The Synthesis and the Biological Evaluation of New Thiazolidin-4-one Derivatives Containing a Xanthine Moiety
Abstract
:1. Introduction

2. Results and Discussion
2.1. Chemistry

2.2. Biological Evaluation
2.2.1. Ferric Reducing Power
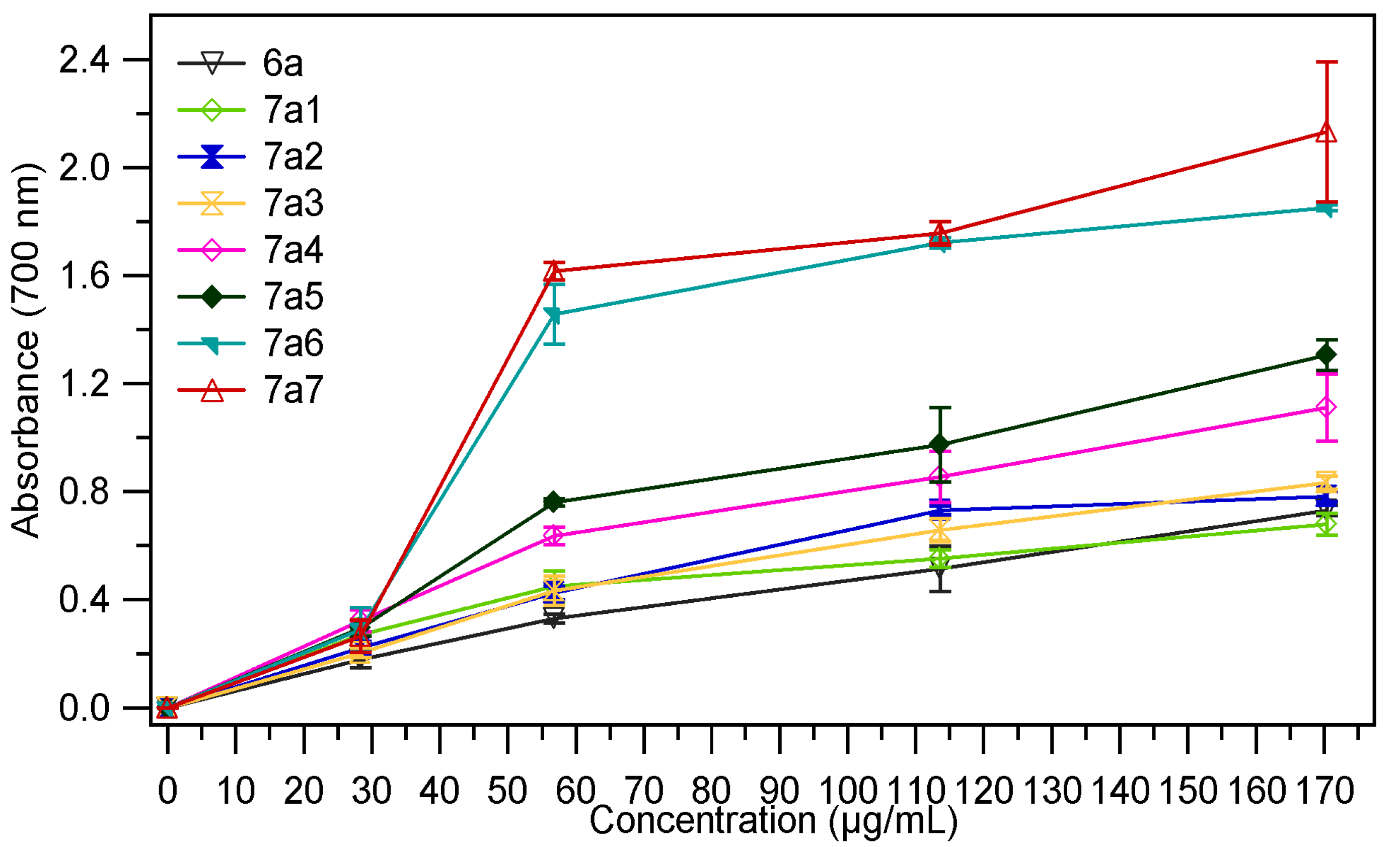
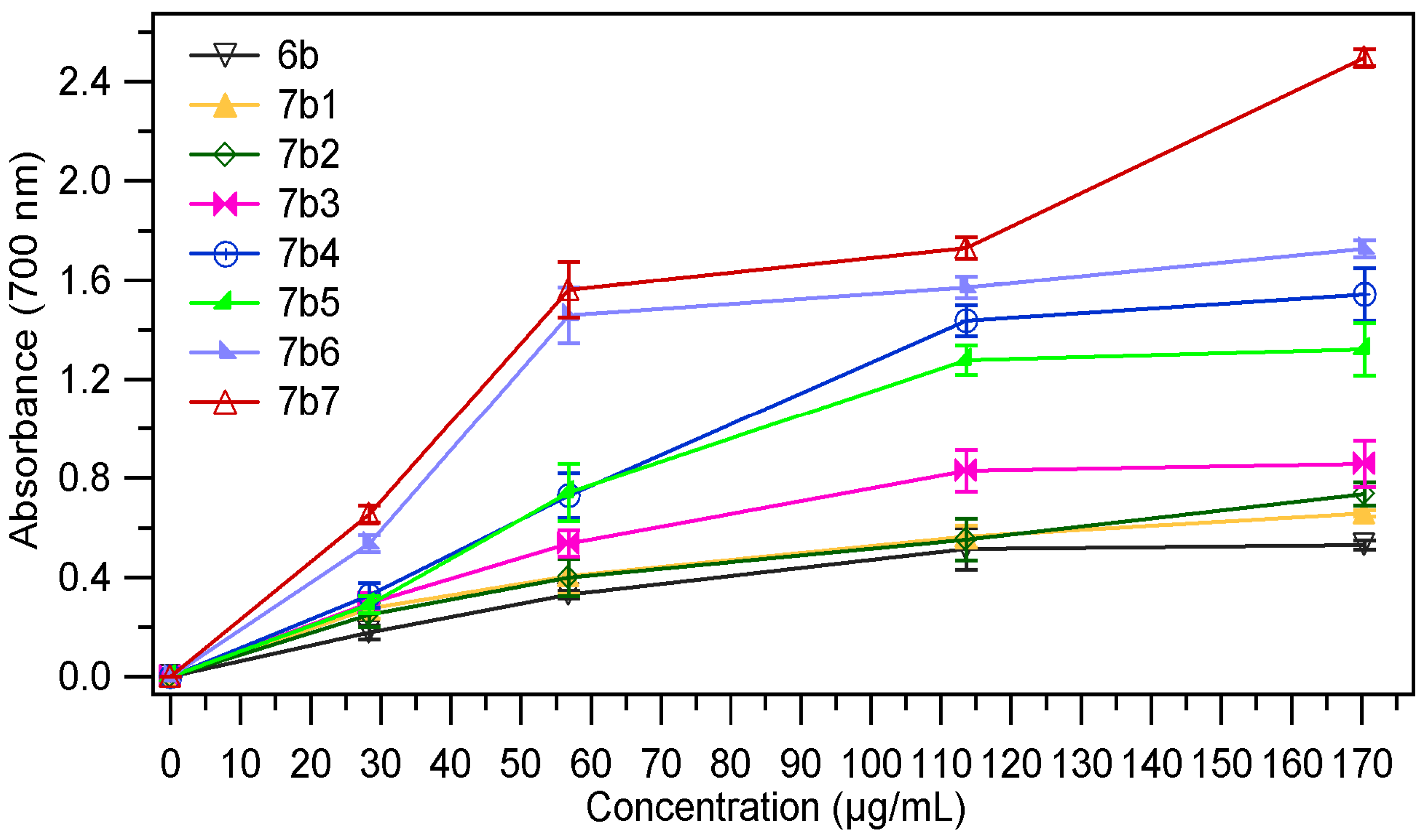
| Sample | EC50 mg/mL | Sample | EC50 mg/mL |
|---|---|---|---|
| 6a | 0.137 ± 0.0036 | 6b | 0.155 ± 0.0038 |
| 7a1 | 0.109 ± 0.0014 | 7b1 | 0.109 ± 0.0045 |
| 7a2 | 0.097 ± 0.0018 | 7b2 | 0.102 ± 0.0024 |
| 7a3 | 0.055 ± 0.0036 | 7b3 | 0.046 ± 0.0015 |
| 7a4 | 0.096 ± 0.0023 | 7b4 | 0.066 ± 0.0017 |
| 7a5 | 0.033 ± 0.0019 | 7b5 | 0.027 ± 0.0013 |
| 7a6 | 0.033 ± 0.0012 | 7b6 | 0.023 ± 0.0011 |
| 7a7 | 0.058 ± 0.0041 | 7b7 | 0.041 ± 0.0010 |
| AA | 0.0075 ± 0.0002 |
2.2.2. Total Antioxidant Activity

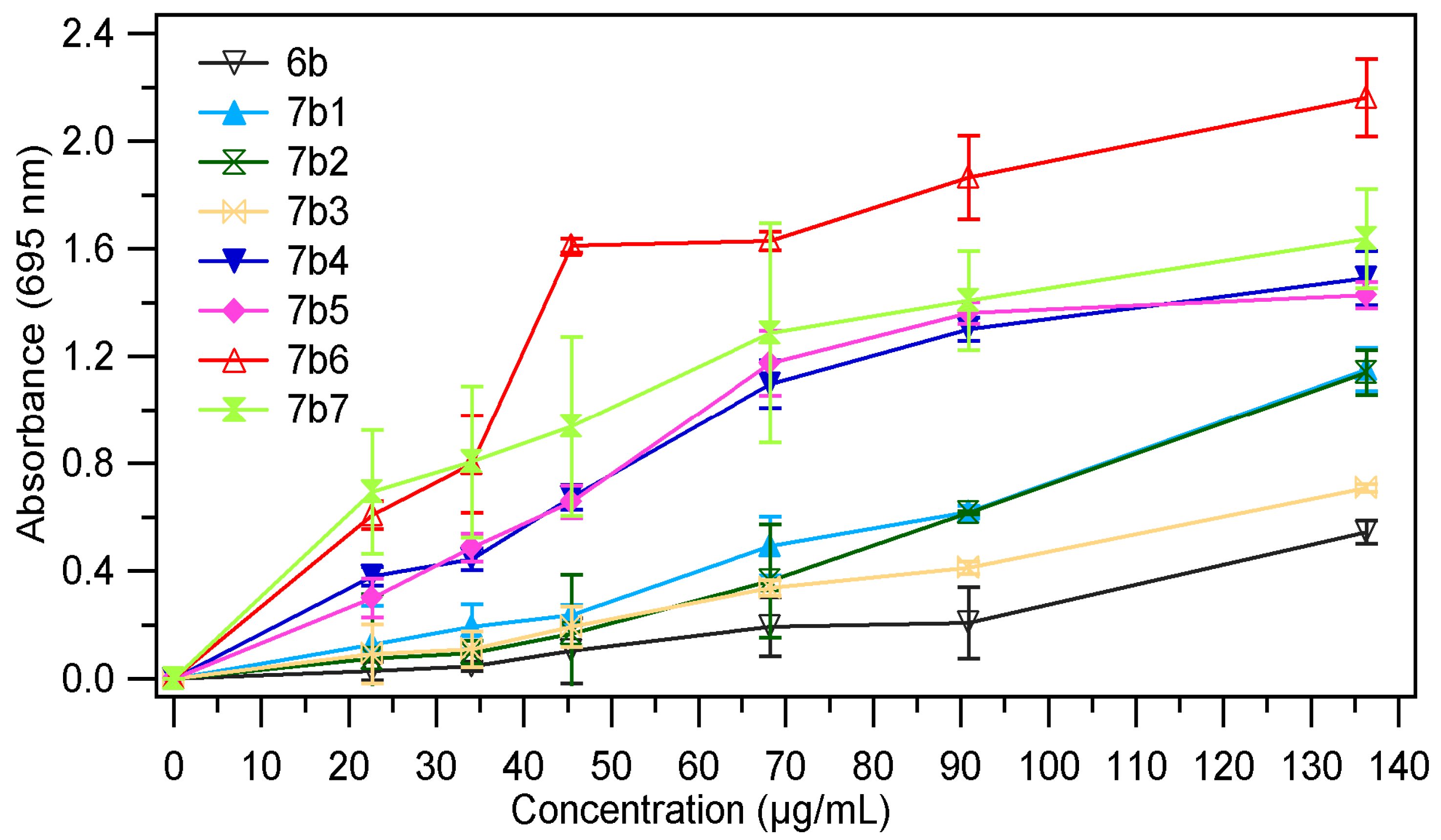
| Sample | EC50 mg/mL | Sample | EC50 mg/mL |
|---|---|---|---|
| 6a | 0.133 ± 0.0045 | 6b | 0.124 ± 0.0009 |
| 7a1 | 0.105 ± 0.0025 | 7b1 | 0.090 ± 0.0018 |
| 7a2 | 0.099 ± 0.0038 | 7b2 | 0.092 ± 0.0008 |
| 7a3 | 0.048 ± 0.0023 | 7b3 | 0.043 ± 0.0026 |
| 7a4 | 0.076 ± 0.0025 | 7b4 | 0.099 ± 0.0024 |
| 7a5 | 0.025 ± 0.0012 | 7b5 | 0.022 ± 0.0013 |
| 7a6 | 0.033 ± 0.0014 | 7b6 | 0.026 ± 0.0018 |
| 7a7 | 0.037 ± 0.0007 | 7b7 | 0.043 ± 0.0028 |
| AA | 0.0067 ± 0.0003 |
2.2.3. DPPH Radical Scavenging Assay
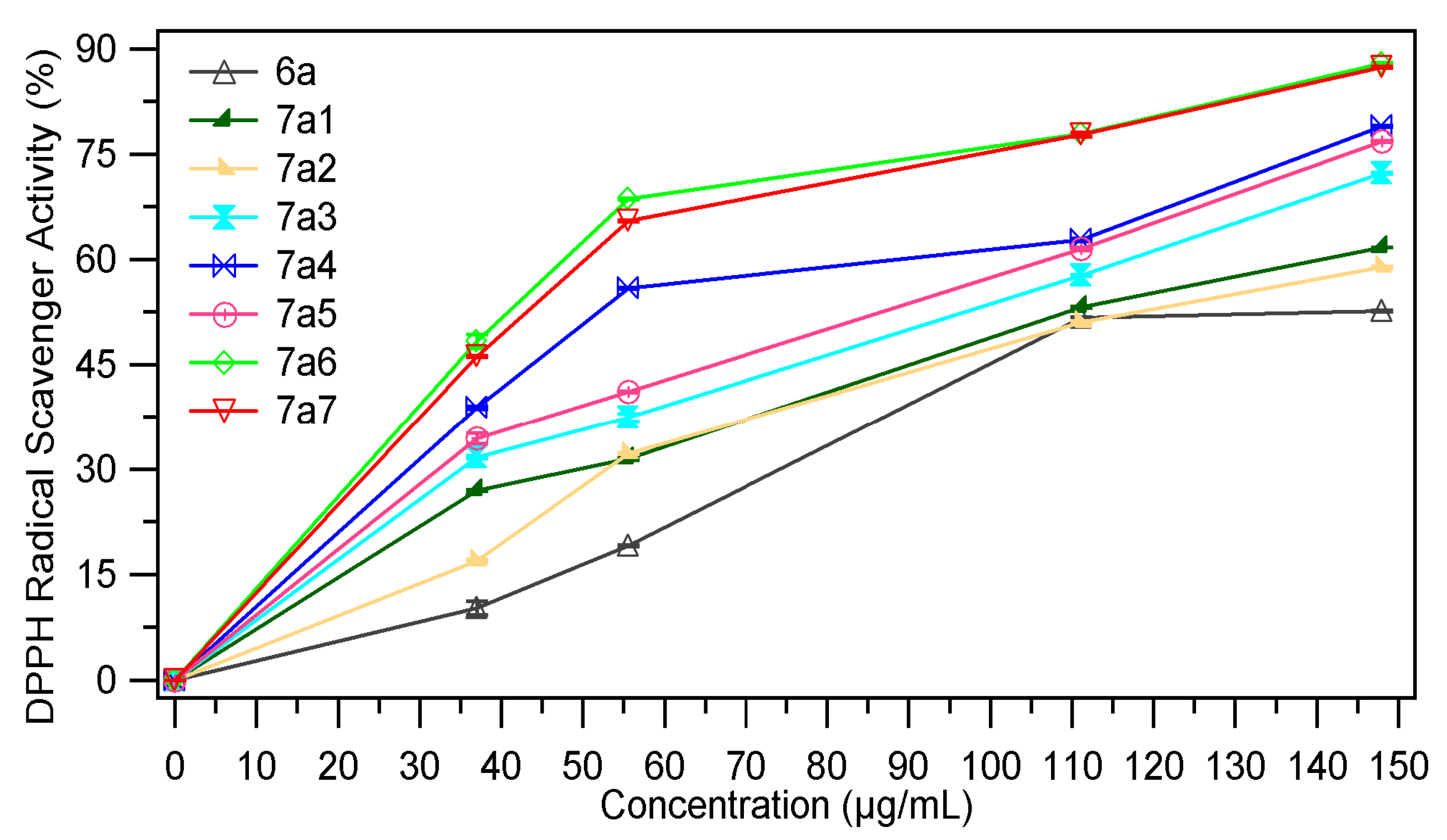
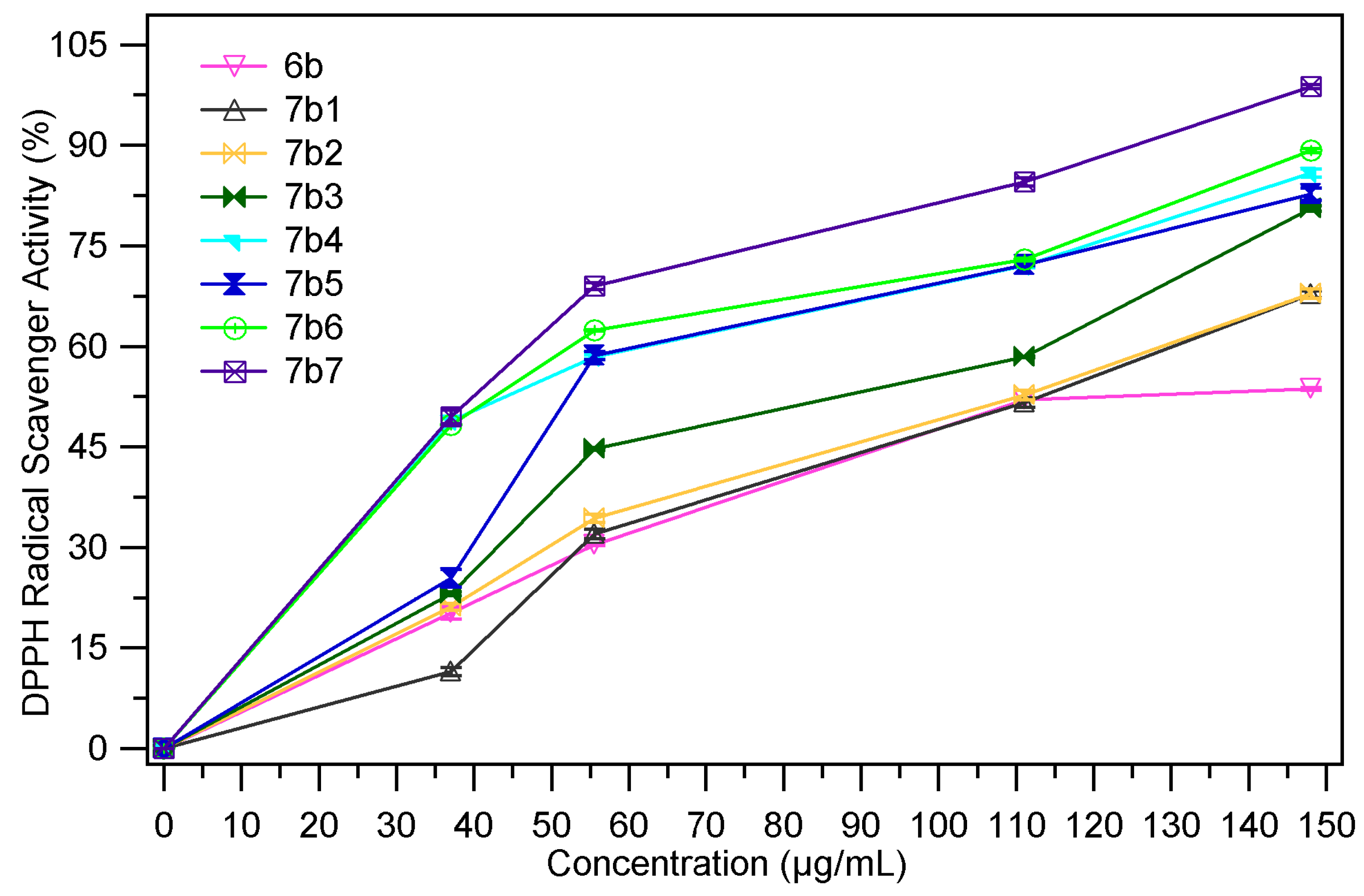
| Sample | EC50 mg/mL | Sample | EC50 mg/mL |
|---|---|---|---|
| 6a | 0.136 ± 0.0006 | 6b | 0.127 ± 0.0013 |
| 7a1 | 0.094 ± 0.0013 | 7b1 | 0.106 ± 0.0011 |
| 7a2 | 0.107 ± 0.0018 | 7b2 | 0.101 ± 0.0044 |
| 7a3 | 0.066 ± 0.0011 | 7b3 | 0.049 ± 0.0006 |
| 7a4 | 0.077 ± 0.0005 | 7b4 | 0.085 ± 0.0017 |
| 7a5 | 0.038 ± 0.0044 | 7b5 | 0.038 ± 0.0024 |
| 7a6 | 0.039 ± 0.0008 | 7b6 | 0.037 ± 0.0037 |
| 7a7 | 0.048 ± 0.0001 | 7b7 | 0.038 ± 0.0014 |
| AA | 0.0065 ± 0.0031 |
2.2.4. ABTS Radical Scavenging Assay
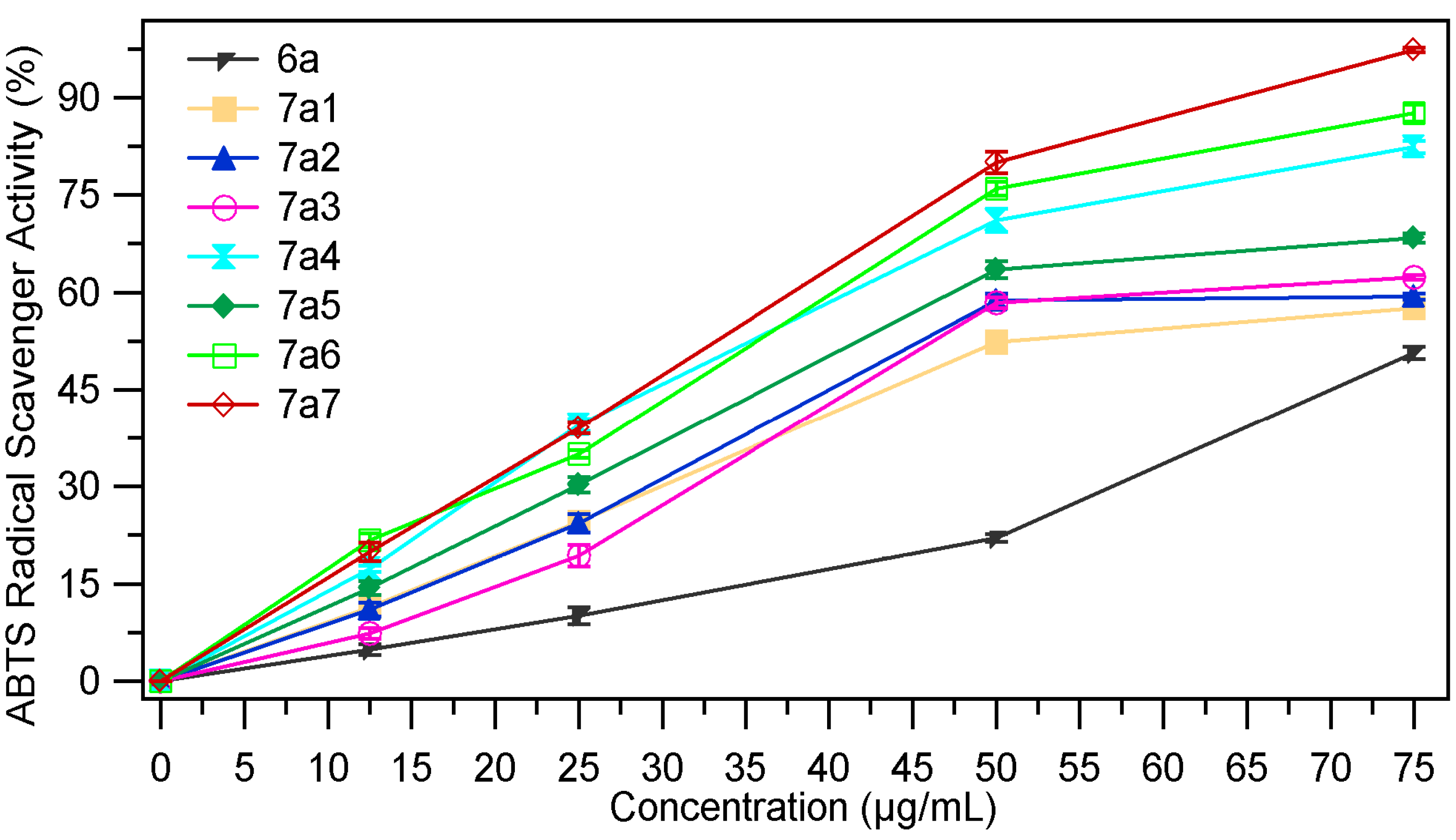
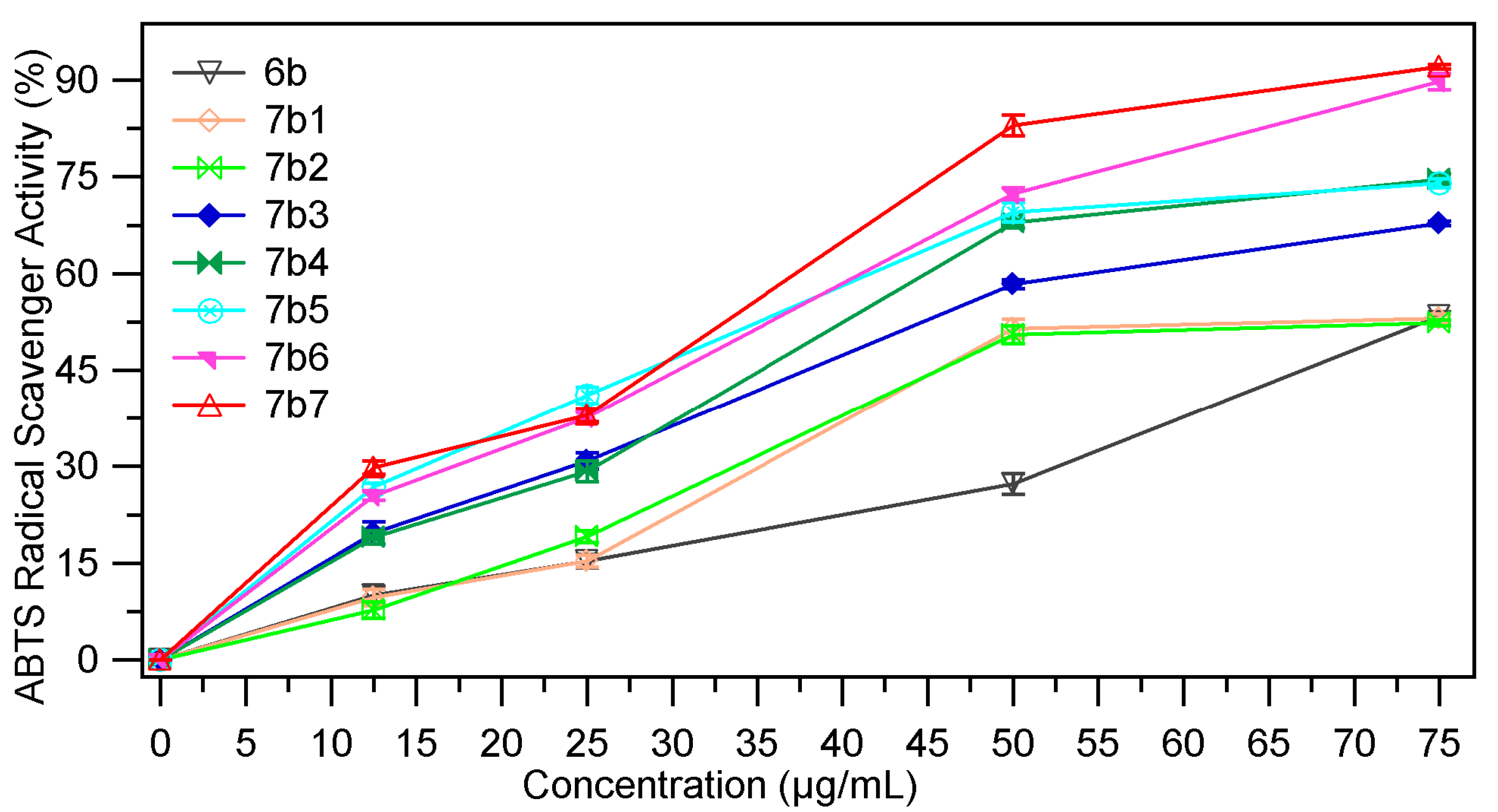
| Sample | EC50 mg/mL | Sample | EC50 mg/mL |
|---|---|---|---|
| 6a | 0.074 ± 0.0039 | 6b | 0.069 ± 0.0031 |
| 7a1 | 0.047 ± 0.0032 | 7b1 | 0.048 ± 0.0014 |
| 7a2 | 0.049 ± 0.0026 | 7b2 | 0.041 ± 0.0041 |
| 7a3 | 0.037 ± 0.0015 | 7b3 | 0.031 ± 0.0035 |
| 7a4 | 0.043 ± 0.0028 | 7b4 | 0.040 ± 0.0029 |
| 7a5 | 0.027 ± 0.0017 | 7b5 | 0.024 ± 0.0047 |
| 7a6 | 0.025 ± 0.0012 | 7b6 | 0.021 ± 0.0041 |
| 7a7 | 0.036 ± 0.0098 | 7b7 | 0.030 ± 0.0017 |
| AA | 0.0055 ± 0.0024 |
3. Experimental
3.1. General Procedures
3.2. Synthetic Procedures
3.2.1. General Procedure for the Preparation of 1-[2-(1,3-Dimethylxanthin-7-yl)acetyl]-4-(4-R-phenyl)-thiosemicarbazides 5a, 5b
3.2.2. General Procedure for the Preparation of 2-{2-[2-(1,3-Dimethylxanthin-7-yl)acetyl]hydrazono}-3-(4-R-phenyl) Thiazolidin-4-ones (R = H, Cl) 6a, 6b
3.2.3. General Procedure for the Preparation of 2-{2-[2-(1,3-Dimethylxanthin-7-yl)acetyl]hydrazono}-3-(4-R1-phenyl-5-(R2-benzyliden)thiazolidin-4-ones 7a1-7, 7b1-7
3.3. Biological Evaluation
3.3.1. Antioxidant Assays
3.3.2. Ferric Reducing Power
3.3.3. Total Antioxidant Activity
3.3.4. DPPH Radical Scavenging Assay
3.3.5. ABTS Radical Scavenging Assay
3.3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Lesyk, R.; Zimenkovsky, B. 4-Thiazolidones: Current status and perspectives for modern organic and medicinal chemistry. Curr. Org. Chem. 2004, 8, 1547–1578. [Google Scholar] [CrossRef]
- Vicini, P.; Geronikaki, A.; Anastasia, K.; Incerti, M.; Zani, F. Synthesis and antimicrobial activity of novel 2-thiazolimino-5-arylidene-4-thiazolidinones. Bioor. Med. Chem. 2006, 14, 3859–3864. [Google Scholar] [CrossRef]
- Alagawadi, K.R.; Alegaon, S.G. Synthesis, characterization and antimicrobial activity evaluation of new 2,4-thiazolidinediones bearing imidazo [2,1-b][1,3,4]thiadiazole moiety. Arab. J. Chem. 2011, 4, 465–472. [Google Scholar] [CrossRef]
- Prasad, D.; Kumar, A.; Kumar, S.P.; Nath, M. Design, synthesis and antimicrobial evaluation of novel 2-aryl-thiazolidin-4-one derivatives. Org. Med. Chem. Lett. 2011, 14, 2–7. [Google Scholar]
- Bhagat, T.M.; Swamy, D.K.; Badne, S.G.; Kuberkar, S.V. Synthesis and antibacterial activity of 4-thiazolidinone containing benzothiazolyl moiety. Rasayan J. Chem. 2011, 4, 24–28. [Google Scholar]
- Sharma, R.; Samadhiya, P.; Srivastava, S.; Srivastava, S. Synthesis of some new thiazolidine derivatives and their biological significance. Latv. J. Chem. 2012, 50, 296–307. [Google Scholar]
- Amin, K.M.; Rahman, D.E.; Al-Eryani, Y.A. Synthesis and preliminary evaluation of some substituted coumarins as anticonvulsant agents. Bioorg. Med. Chem. 2008, 16, 5377–5388. [Google Scholar] [CrossRef]
- Parameswaran, M.; Thengungal, K.R.; Subbuchettiar, G. Synthesis of coumarin heterocyclic derivatives with antioxidant activity and in vitro cytotoxic activity against tumour cells. Acta Pharm. 2009, 59, 159–170. [Google Scholar] [CrossRef]
- Isloor, A.M.; Sunil, D.; Shetty, P.; Malladi, S.; Pai, K.S.R.; Maliyakki, N. Synthesis, characterization, anticancer, and antioxidant activity of some new thiazolidin-4-ones in MCF-7 cells. Med. Chem. Res. 2013, 22, 758–767. [Google Scholar] [CrossRef]
- Liu, Y.; Jing, F.; Xu, Y.; Xie, Y.; Shi, F.; Fangm, H.; Lim, M.; Xu, W. Design, synthesis and biological activity of thiazolidine-4-carboxylic acid derivatives as novel influenza neuraminidase inhibitors. Bioorg. Med. Chem. 2011, 19, 2342–2348. [Google Scholar] [CrossRef]
- Balzarini, J.; Orzeszko, B.; Maurin, J.K.; Orzeszko, A. Synthesis and anti-HIV studies of 2-adamantyl-substituted thiazolidin-4-ones. Eur. J. Med. Chem. 2007, 42, 993–1003. [Google Scholar] [CrossRef]
- Barros, C.D.; Amato, A.A.; de Oliveira, T.B.; Iannini, K.B.; Silva, A.L.; Silva, T.G., Leitem; Hernandes, M.Z.; de Lima, M.C.A.; Galdino, S.L.; et al. Synthesis and anti-inflammatory activity of new arylidene-thiazolidine-2,4-diones as PPARgamma ligands. Bioorg. Med. Chem. 2010, 18, 3805–3811. [Google Scholar] [CrossRef]
- Vazzana, I.; Terranova, E.; Mattioli, F.; Sparatore, F. Aromatic schiff bases and 2,3-disubstituted-1,3-thiazolidin-4-one derivatives as antiinflammatory agents. ARKIVOC 2004, v, 364–374. [Google Scholar]
- Kim, H.; Haluzik, M.; Gavrilova, O.; Yakar, S.; Portas, J.; Sun, H.; Pajvani, U.B.; Scherer, P.E.; LeRoith, D. Thiazolidinediones improve insulin sensitivity in adipose tissue and reduce the hyperlipidemia without affecting the hyperglycaemia in a transgenic model of type 2 diabetes. Diabetologia 2004, 47, 2215–2225. [Google Scholar] [CrossRef]
- Iqbal, A.K.M.; Khan, A.Y.; Kalashetti, M.B.; Belavagi, N.S.; Gong, Y.D.; Khazi, I.A. Synthesis, hypoglycemic and hypolipidemic activities of novel thiazolidinedione derivatives containing thiazole/triazole/oxadiazole ring. Eur. J. Med. Chem. 2012, 53, 308–315. [Google Scholar] [CrossRef]
- Da Costa Leite, L.F.; Veras Mourão, R.H.; de Lima Mdo, C.; Galdino, S.L.; Hernandes, M.Z.; de Assis Rocha Neves, F.; Vidal, S.; Barbe, J.; da Rocha Pitta, I. Synthesis, biological evaluation and molecular modeling studies of arylidene-thiazolidinediones with potential hypoglycemic and hypolipidemic activities. Eur. J. Med. Chem. 2007, 42, 1263–1271. [Google Scholar] [CrossRef]
- Kishore, A.; Nampurath, G.K.; Mathew, S.P.; Zachariah, R.T.; Potu, B.K.; Rao, M.S.; Valiathan, M.; Chamallamudi, M.R. Antidiabetic effect through islet cell protection in streptozotocin diabetes: A preliminary assessment of two thiazolidin-4-ones in Swiss albino mice. Chem. Biol. Interact. 2009, 177, 242–266. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P. Effects of thiazolidinediones and sulfonylureas in patients with diabetes. Diabetes Technol. Ther. 2010, 12, 491–501. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A consensus statement from the international diabetes federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Hughes, T.E. Emerging therapies for metabolic diseases—The focus is on diabetes and obesity. Curr. Opin. Chem. Biol. 2009, 13, 1–6. [Google Scholar] [CrossRef]
- Kushwaha, S.P.; Rawat, S.K.; Abhishekm, P.K.; Tripathim , K. Coupling antioxidant and antidiabetic assests of 2,4-thiazolidinedione derivatives. Asian J. Pharm. 2011, 1, 71–73. [Google Scholar]
- El Nezhawy, A.O.H.; Ramla, M.M.; Khalifa, N.M.; Abdulla, M.M. Synthesis and antioxidant activity of some thiazolidin-4-one derivatives. Monatsh. Chem. 2009, 140, 531–539. [Google Scholar] [CrossRef]
- Lupaşcu, F.; Dragostin, O.M.; Apotrosoaei, M.; Pânzariu, A.; Lupaşcu, D.; Vasile, C.; Profire, L. Synthesis and evaluation of antioxidant activity of some new benzylidene-thiazolidine-xanthine derivatives. Rev. Med. Chir. 2013, 117, 244–249. [Google Scholar]
- Kusano, C.; Ferrari, B. Total Antioxidant Capacity: A biomarker in biomedical and nutritional studies. J. Cell Mol. Biol. 2008, 7, 1–15. [Google Scholar]
- Schwartz, S.L. Treatment of Elderly Patients With Type 2 Diabetes mellitus: A systematic review of the benefits and risks of dipeptidyl peptidase-4 inhibitors. Am. J. Geriatr. Pharmacother. 2010, 5, 405–418. [Google Scholar] [CrossRef]
- Baetta, R.; Corsini, A. Pharmacology of dipeptidyl peptidase-4 inhibitors: Similarities and differences. Drugs 2011, 71, 1441–1467. [Google Scholar] [CrossRef]
- Bhat, V.B.; Madyastha, K.M. Antioxidant and radical scavenging properties of 8-oxo derivatives of xanthine drugs pentoxifylline and lisofylline. Biochem. Biophys. Res. Commun. 2001, 288, 1212–1217. [Google Scholar] [CrossRef]
- Kaptanoglu, E.; Solaroglu, I.; Akbiyik, F.; Demirpence, E.; Ergungor, M.E. The antioxidant effect of aminophylline in rat brain and spinal cord homogenates. Turk. Neurosurg. 2003, 13, 9–13. [Google Scholar]
- Lupascu, F.; Dash, M.; Samal, S.K; Lupusoru, C.E.; Lupusoru, P.; Profire, L.; Dubruel, P. Delivery of hydrophobic antioxidant drugs via chitosan: An approach to enhance their oral bioavailability. J. Mater. Sci. Mater. Med. 2013. submitted. [Google Scholar]
- Lungu Apetrei, C.; Tuchilus, C.; Aprotosoaie, A.C. Chemical, antioxidant and antimicrobial investigations of pinus cembra L. bark and needles. Molecules 2011, 16, 7773–7788. [Google Scholar] [CrossRef]
- Cacic, M.; Molnar, M.; Sarkanj, B.; Has-Schon, E.; Rajkovic, V. Synthesis and antioxidant activity of some new coumarinyl-1,3-Thiazolidine-4-ones. Molecules 2010, 15, 6795–6809. [Google Scholar] [CrossRef]
- Shih, M-H.; Ke, F.-Y. Syntheses and evaluation of antioxidant activity of sydnonyl substituted thiazolidinone and thiazoline derivatives. Bioorg. Med. Chem. 2004, 12, 4633–4643. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 7a1–7, 7b1–7 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lupascu, F.G.; Dragostin, O.M.; Foia, L.; Lupascu, D.; Profire, L. The Synthesis and the Biological Evaluation of New Thiazolidin-4-one Derivatives Containing a Xanthine Moiety. Molecules 2013, 18, 9684-9703. https://doi.org/10.3390/molecules18089684
Lupascu FG, Dragostin OM, Foia L, Lupascu D, Profire L. The Synthesis and the Biological Evaluation of New Thiazolidin-4-one Derivatives Containing a Xanthine Moiety. Molecules. 2013; 18(8):9684-9703. https://doi.org/10.3390/molecules18089684
Chicago/Turabian StyleLupascu, Florentina Geanina, Oana Maria Dragostin, Liliana Foia, Dan Lupascu, and Lenuta Profire. 2013. "The Synthesis and the Biological Evaluation of New Thiazolidin-4-one Derivatives Containing a Xanthine Moiety" Molecules 18, no. 8: 9684-9703. https://doi.org/10.3390/molecules18089684
APA StyleLupascu, F. G., Dragostin, O. M., Foia, L., Lupascu, D., & Profire, L. (2013). The Synthesis and the Biological Evaluation of New Thiazolidin-4-one Derivatives Containing a Xanthine Moiety. Molecules, 18(8), 9684-9703. https://doi.org/10.3390/molecules18089684





