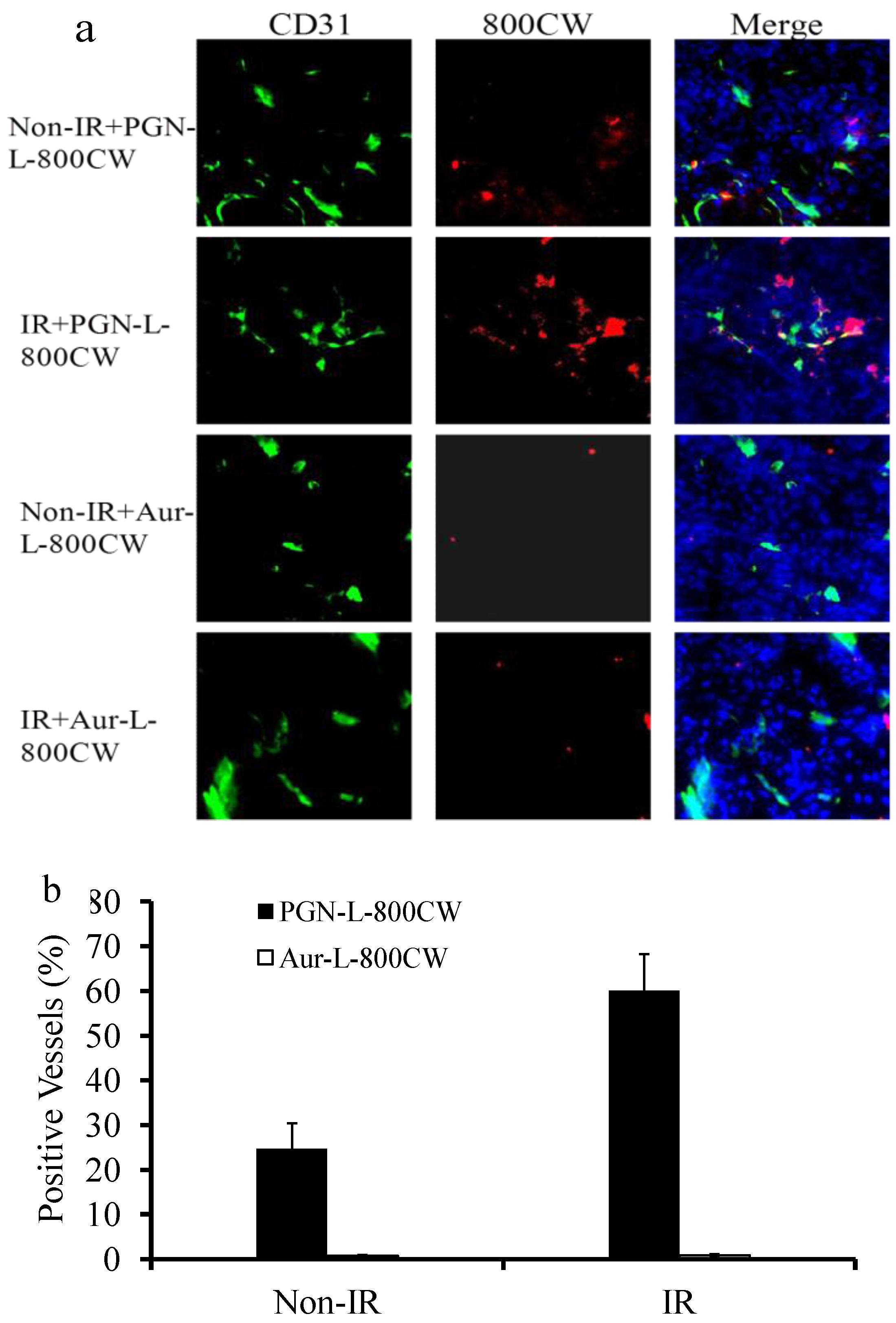
2.5. Histological and Immunohistochemical Analysis
3. Experimental
3.1. Preparation and Characterization of PS-Targeted Liposomes Loaded with Optical Imaging Contrast
3.2. Stability of PGN-L-800CW
3.3. In Vitro Cytotoxicity
3.4. In Vitro Binding Specificity
3.5. Glioma Xenografts in Nude Mice
3.6. In Vivo Near Infrared Optical Imaging of Tumor Targeted PGN-L-800CW
3.7. En Vivo Optical Imaging of Biodistribution
3.8. Immunohistochemical Studies
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Boyle, E.M., Jr.; Pohlman, T.H.; Cornejo, C.J.; Verrier, E.D. Endothelial cell injury in cardiovascular surgery: Ischemia-reperfusion. Ann. Thorac. Surg. 1996, 62, 1868–1875. [Google Scholar] [CrossRef]
- Tait, J.F. Imaging of apoptosis. J. Nucl. Med. 2008, 49, 1573–1576. [Google Scholar] [CrossRef]
- Blankenberg, F.G. In vivo detection of apoptosis. J. Nucl. Med. 2008, 49, 81S–95S. [Google Scholar] [CrossRef]
- Ran, S.; Downes, A.; Thorpe, P.E. Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer Res. 2002, 62, 6132–6140. [Google Scholar]
- Ran, S.; Thorpe, P.E. Phosphatidylserine is a marker of tumor vasculature and a potential target for cancer imaging and therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 54, 1479–1484. [Google Scholar] [CrossRef]
- Hammill, A.K.; Uhr, J.W.; Scheuermann, R.H. Annexin V staining due to loss of membrane asymmetry can be reversible and precede commitment to apoptotic death. Exp. Cell. Res. 1999, 251, 16–21. [Google Scholar] [CrossRef]
- Mirnikjoo, B.; Balasubramanian, K.; Schroit, A.J. Mobilization of lysosomal calcium regulates the externalization of phosphatidylserine during apoptosis. J. Biol. Chem. 2009, 284, 6918–6923. [Google Scholar]
- Balasubramanian, K.; Schroit, A.J. Aminophospholipid asymmetry: A matter of life and death. Annu. Rev. Physiol. 2003, 65, 701–734. [Google Scholar] [CrossRef]
- He, J.; Luster, T.A.; Thorpe, P.E. Radiation-enhanced vascular targeting of human lung cancers in mice with a monoclonal antibody that binds anionic phospholipids. Clin. Cancer Res. 2007, 13, 5211–5218. [Google Scholar] [CrossRef]
- Zhao, D.; Stafford, J.H.; Zhou, H.; Thorpe, P.E. Near-infrared optical imaging of exposed phosphatidylserine in a mouse glioma model. Transl. Oncol. 2011, 4, 355–364. [Google Scholar]
- Nie, S. Understanding and overcoming major barriers in cancer nanomedicine. Nanomedicine 2010, 5, 523–528. [Google Scholar] [CrossRef]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Pastorino, F.; Brignole, C.; Marimpietri, D.; Sapra, P.; Moase, E.H.; Allen, T.M.; Ponzoni, M. Doxorubicin-loaded Fab’ fragments of anti-disialoganglioside immunoliposomes selectively inhibit the growth and dissemination of human neuroblastoma in nude mice. Cancer Res. 2003, 63, 86–92. [Google Scholar]
- Klibanov, A.L.; Maruyama, K.; Torchilin, V.P.; Huang, L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990, 268, 235–237. [Google Scholar] [CrossRef]
- Gabizon, A.A. Pegylated liposomal doxorubicin: Metamorphosis of an old drug into a new form of chemotherapy. Cancer Invest. 2001, 19, 424–436. [Google Scholar] [CrossRef]
- Luster, T.A.; He, J.; Huang, X.; Maiti, S.N.; Schroit, A.J.; de Groot, P.G.; Thorpe, P.E. Plasma protein beta-2-glycoprotein 1 mediates interaction between the anti-tumor monoclonal antibody 3G4 and anionic phospholipids on endothelial cells. J. Biol. Chem. 2006, 281, 29863–29871. [Google Scholar]
- Stafford, J.H.; Thorpe, P.E. Increased exposure of phosphatidylethanolamine on the surface of tumor vascular endothelium. Neoplasia 2011, 13, 299–308. [Google Scholar]
- Soman, N.R.; Baldwin, S.L.; Hu, G.; Marsh, J.N.; Lanza, G.M.; Heuser, J.E.; Arbeit, J.M.; Wickline, S.A.; Schlesinger, P.H. Molecularly targeted nanocarriers deliver the cytolytic peptide melittin specifically to tumor cells in mice, reducing tumor growth. J. Clin. Invest. 2009, 119, 2830–2842. [Google Scholar] [CrossRef]
- Kirpotin, D.B.; Drummond, D.C.; Shao, Y.; Shalaby, M.R.; Hong, K.; Nielsen, U.B.; Marks, J.D.; Benz, C.C.; Park, J.W. Antibody targeting of long-circulating lipidic nanoparticles does not increase tumor localization but does increase internalization in animal models. Cancer Res. 2006, 66, 6732–6740. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nat. Nanotechnol. 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Andresen, T.L.; Jensen, S.S.; Jorgensen, K. Advanced strategies in liposomal cancer therapy: Problems and prospects of active and tumor specific drug release. Prog. Lipid Res. 2005, 44, 68–97. [Google Scholar] [CrossRef]
- Park, J.W.; Kirpotin, D.B.; Hong, K.; Shalaby, R.; Shao, Y.; Nielsen, U.B.; Marks, J.D.; Papahadjopoulos, D.; Benz, C.C. Tumor targeting using anti-her2 immunoliposomes. J. Control. Release 2001, 74, 95–113. [Google Scholar] [CrossRef]
- Sapra, P.; Allen, T.M. Internalizing antibodies are necessary for improved therapeutic efficacy of antibody-targeted liposomal drugs. Cancer Res. 2002, 62, 7190–7194. [Google Scholar]
- Cai, W.; Chen, X. Multimodality molecular imaging of tumor angiogenesis. J. Nucl. Med. 2008, 49, 113S–128S. [Google Scholar] [CrossRef]
- Sipkins, D.A.; Cheresh, D.A.; Kazemi, M.R.; Nevin, L.M.; Bednarski, M.D.; Li, K.C. Detection of tumor angiogenesis in vivo by alphaVbeta3-targeted magnetic resonance imaging. Nat. Med. 1998, 4, 623–626. [Google Scholar] [CrossRef]
- Khemtong, C.; Kessinger, C.W.; Ren, J.; Bey, E.A.; Yang, S.G.; Guthi, J.S.; Boothman, D.A.; Sherry, A.D.; Gao, J. In vivo off-resonance saturation magnetic resonance imaging of alphavbeta3-targeted superparamagnetic nanoparticles. Cancer Res. 2009, 69, 1651–1658. [Google Scholar] [CrossRef]
- Andree, H.A.; Reutelingsperger, C.P.; Hauptmann, R.; Hemker, H.C.; Hermens, W.T.; Willems, G.M. Binding of vascular anticoagulant alpha (VAC alpha) to planar phospholipid bilayers. J. Biol. Chem. 1990, 265, 4923–4928. [Google Scholar]
- Schlaepfer, D.D.; Mehlman, T.; Burgess, W.H.; Haigler, H.T. Structural and functional characterization of endonexin II, a calcium- and phospholipid-binding protein. Proc. Natl. Acad. Sci. USA 1987, 84, 6078–6082. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, H.J.; Yu, Y.; Zhang, Y.; Li, R.J.; Ju, R.J.; Wang, X.X.; Sun, M.G.; Shi, J.F.; Lu, W.L. Mitochondrial targeting liposomes incorporating daunorubicin and quinacrine for treatment of relapsed breast cancer arising from cancer stem cells. Biomaterials 2012, 33, 565–582. [Google Scholar] [CrossRef]
- Zhou, H.; Luby-Phelps, K.; Mickey, B.E.; Habib, A.A.; Mason, R.P.; Zhao, D. Dynamic near-infrared optical imaging of 2-deoxyglucose uptake by intracranial glioma of athymic mice. PLoS One 2009, 4, e8051. [Google Scholar]
- Samples Availability: Samples of the compounds are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhang, L.; Zhao, D. Liposomal Encapsulation Enhances In Vivo Near Infrared Imaging of Exposed Phosphatidylserine in a Mouse Glioma Model. Molecules 2013, 18, 14613-14628. https://doi.org/10.3390/molecules181214613
Zhang L, Zhao D. Liposomal Encapsulation Enhances In Vivo Near Infrared Imaging of Exposed Phosphatidylserine in a Mouse Glioma Model. Molecules. 2013; 18(12):14613-14628. https://doi.org/10.3390/molecules181214613
Chicago/Turabian StyleZhang, Liang, and Dawen Zhao. 2013. "Liposomal Encapsulation Enhances In Vivo Near Infrared Imaging of Exposed Phosphatidylserine in a Mouse Glioma Model" Molecules 18, no. 12: 14613-14628. https://doi.org/10.3390/molecules181214613




