Biosynthesis of Panaxynol and Panaxydol in Panax ginseng
Abstract
:1. Introduction
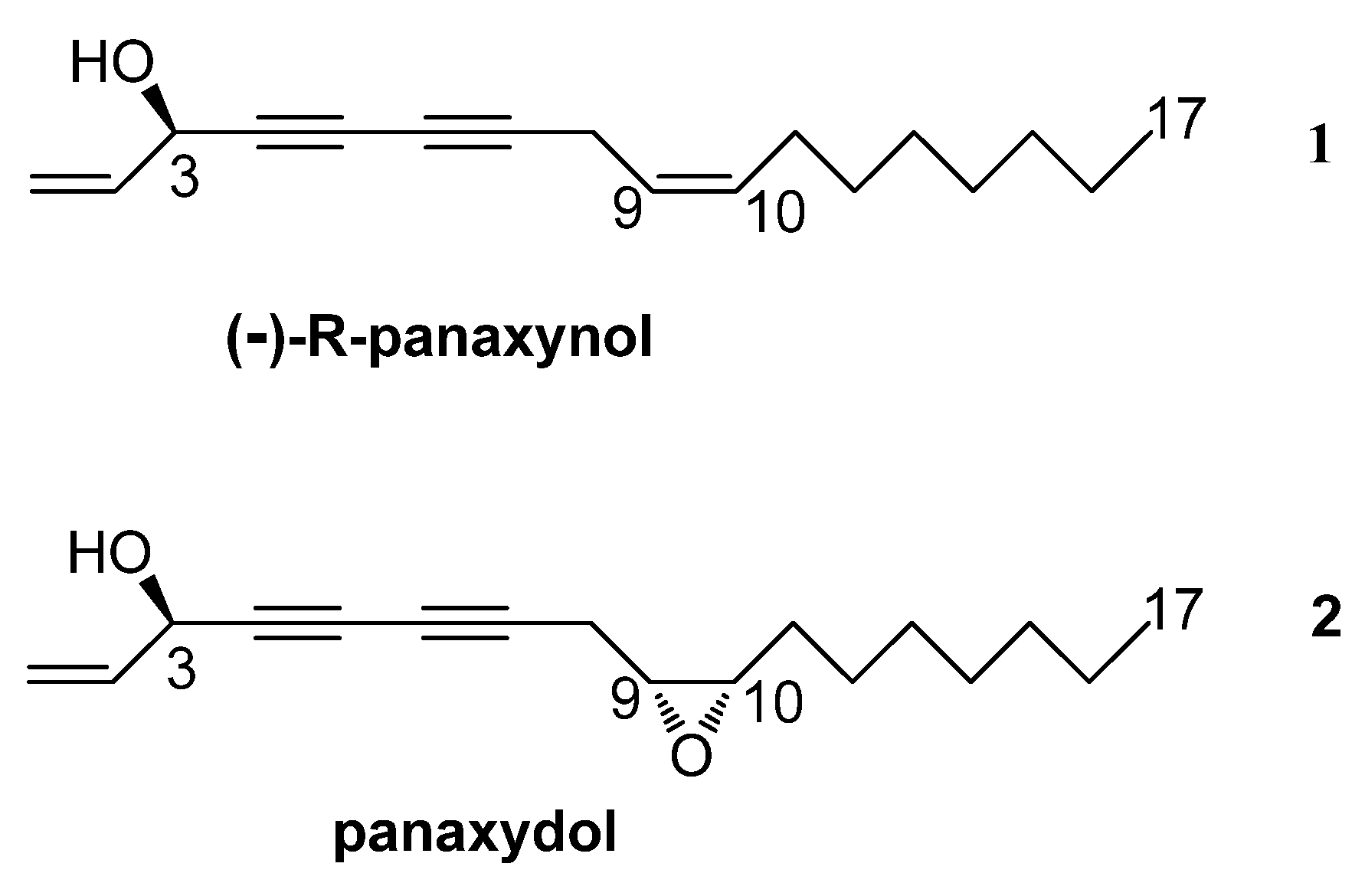
2. Results and Discussion
2.1. Isolation and Identification of Panaxynol (1) and Panaxydol (2)
2.2. Biosynthesis of Panaxynol and Panaxydol in P. ginseng
2.2.1. In planta Experiments with 13CO2

| Atom | 1H (δ) | JHH (Hz) | Atom | 13C (δ) | JCC (Hz) |
|---|---|---|---|---|---|
| 1a 1b 2 3 8a 8b 9 10 11 12 13 14 15 16 17 | 5.26 5.47 5.95 4.92 2.39 2.70 3.14 2.96 1.45–1.55 1.25–1.40 1.25–1.40 1.25–1.40 1.25–1.40 1.25–1.40 0.89 | 1H, ddd; 10.2, 1.5, 1.0 1H, ddd; 17.1, 1.5, 1.0 1H, ddd;16.8, 10.2, 5.4 1H, br d; 5.2 1H, ddd;17.7, 7.1, 0.9 1H, ddd; 17.7, 5.5, 0.9 1H, ddd; 7.1, 5.5, 4.2 1H, br td; 6.1, 4.1 2H, m 10H, m 10H, m 10H, m 10H, m 10H, m 3H, br t; 6.8 | 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 | 117.4 136.1 63.7 75.0 71.0 66.4 77.4 19.6 54.4 57.1 27.7 26.6 29.6 29.3 31.9 22.8 14.3 | 70.9 70.9 75.8 75.8 156.9 157.0 nd * 68.2 29.9 29.9 33.8 33.9 45.7 45.3 34.5 34.5 - |
| Atom | 1H (δ) | JHH (Hz) | Atom | 13C (δ) | JCC (Hz) |
|---|---|---|---|---|---|
| 1a 1b 2 3 8 9 10 11 12 13 14 15 16 17 | 5.47 5.24 5.94 4.91 3.03 5.39 5.52 2.03 1.24–1.39 1.24–1.39 1.24–1.39 1.24–1.39 1.24–1.39 0.88 | 1H, ddd; 17.1, 1.2 1H, ddd; 10.1, 1.2 1H, ddd; 17.0, 10.2, 5.4 3H, t; 5.9 2H, d; 6.9 1H, ddddd; 11.3, 6.1, 1.6 1H, ddddd; 9.8, 8.1, 1.7 2H, ddd; 10.7, 6.9 10H, m 10H, m 10H, m 10H, m 10H, m 3H, t; 6.9 | 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 | 117.2 136.3 63.7 74.3 71.5 64.1 80.5 17.8 122.0 133.3 27.4 29.3 29.3 29.3 31.7 22.8 14.3 | 71.0 70.7 76.0 76.0 156.6 nd * 68.1 67.8 71.4 71.3 34.0 34.0 34.6 34.6 34.5 34.5 - |
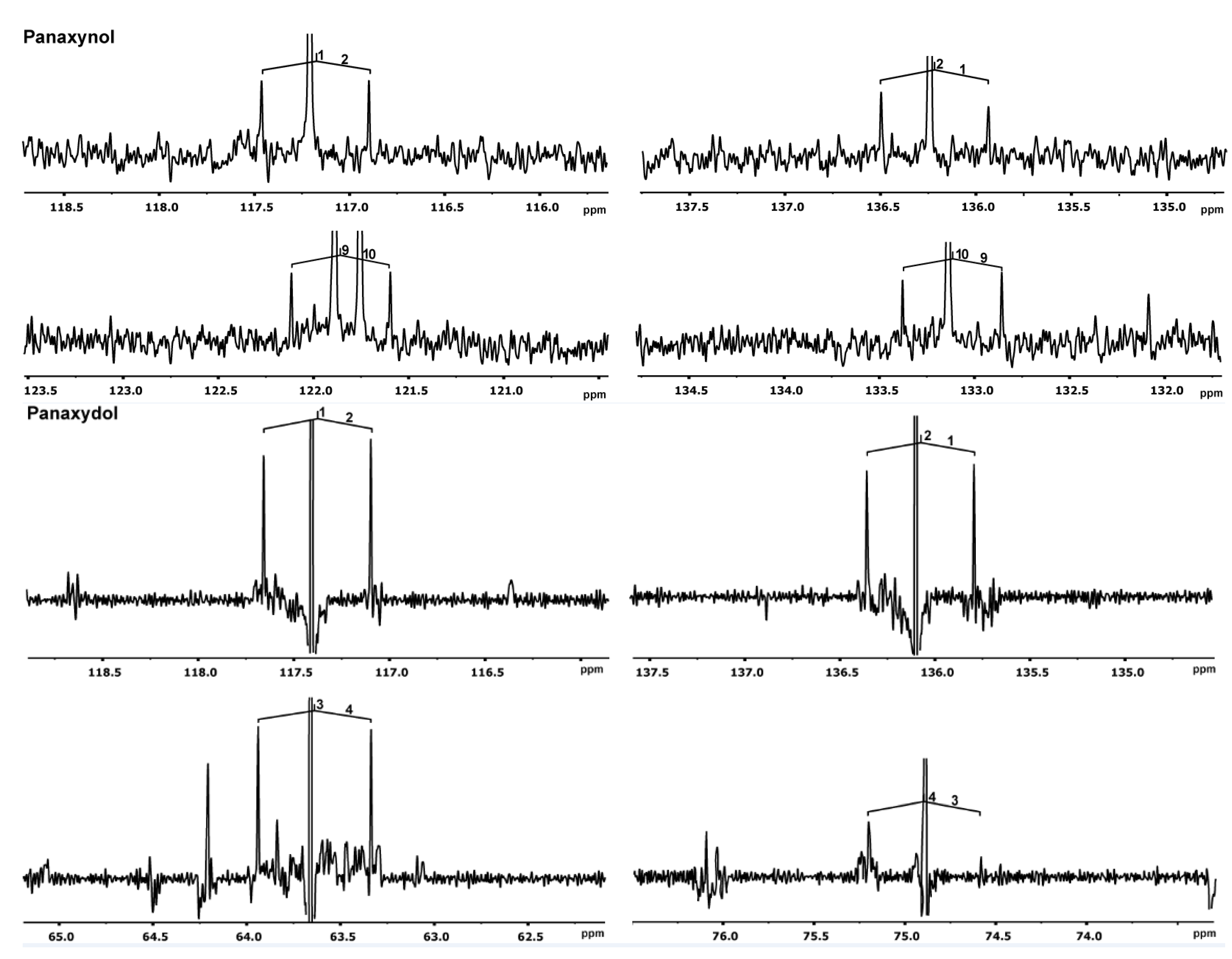
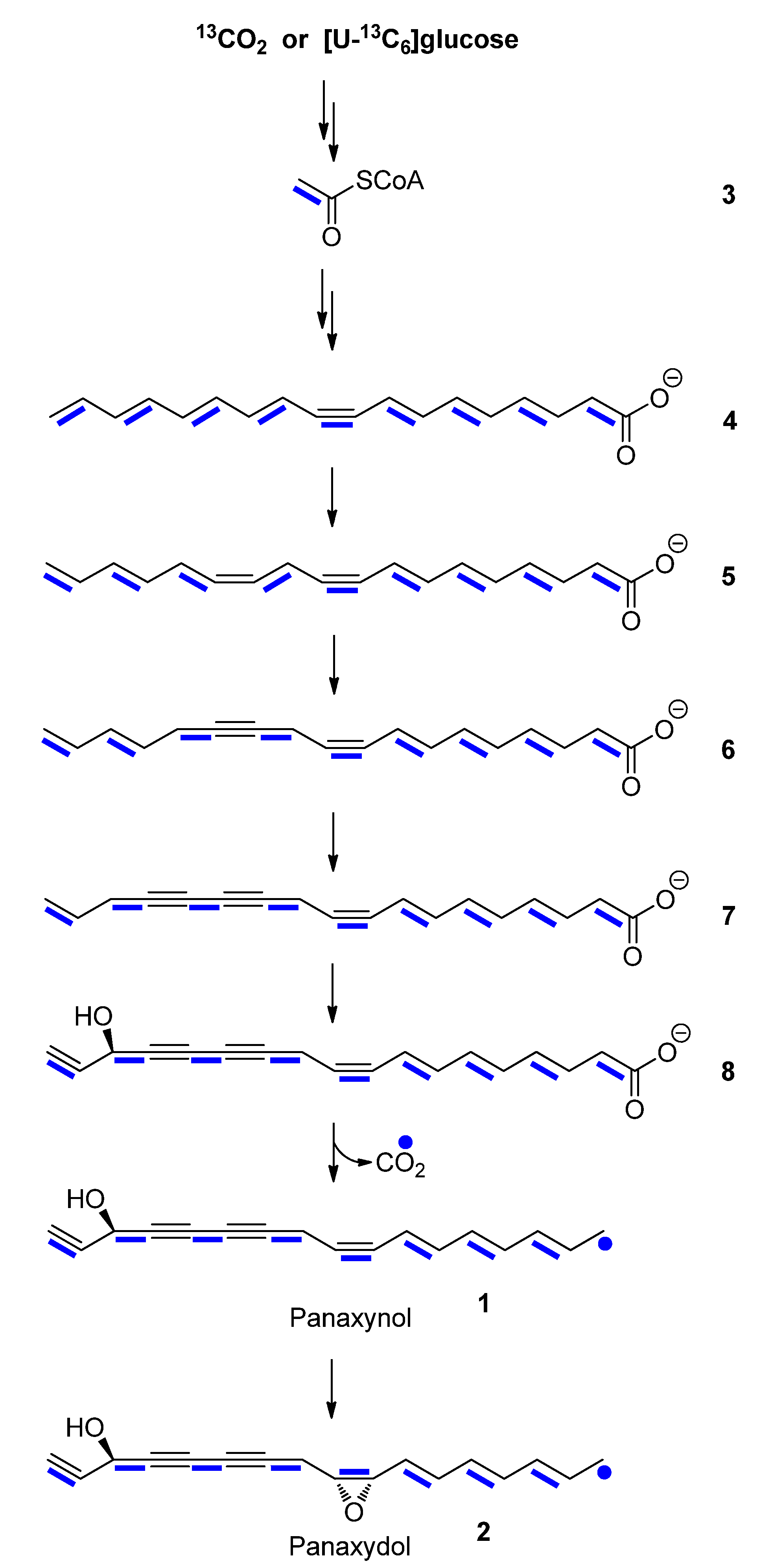
2.2.2. Experiments in Root Cultures with [U-13C6]glucose
3. Experimental
3.1. Chemicals
3.2. Plants and Labeling Experiments with 13CO2
3.3. Root Cultures
3.4. Labeling Experiments with [U-13C6]Glucose
3.5. Isolation of Panaxynol (1) and Panaxydol (2)
3.6. Chromatography
3.7. NMR Spectroscopy and Optical Rotation
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Jia, L.; Zhao, Y. Current evaluation of the millennium phytomedicine—ginseng (I): Etymology, Pharmacognosy, Phytochemistry, Market and regulations. Curr. Med. Chem. 2009, 16, 2475–2484. [Google Scholar] [CrossRef]
- Qi, L.-W.; Wang, C.-Z.; Yuan, C.-S. Ginsenosides from American ginseng: Chemical and pharmacological diversity. Phytochemistry 2011, 72, 689–699. [Google Scholar] [CrossRef]
- Baek, S.-H.; Bae, O.-N.; Park, J.H. Recent methodology in Ginseng analysis. J. Ginseng Res. 2012, 36, 119–134. [Google Scholar] [CrossRef]
- Herrmann, F.; Sporer, F.; Tahrani, A.; Wink, M. Antitrypanosomal properties of Panax ginseng C.A. Meyer: New possibilities for a remarkable traditional drug. Phytother. Res. 2013, 27, 86–98. [Google Scholar] [CrossRef]
- Christensen, L.P. Aliphatic C17-polyacetylenes of the falcarinol type as potential health promoting compounds in food plants of the Apiaceae family. Recent Pat. Food Nutr. Agric. 2011, 3, 64–77. [Google Scholar] [CrossRef]
- Takahashi, M.; Isoi, K.; Kimura, Y.; Yoshikura, M. Studies on the components of Panax ginseng C.A. Meyer. J. Pharm. Soc. Japan 1964, 84, 757–759. [Google Scholar]
- Hirakura, K.; Takagi, H.; Morita, M.; Nakajima, K.; Niitsu, K.; Sasaki, H.; Maruno, M.; Okada, M. Cytotoxic activity of acetylenic compounds from Panax ginseng. Nat. Med. 2000, 54, 342–345. [Google Scholar]
- Zidorn, C.; Jöhrer, K.; Ganzera, M.; Schubert, B.; Sigmund, E.M.; Mader, J.; Greil, R.; Ellmerer, E.P.; Stuppner, H. Polyacetylenes from the Apiaceae vegetables carrot, celery, fennel, parsley, and parsnip and their cytotoxic activities. J. Agric. Food Chem. 2005, 53, 2518–2523. [Google Scholar] [CrossRef]
- Minto, R.E.; Blacklock, B.J. Biosynthesis and function of polyacetylenes and allied natural products. Prog. Lipid Res. 2008, 47, 233–306. [Google Scholar] [CrossRef]
- Xu, L.-L.; Han, T.; Wu, J.-Z.; Zhang, Q.-Y.; Zhang, H.; Huang, B.-K.; Rahman, K.; Qin, L.-P. Comparative research of chemical constituents, antifungal and antitumor properties of ether extracts of Panax ginseng and its endophytic fungus. Phytomedicine 2009, 16, 609–616. [Google Scholar] [CrossRef]
- Hansen, S.L.; Purup, S.; Christensen, L.P. Bioactivity of falcarinol and the influence of processing and storage on its content in carrots (Daucus carota L). J. Sci. Food Agric. 2003, 83, 1010–1017. [Google Scholar] [CrossRef]
- Saita, T.; Katano, M.; Matsunaga, H.; Yamamoto, H.; Fujito, H.; Mori, M. The first specific antibody against cytotoxic polyacetylenic alcohol, panaxynol. Chem. Pharm. Bull. 1993, 41, 549–552. [Google Scholar] [CrossRef]
- Bernart, M.W.; Cardellina, J.H.; Balaschak, M.S.; Alexander, M.; Shoemaker, R.H.; Boyd, M.R. Cytotoxic falcarinol oxylipins from Dendropanax arboreus. J. Nat. Prod. 1996, 59, 748–753. [Google Scholar] [CrossRef]
- Matsunaga, H.; Katano, M.; Yamamoto, H.; Fujito, H.; Mori, M.; Takata, K. Cytotoxyc activity of polyacetylene compounds in Panax ginseng C.A. Meyer. Chem. Pharm. Bull. 1990, 38, 3480–3482. [Google Scholar] [CrossRef]
- Kuo, Y-C.; Lin, Y-L.; Huang, C-P.; Shu, J-W.; Tsai, W-J. A tumor cell growth inhibitor from Saposhnikovae divaricata. Cancer Invest. 2002, 20, 955–964. [Google Scholar] [CrossRef]
- Kobaisy, M.; Abramowski, Z.; Lermer, L.; Saxena, G.; Hancock, R.E.W.; Towers, G.H.N. Antimycobacterial polyynes of devil’s club (Oplopanax horridus), a North American native medicinal plant. J. Nat. Prod. 1997, 60, 1210–1213. [Google Scholar] [CrossRef]
- Kemp, M.S. Falcarindiol: An antifungal polyacetylene from Aegopodium podagraria. Phytochemistry 1978, 17, 1002. [Google Scholar] [CrossRef]
- Harding, V.K.; Heale, J.B. Isolation and identification of the antifungal compounds accumulating in the induced resistance response of carrot root slices to Botrytis cinerea. Physiol. Plant. Pathol. 1980, 17, 277–289. [Google Scholar]
- Hansen, L.; Boll, P.M. Polyacetylenes in Araliaceae: Their chemistry, biosynthesis and biological significance. Phytochemistry 1986, 25, 285–293. [Google Scholar] [CrossRef]
- Otsuka, H.; Komiya, T.; Fujioka, S.; Goto, M.; Hiramatsu, Y.; Fujimura, H. Studies on anti-inflammatory agents. IV. Anti-inflammatory constituents from roots of Panax ginseng C.A. Meyer. Yakugaku Zasshi 1981, 101, 1113. [Google Scholar]
- Baba, K.; Tabata, Y.; Kozawa, M.; Kimura, Y.; Arichi, S. Studies on Chinese traditional medicine Fang-feng (I). Structures and physiological activities of polyacetylene compounds from Saposhnikoviae radix. Shoyakugaku Zasshi 1987, 41, 189–194. [Google Scholar]
- Teng, C.-M.; Kuo, S.-C.; Ko, F.-N.; Lee, J.C.; Lee, L.-G.; Chen, S.-C.; Huang, T.-F. Antiplatelet actions of panaxynol and ginsenosides isolated from ginseng. Biochim. Biophys. Acta 1989, 990, 315–320. [Google Scholar] [CrossRef]
- Alanko, J.; Kurahashi, Y.; Yoshimoto, T.; Yamamoto, S.; Baba, K. Panaxynol, a polyacetylene compound isolated from oriental medicines, inhibits mammalian lipoxygenases. Biochem. Pharmacol. 1994, 48l, 1979–1981. [Google Scholar]
- Nie, B.M.; Jiang, X.Y.; Cai, J.X.; Fu, S.L.; Yang, L.M.; Lin, L.; Hang, Q.; Lu, P.L.; Lu, Y. Panaxydol and panaxynol protect cultured cortical neurons against Abeta25-35-induced toxicity. Neuropharmacology 2008, 54, 845–853. [Google Scholar] [CrossRef]
- Yang, Z.-H.; Sun, K.; Yan, Z.-H.; Suo, W.-H.; Fu, G.-H.; Lu, Y. Panaxynol protects cortical neurons from ischemia-like injury by up-regulation of HIF-1α expression and inhibition of apoptotic cascade. Chem. Biol. Interact. 2010, 183, 165–171. [Google Scholar] [CrossRef]
- Ahn, B.-Z.; Kim, S.-I. Beziehung zwischen Struktur und cytotoxischer Aktivität von Panaxydol-Analogen gegen L1210 Zellen. Arch. Pharm. 1988, 321, 61–63. [Google Scholar] [CrossRef]
- Matsunaga, H.; Katano, M.; Yamamoto, H.; Mori, M.; Takata, K. Studies on the panaxytriol of Panax ginseng C.A. Meyer. Isolation, Determination and antitumor activity. Chem. Pharm. Bull. 1989, 37, 1279–81. [Google Scholar] [CrossRef]
- Kobæk-Larsen, M.; Christensen, L.P.; Vach, W.; Ritskes-Hoitinga, J.; Brandt, K. Inhibitory effects of feeding with carrots or (−)-falcarinol on development of azoxymethane-induced preneoplastic lesions in the rat colon. J. Agric. Food Chem. 2005, 53, 1823–1827. [Google Scholar] [CrossRef]
- Ahn, B.Z.; Kim, S.I.; Lee, Y.H. Acetylpanaxydol und Panaxydolchlorhydrin, zwei neue, gegen L1210-Zellen cytotoxische Polyine aus Koreanischem Ginseng. Arch. Pharm. 1989, 322, 223. [Google Scholar] [CrossRef]
- Matsunaga, H.; Saita, T.; Nagamo, F.; Mori, M.; Katano, M. A possible mechanism for the cytotoxicity of a polyacetylenic alcohol, panaxytriol: inhibition of mitochondrial respiration. Cancer Chemother. Pharmacol. 1995, 35, 291–296. [Google Scholar] [CrossRef]
- Hansen, L.; Hammershøy, O.; Boll, P.M. Allergic contact dermatitis from falcarinol isolated from Schefflera arboricola. Contact Dermat. 1986, 14, 91–93. [Google Scholar] [CrossRef]
- Hausen, B.M.; Bröhan, J.; König, W.A.; Faasch, H.; Hahn, H.; Bruhn, G. Allergic and irritant contact dermatitis from falcarinol and didehydrofalcarinol in common ivy (Hedera helix L.). Contact Dermat. 1987, 17, 1–9. [Google Scholar] [CrossRef]
- Gafner, F.; Epstein, W.; Reynolds, G.; Rodriguez, E. Human maximization test of falcarinol, the principal contact allergen of English ivy and Algerian ivy (Hedera helix, H. canariensis). Contact Dermat. 1988, 19, 125–128. [Google Scholar] [CrossRef]
- Machado, S.; Silva, E.; Massa, A. Occupational allergic contact dermatitis from falcarinol. Contact Dermat. 2002, 47, 113–114. [Google Scholar]
- Barley, G.C.; Jones, E.H.R.; Thaller, V. Crepenynate as a precursor of falcarinol in carrot tissue culture. In Chemistry and Biology of Naturally-Occurring Acetylenes and Related Compounds; Lam, J., Breteler, H., Arnason, T., Hansen, L., Eds.; Elsevier: Amsterdam, The Netherlands, 1988; pp. 85–91. [Google Scholar]
- Bohlmann, F.; Burkhardt, T. Polyacetylenverbindungen. 166. Über die Biogenese von C17-Polyinen. Chem. Ber. 1969, 102, 1702–1706. [Google Scholar] [CrossRef]
- Bu’Lock, J.D.; Smalley, H.M. The biosynthesis of polyacetylenes. Part V. The role of malonate derivatives, and the common origin of fatty acids, polyacetylenes, and “acetate-derived” phenols. J. Chem. Soc. 1967, 332–336. [Google Scholar]
- Poplawski, J.; Wrobel, J.T.; Glinka, T. Panaxydol, a new polyacetylenic epoxide from Panax ginseng roots. Phytochemistry 1980, 19, 1539–1541. [Google Scholar]
- Hirakura, K.; Morita, M.; Nakajima, K.; Ikeya, Y.; Mitsuhashi, H. Polyacetylenes from the roots of Panax Ginseng. Phytochemistry 1991, 30, 3327–3333. [Google Scholar] [CrossRef]
- Kobayashi, M.; Mahmud, T.; Umezome, T.; Wang, W.; Murakami, N.; Kitagawa, I. The absolute stereostructures of the polyacetylenic constituents of Ginseng Radix Rubra. Tetrahedron 1997, 53, 15691–15700. [Google Scholar]
- Seger, C.; Godejohann, M.; Spraul, M.; Stuppner, H.; Hadacek, F. Reaction product analysis by high-performance liquid chromatography-solid-phase extraction-nuclear magnetic resonance. Application to the absolute configuration determination of naturally occurring polyyne alcohols. J. Chromatogr. A 1136, 82–88. [Google Scholar]
- Bohlmann, F.; Niedballa, U.; Rode, K.M. New polyines with a C17-chain. Chem. Ber. 1966, 99, 3552–3558. [Google Scholar] [CrossRef]
- Crosby, D.G.; Aharonson, N. The structure of carotatoxin, a natural toxicant from carrot. Tetrahedron 1967, 23, 465–472. [Google Scholar] [CrossRef]
- Larsen, P.K.; Nielsen, B.E.; Lemmich, J. The absolute configuration of falcarinol, an acetylenic compound from the roots of Seseli gummiferum Pall. Acta Chem. Scand. 1969, 23, 2552–2554. [Google Scholar] [CrossRef]
- Shim, S.C.; Koh, H.Y.; Chang, S. Determination of absolute stereochemistry of panaxynol. Tetrahedron Lett. 1985, 26, 5775–5776. [Google Scholar] [CrossRef]
- Shim, S.C.; Koh, H.Y.; Chang, S.; Moon, S.K.; Min, T.-J. Absolute configuration of p-substituted benzoates of panaxynol. Bull. Korean Chem. Soc. 1986, 7, 106–108. [Google Scholar]
- Bernart, M.W.; Hallock, Y.F.; Cardellina, J.H., II; Boyd, M.R. Stereochemistry of enynols—A caveat on the excition chirality method. Tetrahedron Lett. 1994, 35, 993–994. [Google Scholar] [CrossRef]
- Zheng, G.; Lu, W.; Aisa, H.A.; Cai, J. Absolute configuration of falcarinol, a potent antitumor agent commonly occurring in plants. Tetrahedron Lett. 1999, 40, 2181–2182. [Google Scholar] [CrossRef]
- Römisch-Margl, W.; Schramek, N.; Radykewicz, T.; Ettenhuber, C.; Eylert, E.; Huber, C.; Römisch-Margl, L.; Schwarz, C.; Dobner, M.; Demmel, N.; et al. 13CO2 as a universal metabolic tracer in isotopologue perturbation experiments. Phytochemistry 2007, 68, 2273–2289. [Google Scholar] [CrossRef]
- Ostrozhenkova, E.; Eylert, E.; Schramek, N.; Golan-Goldhirsh, A.; Bacher, A.; Eisenreich, W. Biosynthesis of the chromogen hermidin from Mercurialis annua L. Phytochemistry 2007, 68, 2816–2824. [Google Scholar] [CrossRef]
- Schramek, N.; Wang, H.; Römisch-Margl, W.; Keil, B.; Radykewicz, T.; Winzenhörlein, B.; Beerhues, L.; Bacher, A.; Rohdich, F.; Gershenzon, J.; et al. Artemisinin biosynthesis in growing plants of Artemisia annua. A 13CO2 study. Phytochemistry 2010, 71, 179–187. [Google Scholar] [CrossRef]
- Eisenreich, W.; Huber, C.; Kutzner, E.; Knispel, N.; Schramek, N. Isotopologue profiling—Toward a better understanding of metabolic pathways. In The Handbook of Plant Metabolomics, Metabolite Profiling and Networking; Weckwerth, W., Kahl, G., Eds.; Wiley: Weinheim, Germany, 2013; pp. 25–56. [Google Scholar]
- Eisenreich, W.; Bacher, A. Advances of high-resolution NMR techniques in the structural and metabolic analysis of plant biochemistry. Phytochemistry 2007, 68, 2799–2815. [Google Scholar] [CrossRef]
- Vervliet, G.; Holsters, M.; Teuchy, H.; Van, M.M.; Schell, J. Characterization of different plaque-forming and defective temperate phages in Agrobacterium. J. Gen. Virol. 1975, 26, 33–48. [Google Scholar] [CrossRef]
- Schenk, R.U.; Hildebrandt, A. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- Mallol, A.; Cusidó, R.M.; Palazón, J.; Bonfill, M.; Morales, C.; Piñol, M.T. Ginsenoside production in different phenotypes of Panax ginseng transformed roots. Phytochemistry 2001, 57, 365–371. [Google Scholar] [CrossRef]
- McLaughlin, N.P.; Butler, E.; Evans, P.; Brunton, N.P.; Koidis, A.; Rai, D.K. A short synthesis of (+) and (−)-falcarinol. Tetrahedron 2010, 66, 9681–9687. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds panaxynol and panaxydol are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Knispel, N.; Ostrozhenkova, E.; Schramek, N.; Huber, C.; Peña-Rodríguez, L.M.; Bonfill, M.; Palazón, J.; Wischmann, G.; Cusidó, R.M.; Eisenreich, W. Biosynthesis of Panaxynol and Panaxydol in Panax ginseng. Molecules 2013, 18, 7686-7698. https://doi.org/10.3390/molecules18077686
Knispel N, Ostrozhenkova E, Schramek N, Huber C, Peña-Rodríguez LM, Bonfill M, Palazón J, Wischmann G, Cusidó RM, Eisenreich W. Biosynthesis of Panaxynol and Panaxydol in Panax ginseng. Molecules. 2013; 18(7):7686-7698. https://doi.org/10.3390/molecules18077686
Chicago/Turabian StyleKnispel, Nihat, Elena Ostrozhenkova, Nicholas Schramek, Claudia Huber, Luis M. Peña-Rodríguez, Mercedes Bonfill, Javier Palazón, Gesine Wischmann, Rosa M. Cusidó, and Wolfgang Eisenreich. 2013. "Biosynthesis of Panaxynol and Panaxydol in Panax ginseng" Molecules 18, no. 7: 7686-7698. https://doi.org/10.3390/molecules18077686







