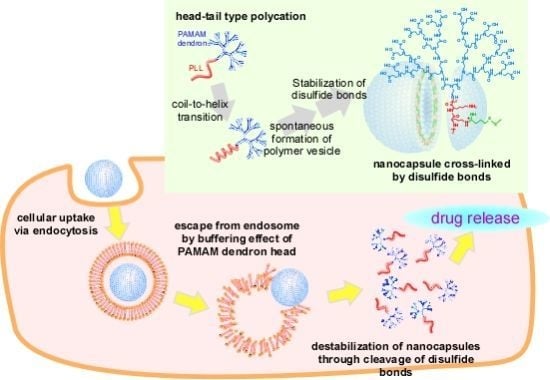
Intracellular Environment-Responsive Stabilization of Polymer Vesicles Formed from Head-Tail Type Polycations Composed of a Polyamidoamine Dendron and Poly(L-lysine)
Abstract
:1. Introduction
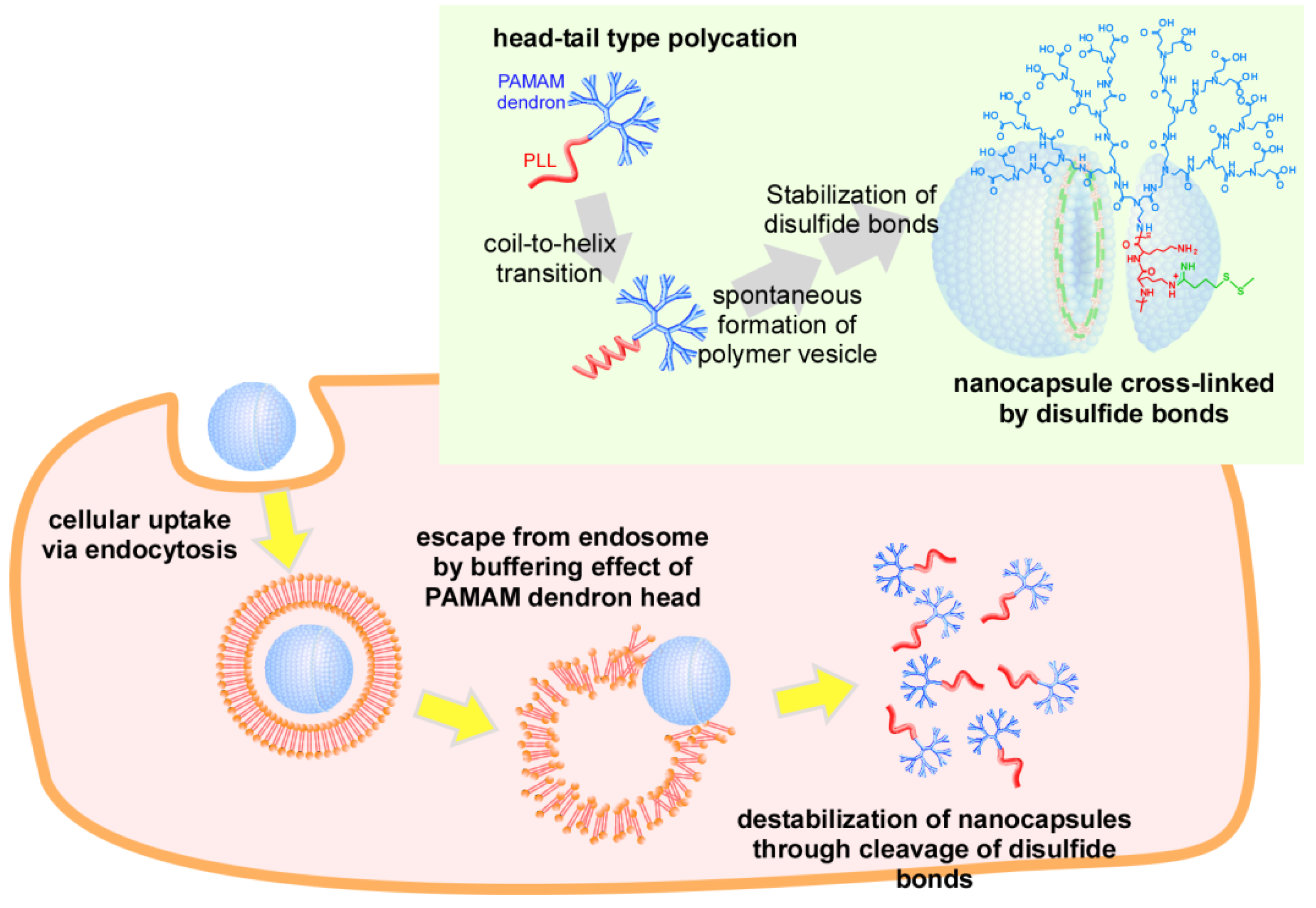
2. Results and Discussion
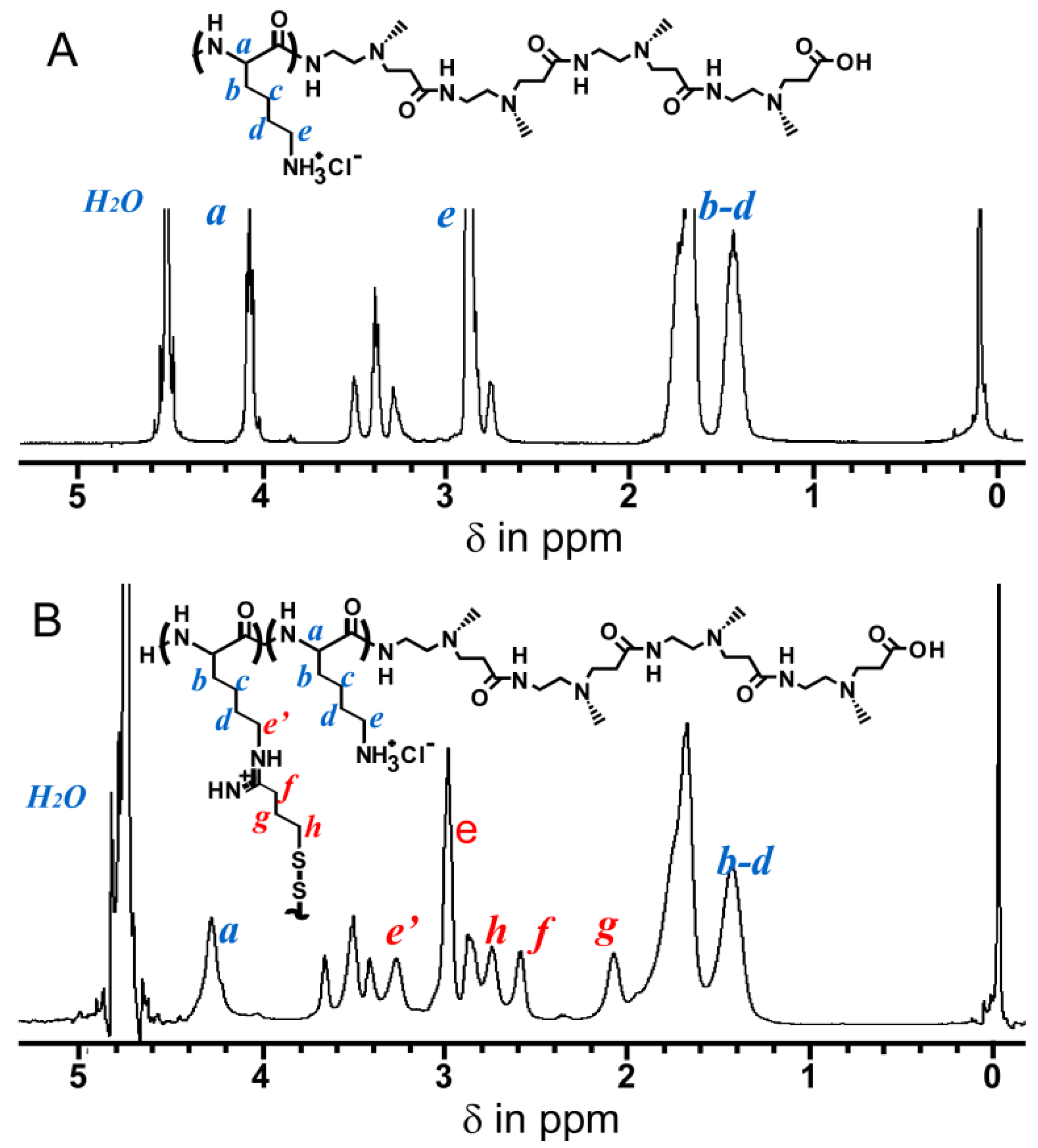
2.1. Preparation of Nanocapsules Stabilized by Disulfide Bonds
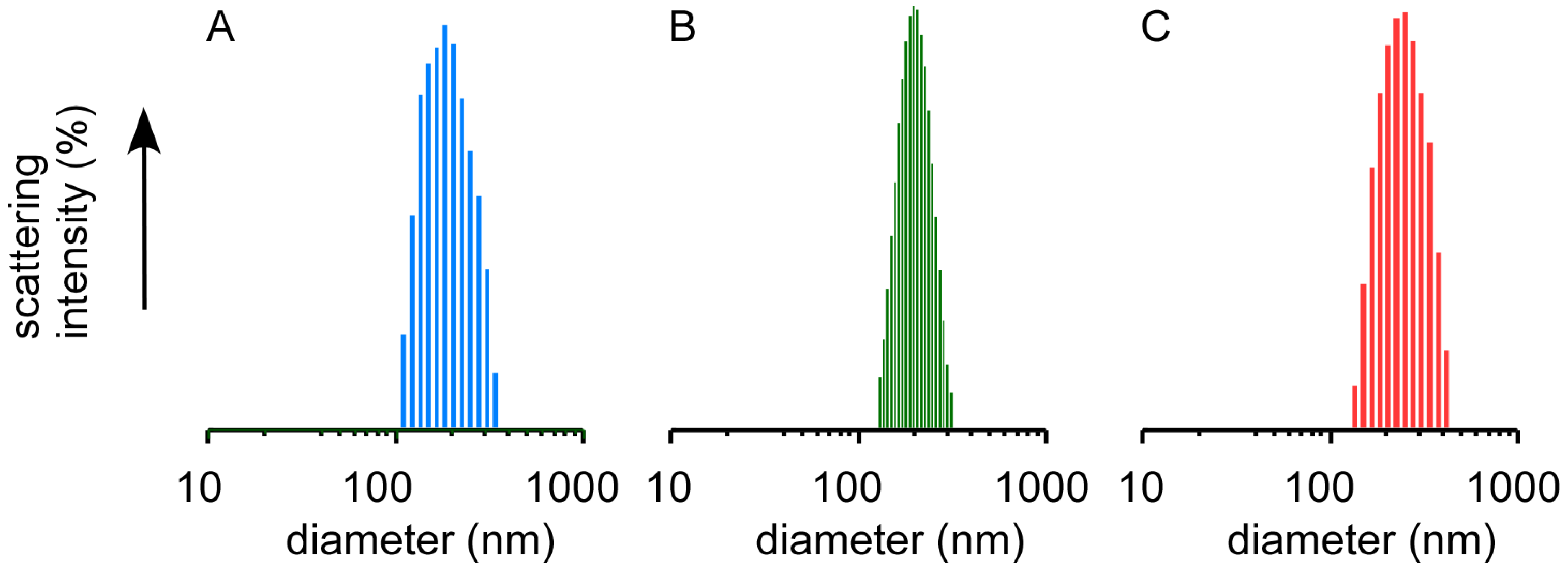
| Cross-linker | Average diameter (nm) [polydispersity index] | ||
|---|---|---|---|
| Before cross-linking | After cross-linking | After dialysis | |
| 2-iminothiolane (IT) | 187 [0.08] | 194 [0.06] | 218 [0.09] |
| ethylene glycol diglycidyl ether (EGDE) | 187 [0.08] | 194 [0.07] | 238 [0.08 |
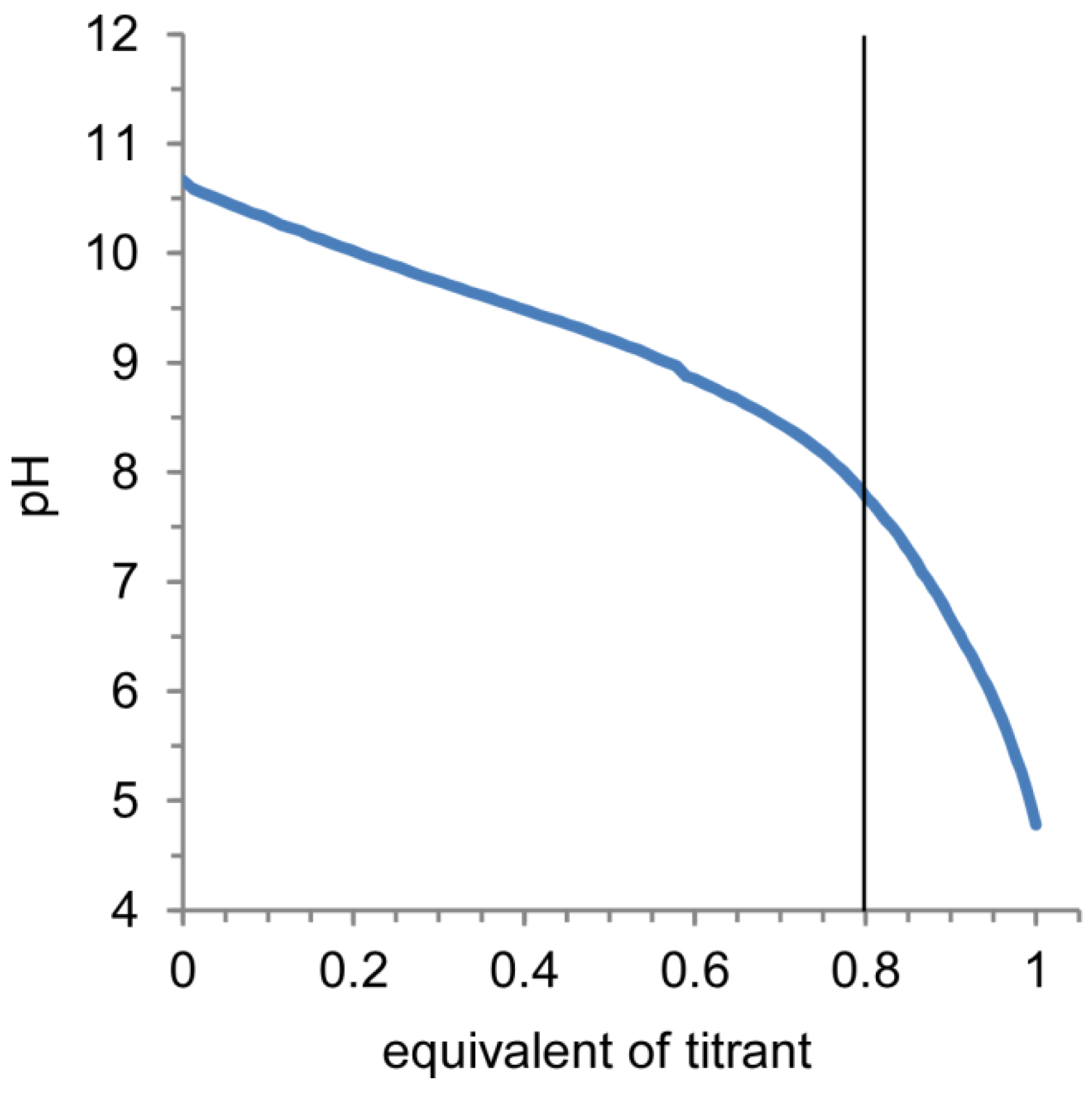
2.2. Release of Model Compounds from Nanocapsules and Cytotoxicity of Nanocapsules
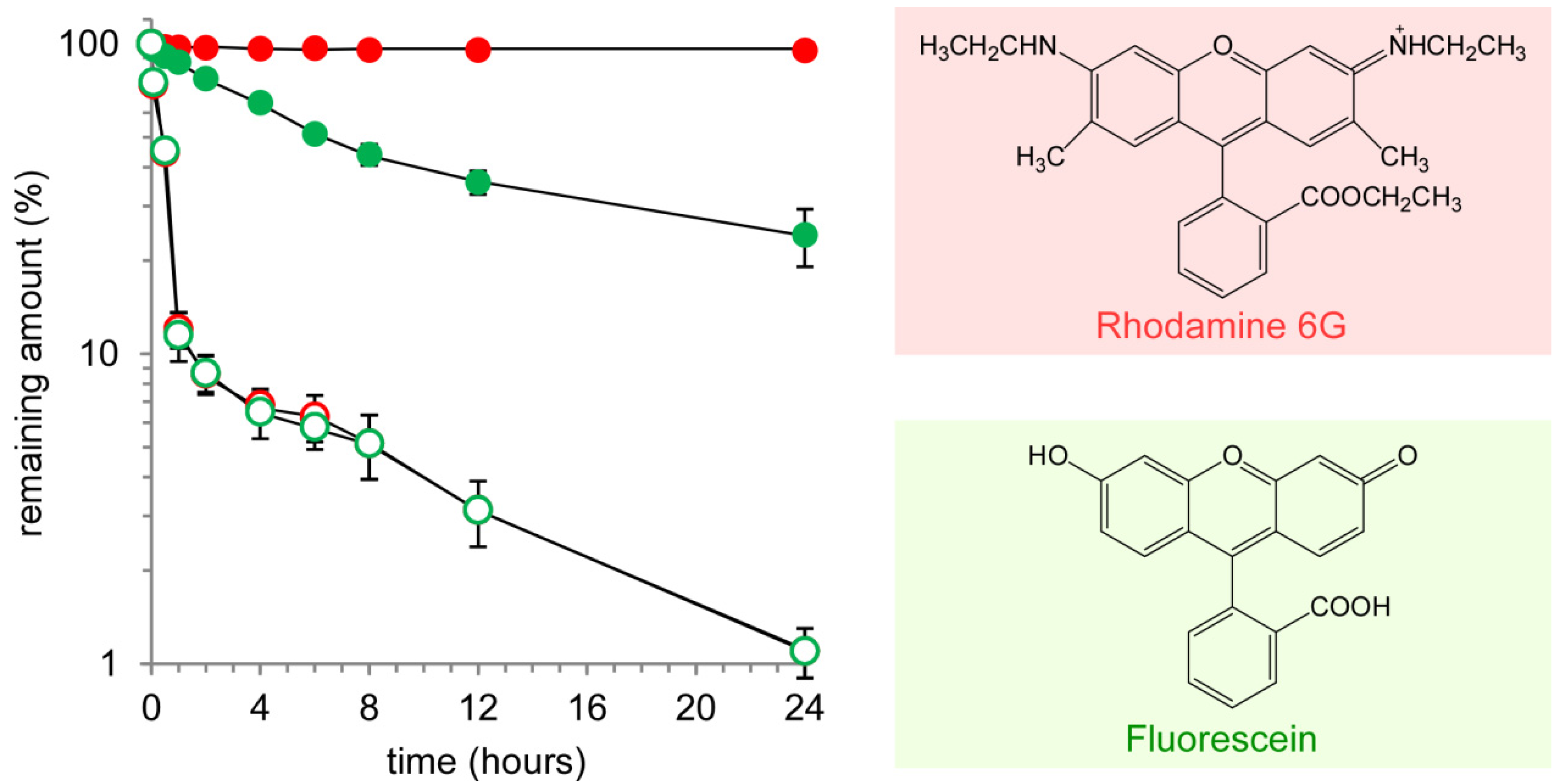

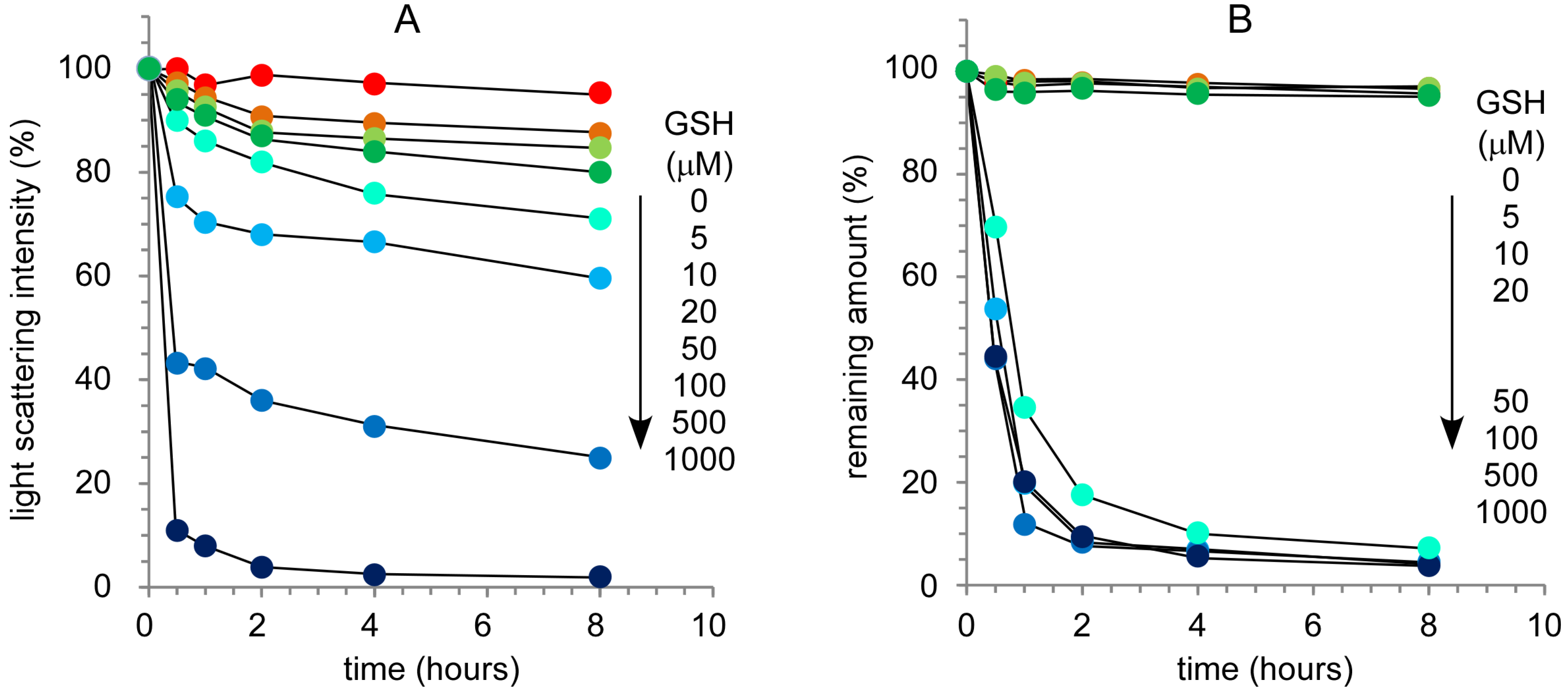
2.3. Response of Nanocapsules to a Reductive Environment

3. Experimental
3.1. Materials
3.2. Preparation of Nanocapsules Cross-Linked by Disulfide Bonds
3.3. Dye Release Experiments from Nanocapsules
3.4. In vitro Cytotoxicity and Laser Confocal Microscope Observations
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Cai, C.; Wang, L.; Lin, J. Self-assembly of polypeptide-based copolymers into diverse aggregates. Chem. Commum. 2011, 47, 11189–11203. [Google Scholar] [CrossRef]
- Herr, D.J.C. Directed block copolymer self-assembly for nanoelectronics fabrication. J. Mater. Res. 2011, 26, 122–139. [Google Scholar] [CrossRef]
- Kim, J.K.; Yang, S.Y.; Lee, Y.; Kim, Y. Functional nanomaterials based on block copolymer self-assembly. Prog. Polym. Sci. 2010, 35, 1325–1349. [Google Scholar] [CrossRef]
- Hu, J.; Liu, S. Responsive polymers for detection and sensing application: Current status and future developments. Macromolecules 2010, 43, 8315–8330. [Google Scholar] [CrossRef]
- Bang, J.; Jeong, U.; Ryu, D.Y.; Russell, T.P.; Hawker, C.J. Block copolymer nanolithography: Translation of molecular level control to nanoscale patterns. Adv. Mater. 2009, 21, 4769–4792. [Google Scholar] [CrossRef]
- Matyjaszewski, K.; Tsarevsky, N.V. Nanostructured functional materials prepared by atom transfer radical polymerization. Nat. Chem. 2009, 1, 276–288. [Google Scholar] [CrossRef]
- Harada, A.; Kataoka, K. Supramolecular assemblies of block copolymers in aqueous media as nanocontainers relevant to biological applications. Prog. Polym. Sci. 2006, 31, 949–982. [Google Scholar] [CrossRef]
- Ahmed, F.; Pakunlu, G.; Srinivas, G.; Brannan, A.; Bates, F.; Klein, M.K.; Minko, T.; Discher, D.E. Shrinkage of a rapidly growing tumor by drug-loaded polymersomes: pH-Triggered release through copolymer degradation. Mol. Pharm. 2006, 3, 340–350. [Google Scholar] [CrossRef]
- EI-Sayed, M.E.H.; Hoffman, A.S.; Stayton, P.S. Rational design of composition and activity correlations for pH-sensitive and glutathione-reactive polymer therapeutics. J. Controll. Release 2005, 101, 47–58. [Google Scholar] [CrossRef]
- Napoli, A.; Boerakker, M.J.; Tirelli, N.; Nolte, R.J.M.; Sommerdijk, N.; Hubbel, J.A. Glucose-oxidase based self-destructing polymeric vesicles. Langmuir 2004, 20, 3487–3491. [Google Scholar] [CrossRef]
- Napoli, A.; Valentini, M.; Tirelli, N.; Muller, M.; Hubbel, J.A. Oxidation-responsive polymeric vesicles. Nat. Mater. 2004, 3, 183–189. [Google Scholar] [CrossRef]
- Lee, G.Y.; Park, K.; Kim, S.Y.; Byun, Y. MMPs-specific PEGylated peptide-DOX conjugate micelles that can contain free doxorubicin. Eur. J. Pharm. Biopharm. 2007, 67, 646–654. [Google Scholar] [CrossRef]
- Harada, A.; Kawamura, M.; Matsuo, T.; Takahashi, T.; Kono, K. Synthesis and characterization of head-tail type polycation block copolymer as non-viral gene vector. Bioconjug. Chem. 2006, 17, 3–5. [Google Scholar]
- Harada, A.; Kawamura, M.; Kimura, Y.; Takahashi, T.; Kojima, C.; Kono, K. Effect of head size in head-tail type polycations on their in vitro performances as non-viral gene vectors. Macromol. Biosci. 2009, 9, 605–612. [Google Scholar] [CrossRef]
- Harada, A.; Kimura, Y.; Kojima, C.; Kono, K. Effective tolerance to serum proteins of head-tail type polycation vectors by PEGylation at the periphery of the head block. Biomacromolecules 2010, 11, 1036–1042. [Google Scholar] [CrossRef]
- Harada, A.; Kimura, Y.; Kono, K. Cationic polymers with inhibition ability of DNA condensation elevate gene expression. Chem. Biol. Chem. 2010, 11, 1985–1988. [Google Scholar] [CrossRef]
- Harada, A.; Nakanishi, K.; Ichimura, S.; Kojima, C.; Kono, K. Spontaneous formation of narrowly-distributed self-assembly from polyamidoamine dendron-poly(l-lysine) block copolymers through helix-coil transition of poly(l-lysine) block. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 1217–1223. [Google Scholar] [CrossRef]
- Harada, A.; Ichimura, S.; Yuba, E.; Kono, K. Hollow nanocapsules prepared through stabilization of polymer vesicles formed from head-tail type polycations by introducing cross-linkages. Soft Matter 2011, 7, 4629–4635. [Google Scholar] [CrossRef]
- Meister, A.; Anderson, M.E. Glutathione. Ann. Rev. Biochem. 1983, 52, 711–760. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Harada, A.; Matsuki, R.; Ichimura, S.-i.; Yuba, E.; Kono, K. Intracellular Environment-Responsive Stabilization of Polymer Vesicles Formed from Head-Tail Type Polycations Composed of a Polyamidoamine Dendron and Poly(L-lysine). Molecules 2013, 18, 12168-12179. https://doi.org/10.3390/molecules181012168
Harada A, Matsuki R, Ichimura S-i, Yuba E, Kono K. Intracellular Environment-Responsive Stabilization of Polymer Vesicles Formed from Head-Tail Type Polycations Composed of a Polyamidoamine Dendron and Poly(L-lysine). Molecules. 2013; 18(10):12168-12179. https://doi.org/10.3390/molecules181012168
Chicago/Turabian StyleHarada, Atsushi, Ryota Matsuki, Shin-ichi Ichimura, Eiji Yuba, and Kenji Kono. 2013. "Intracellular Environment-Responsive Stabilization of Polymer Vesicles Formed from Head-Tail Type Polycations Composed of a Polyamidoamine Dendron and Poly(L-lysine)" Molecules 18, no. 10: 12168-12179. https://doi.org/10.3390/molecules181012168
APA StyleHarada, A., Matsuki, R., Ichimura, S.-i., Yuba, E., & Kono, K. (2013). Intracellular Environment-Responsive Stabilization of Polymer Vesicles Formed from Head-Tail Type Polycations Composed of a Polyamidoamine Dendron and Poly(L-lysine). Molecules, 18(10), 12168-12179. https://doi.org/10.3390/molecules181012168