Superoxide Scavenging Effects of Some Novel Bis-Ligands and Their Solvated Metal Complexes Prepared by the Reaction of Ligands with Aluminum, Copper and Lanthanum Ions
Abstract
:1. Introduction
2. Results and Discussion
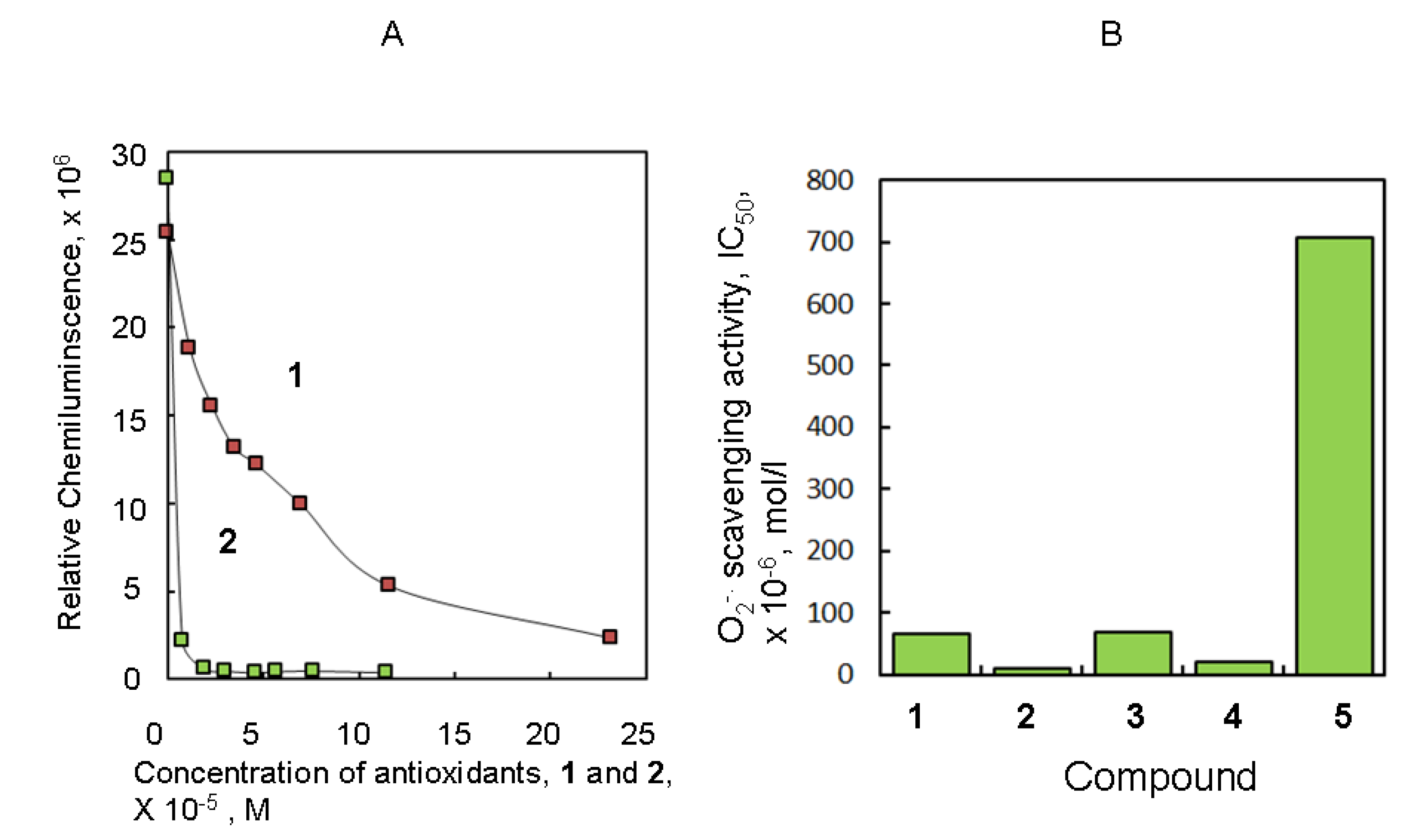
2.1. Antioxidant Activity of Several Natural Producuts
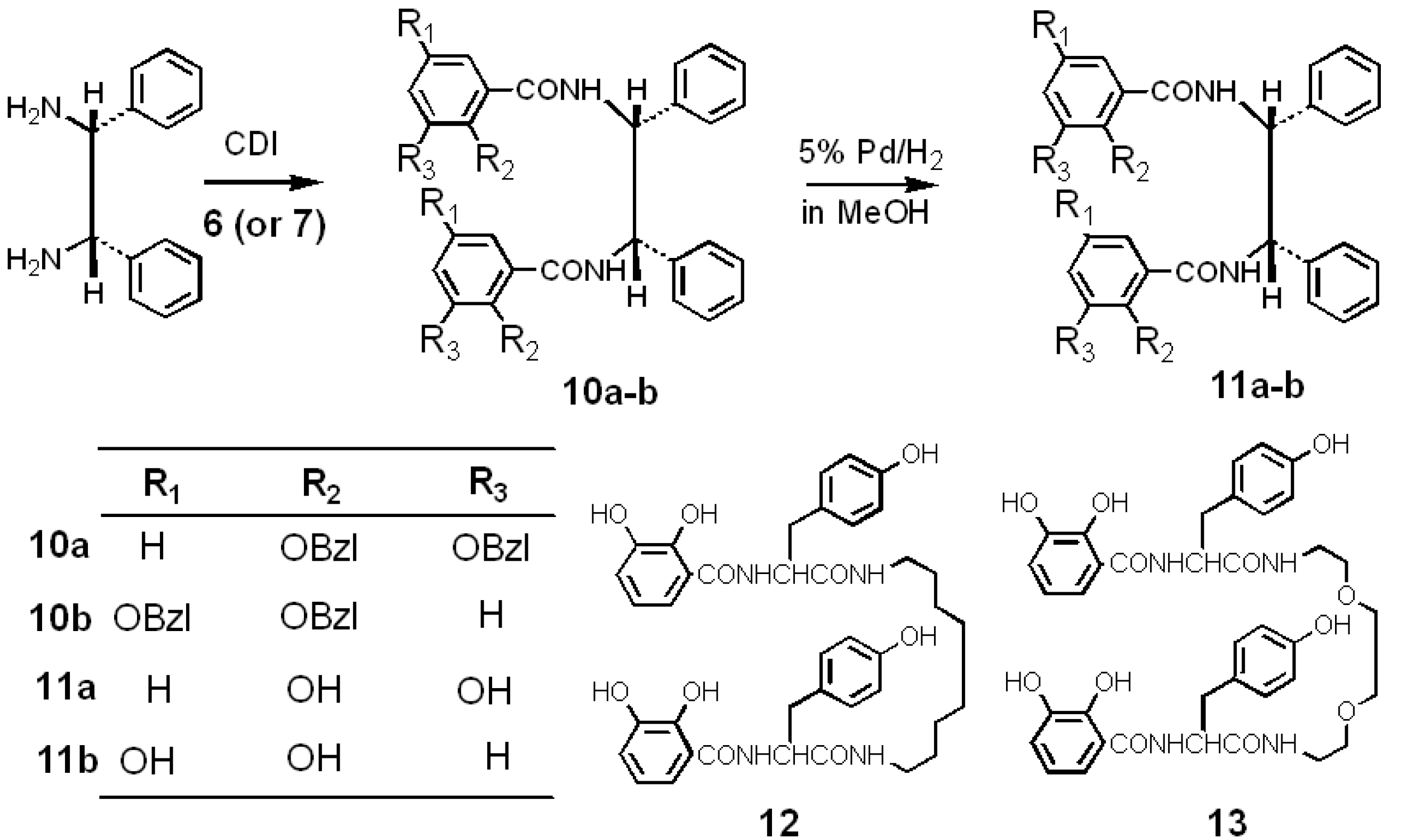
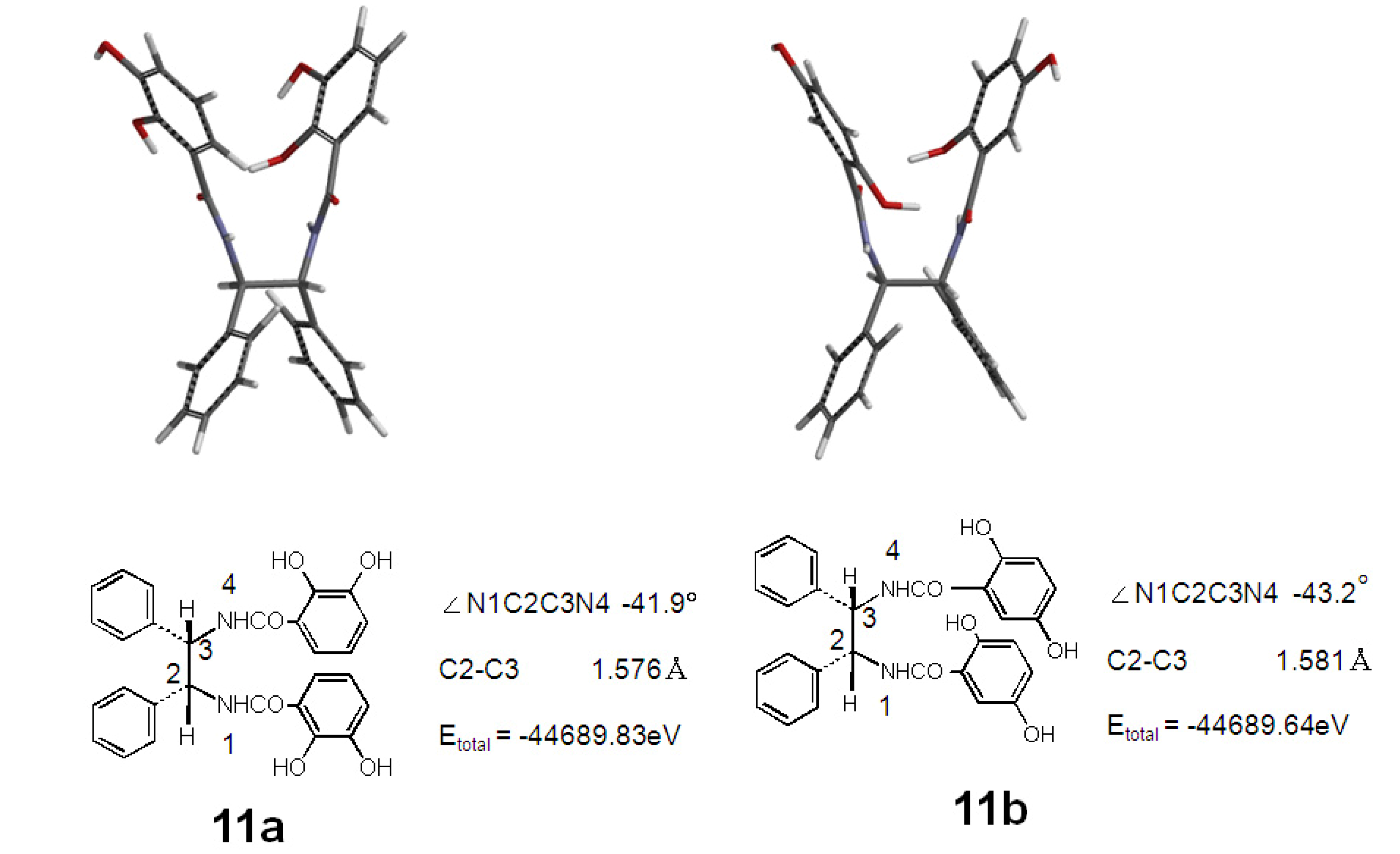
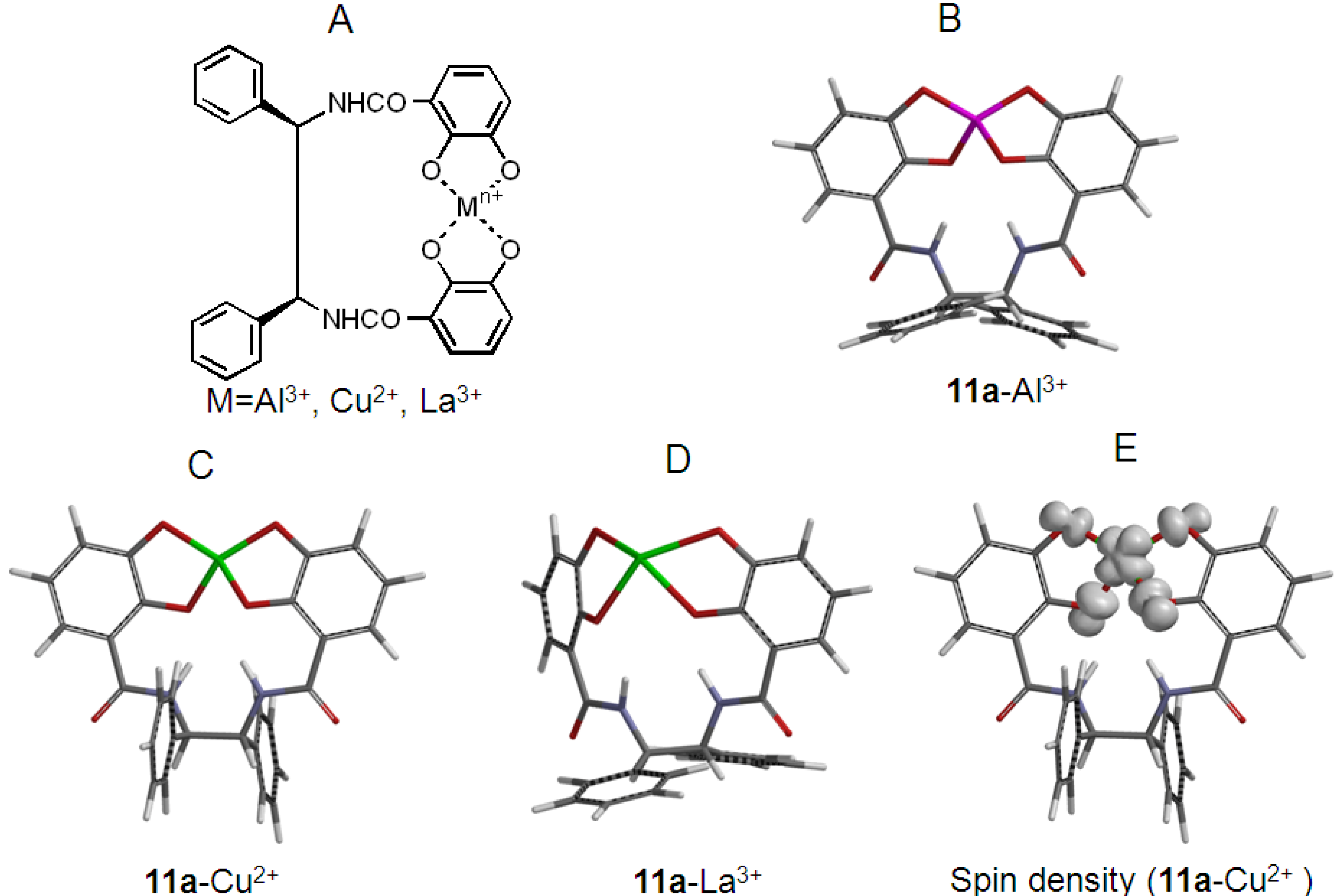
2.2. Synthesis and Stable Conformation of 11a and 11b
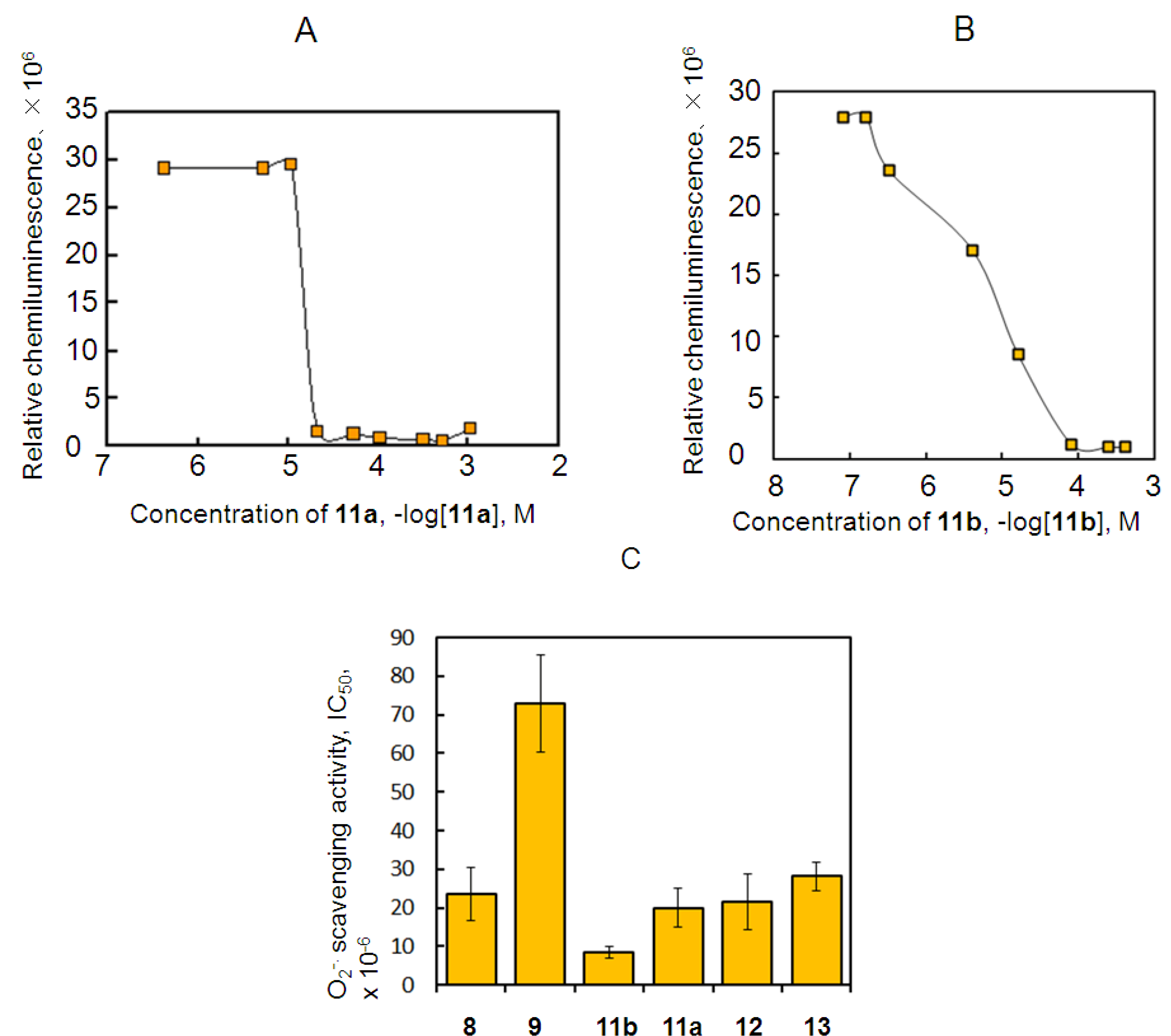
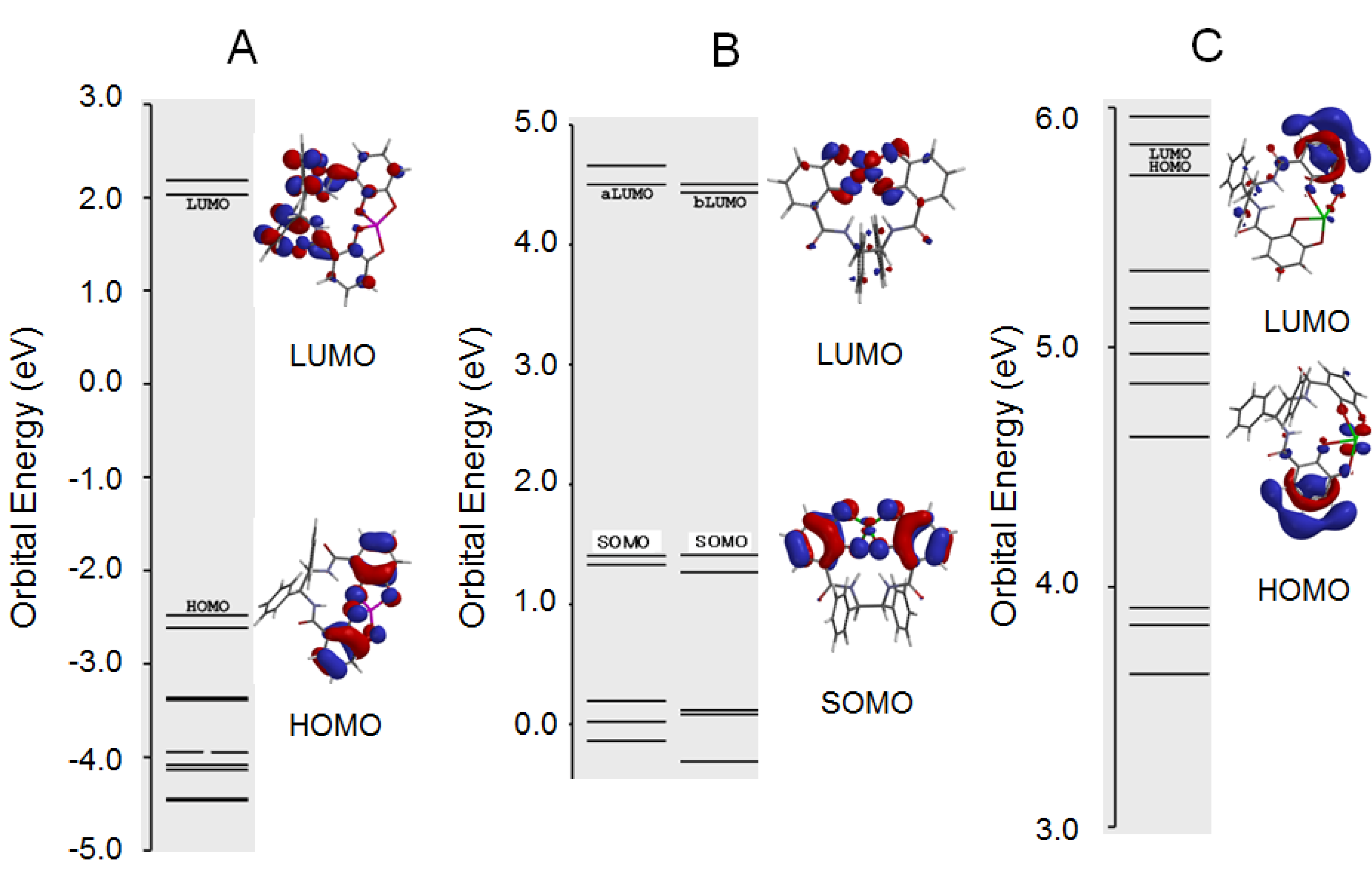
2.3. Antioxidant Activity of 11a and 11b
2.4. Antioxidant Activity of the Metal Complexes 11a–Mn+

3. Experimental
3.1. Materials and Methods
3.2. Synthesis
3.3. O2−. Scavenging Assay
3.4. O2−.Scavenging Assay of the 11a–Metal Ion Complexes and Metal Ions
3.5. Computational Chemistry
4. Conclusions
Conflicts of Interest
References
- Droge, W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Cross, C.E. Oxygen-derived species: Their relation to human disease and environmental stress. Environ. Health Perspect. 1994, 102, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; Poel, V.B.; Piters, L.; Vlietick, A.J.; Berghe, D.V. Structure-activity relationships and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Okawa, M.; Kinjo, J.; Nohara, T.; Ono, M. DPPH(1,1-diphenyl-2-picrylhydrazyl) radical scavenging activity of flavonoids obtained from some medicinal plants. Biol. Pharm. Bull. 2001, 24, 1202–1205. [Google Scholar] [CrossRef] [PubMed]
- Iwatsuki, M.; Tsuchiya, J.; Komuro, E.; Yamamoto, Y.; Niki, E. Effects of solvents and media on the antioxidant activity of α-tocopherol. Biochim. Biophys. Acta 1994, 1200, 19–26. [Google Scholar] [CrossRef]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Antioxidant properties of phenolic compounds: H-atom versus electron transfer mechanism. J. Phys. Chem. A 2004, 108, 4916–4922. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Chiodo, S.; Toscano, M. Iron chelation by the powerful antioxidant flavonoid quercetin. J. Agric. Food Chem. 2006, 54, 6343–6351. [Google Scholar] [CrossRef] [PubMed]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–305. [Google Scholar] [CrossRef]
- Nakanishi, I.; Miyazaki, K.; Shimada, T.; Ohkubo, K.; Urano, S.; Ikota, N.; Ozawa, T.; Fukuzumi, S.; Fukuhara, S. Effects of metal ions distinguishing between one-step hydrogen- and electron-transfer mechanisms for the radical-scavenging reaction of (+)-catechin. J. Phys. Chem. A 2002, 106, 11123–11126. [Google Scholar] [CrossRef]
- Waki, T.; Nakanishi, I.; Matsumoto, K.; Kitajima, J.; Chikuma, T.; Kobayashi, S. Key role of chemical hardness to compare 2,2-diphenyl-1-picrylhydrazyl radical scavenging power of flavone and flavanol O-glycoside and C-glycoside derivatives. Chem. Pharm. Bull. 2012, 60, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Waki, T.; Nakanishi, I.; Matsumoto, K.; Anzai, K. Potent 2,2-diphenyl-1-picrylhydrazyl radical-scavenging activity of novel antioxidants, double-stranded tyrosine residues conjugating pyrocatechol. Chem. Pharm. Bull. 2010, 58, 1442–1446. [Google Scholar] [CrossRef] [PubMed]
- Parr, R.G.; Pearson, R. Absolute hardness: Companion parameter to absolute electronegativity. J. Am. Chem. Soc. 1983, 105, 7512–7516. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density Functional Theory of Atoms and Molecules, 1st ed.; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Pearson, R. The principle of maximum hardness. Acc. Chem. Res. 1993, 26, 250–255. [Google Scholar] [CrossRef]
- Kobayashi, S.; Mizushima, A.; Sasuga, A.; Watanabe, M. Development of soft-based double-stranded peptide chelators which selectively separate europium and lanthanum ions based on the hardness concept. Chem. Pharm. Bull. 2006, 54, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Watanabe, M.; Chikuma, T. Development of new double-stranded phenylalanyl chelators using η–χ diagrams and binding constants for chelators and lanthanide ions. Chem. Pharm. Bull. 2010, 58, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Kawahara, M. Aggregation of Amyloid β-protein and its neurotoxicity: Enhancement by aluminum and other metals. Tohoku J. Exp. Med. 1994, 174, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, S.L.; Dupuis, N.F.; Lazo, N.D.; Wyttenbach, T.; Condron, M.M.; Bitan, G.; Teplow, D.B.; Shea, J.-E.; Ruotolo, B.T.; Robinson, C.V.; et al. Amyloid-β protein oligomerization and the importance of tetramers and dodecamers in the aetiology of Alzheimer’s disease. Nat. Chem. 2009, 1, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, O.; Wu, C.; Murai, A.; Nakamura, H. Evaluation of five imidazopyrazione-type chemiluminescent superoxide probs and their application to the measurement of superoxide anion generated by listeria monocytogenes. Anal. Biochem. 1998, 258, 923–235. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, D.V.; Rao, M.N.A. Antioxidant properties of phenylstyryl ketones. Free Radic. Res. 1995, 22, 309–317. [Google Scholar] [CrossRef]
- Kobayashi, S.; Atuchi, N.; Kobayashi, H.; Shiraishi, A.; Hamashima, H.; Tanaka, A. Diastereomer selective effects for growth inhibition of synthesized mini parallel double-stranded peptides on Escherichia coli and Staphylococcus aureus. Chem. Pharm. Bull. 2004, 52, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Milton, N.G. Role of hydrogen peroxide in the aetiology of Alzheimer’s disease: Implications for treatment. Drugs Aging 2004, 21, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, A.V.; Ivanova, M.V.; Timoshin, A.A.; Ruuge, E.K. Effect of group II metal cation on catecholate oxidation. ChemPhysChem 2007, 8, 186–1869. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Wang, P.-C.; Iwasaki, A. Synthesis of porphyrin-incorporated polymers and their application for simultaneous detection of multimetal components by using spectrophotometry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 75, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Asano, T. Development of the analytical method of heavy metals using the UV/Vis absorption characteristics of porphirin. Ph.D. Thesis; University of Tsukuba: Ibaraki, Japan, March 2010. Available online: http://www.tulips.tsukuba.ac.jp/limedio/dlam/B29/B2959292/1.pdf (accessed on 20 December 2012).
- Hay, P.J.; Walt, W.E. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 8a and 8b are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kobayashi, S.; Kanai, S. Superoxide Scavenging Effects of Some Novel Bis-Ligands and Their Solvated Metal Complexes Prepared by the Reaction of Ligands with Aluminum, Copper and Lanthanum Ions. Molecules 2013, 18, 6128-6141. https://doi.org/10.3390/molecules18066128
Kobayashi S, Kanai S. Superoxide Scavenging Effects of Some Novel Bis-Ligands and Their Solvated Metal Complexes Prepared by the Reaction of Ligands with Aluminum, Copper and Lanthanum Ions. Molecules. 2013; 18(6):6128-6141. https://doi.org/10.3390/molecules18066128
Chicago/Turabian StyleKobayashi, Shigeki, and Sachi Kanai. 2013. "Superoxide Scavenging Effects of Some Novel Bis-Ligands and Their Solvated Metal Complexes Prepared by the Reaction of Ligands with Aluminum, Copper and Lanthanum Ions" Molecules 18, no. 6: 6128-6141. https://doi.org/10.3390/molecules18066128



