The Role of Bioactive Compounds on the Promotion of Neurite Outgrowth
Abstract
:Abbreviations
| Ntrk1: neurotrophic tyrosine kinase receptor type 1 | NGF: Nerve growth Factor, |
| B-Raf: Serine/threonine-protein kinase | GTPP: Green tea polyphenol |
| MEKK: mitogen-activated protein kinase kinase kinase | CREB: cAMP response element-binding |
| MEK: mitogen-activated protein kinase kinase | Rap1: Ras-Proximate-1 |
| ERK1/2: extracellular signal-regulated kinase | Ras: GTPasep21ras |
| p38-MAPK: p38 mitogen-activated protein kinase | ROS: Reactive oxygen species |
1. Introduction
| Main Biological Source | Compounds | Effective dose | Activity | Ref. |
|---|---|---|---|---|
| Panax ginseng | Ginsenoside Rb1 | 40 mg | Neuritogenesis in rats | [21] |
| Panax ginseng | Ginsenoside Rg1 | 10 mM | Survival of dopaminergic neurons | [22] |
| Curcuma longa | Curcumin | 10 & 20 μM/ 0.2 mg | Neurite outgrowth in PC12 cells/Neurogenesis in mouse | [23,24] |
| Withania somnifera | Withanoside IV & VI | 1 μM | Axon & dendritic extension in rat cortical neurons | [4] |
| Camellia sinensis | EGCG | 0.1–1 μM | Neurite outgrowth in PC12 cells | [25] |
| Picrorhiza scrophulariiflora | Picroside I & II | 60 μM | Potentiating NGF induced neurite outgrowth in PC12D cells | [26] |
| Propolis | Artepillin C | 10, 20 & 50 μM | Potentiating NGF induced neurite outgrowth | [16] |
| Rehmannia glutinosa | Catalpol | 5, 15 & 50 mg | Increase in the number of mouse tyrosine hydroxylase positive cells | [27] |
| Citrus depressa | Nobiletin | 100 μM | Neurite outgrowth in PC12 cells | [28] |
| Sargassum macrocarpum | Sargaquinoic acid | 1.25–100 ng | Potentiating NGF induced neurite outgrowth in PC12D cells | [29] |
| Tripterygium wilfordii | Tripchlorolide | 10−10 M | Neurite outgrowth & survival of dopaminergic neurons. | [30] |
| Scutellaria baicalensis | Baicalein | 5 μg/ 50 & 200 mg | Neurite outgrowth in PC12cells/Increase & survival of rat TH-positive cells | [31] |
2. Natural Herbs and their Active Constituents with Neuritogenic Activity
2.1. Ginsenosides from ginseng

2.2. Curcumin from Curcuma longa
2.3. Withanosides from Withania somnifera
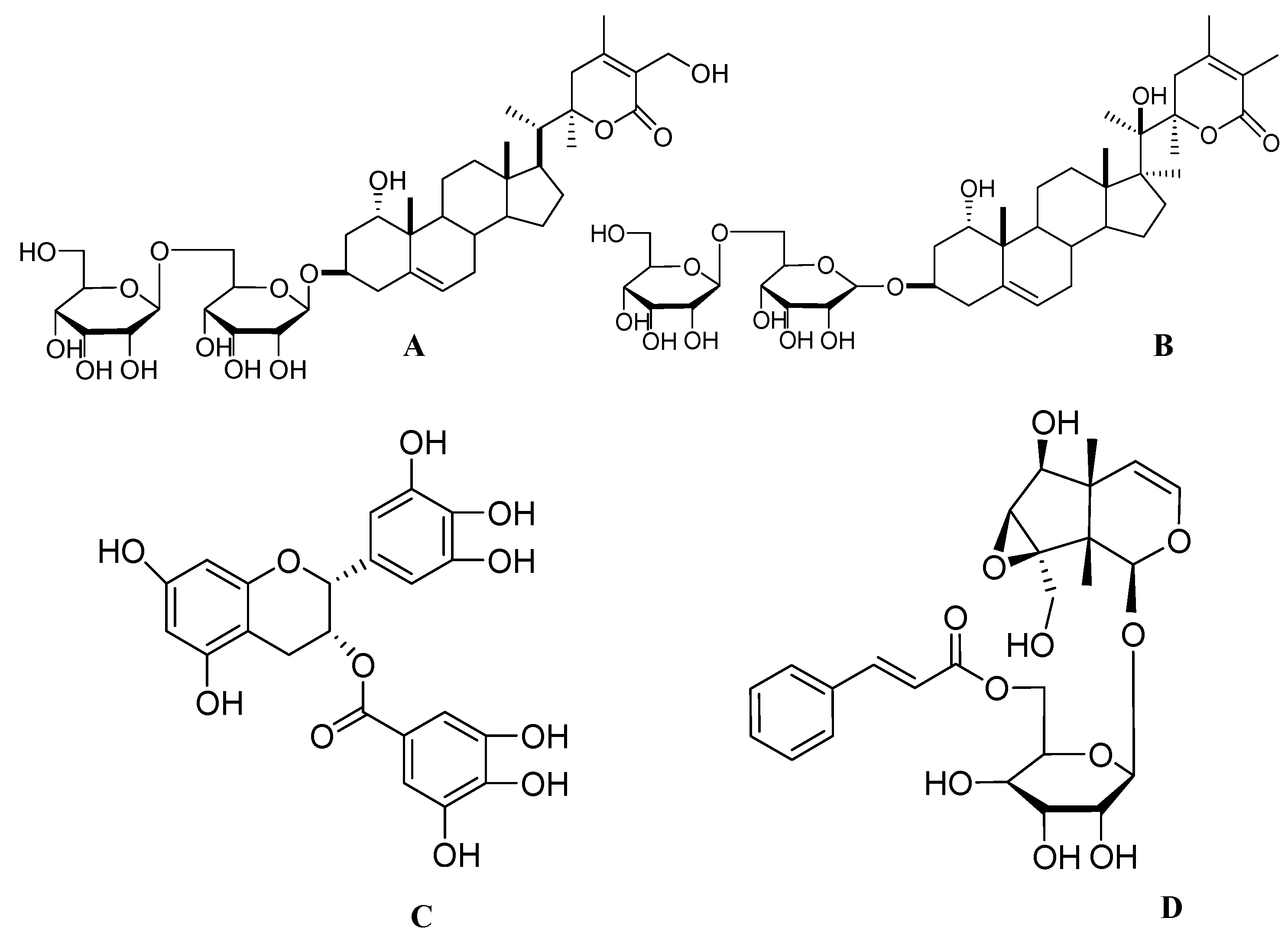
2.4. Green Tea Polyphenols from Camellia sinensis
2.5. Picrosides from Picrorhiza scrophulariiflora
3. Other Miscellaneous Compounds and Extracts from Natural Sources that Show Neuritogenic Activity
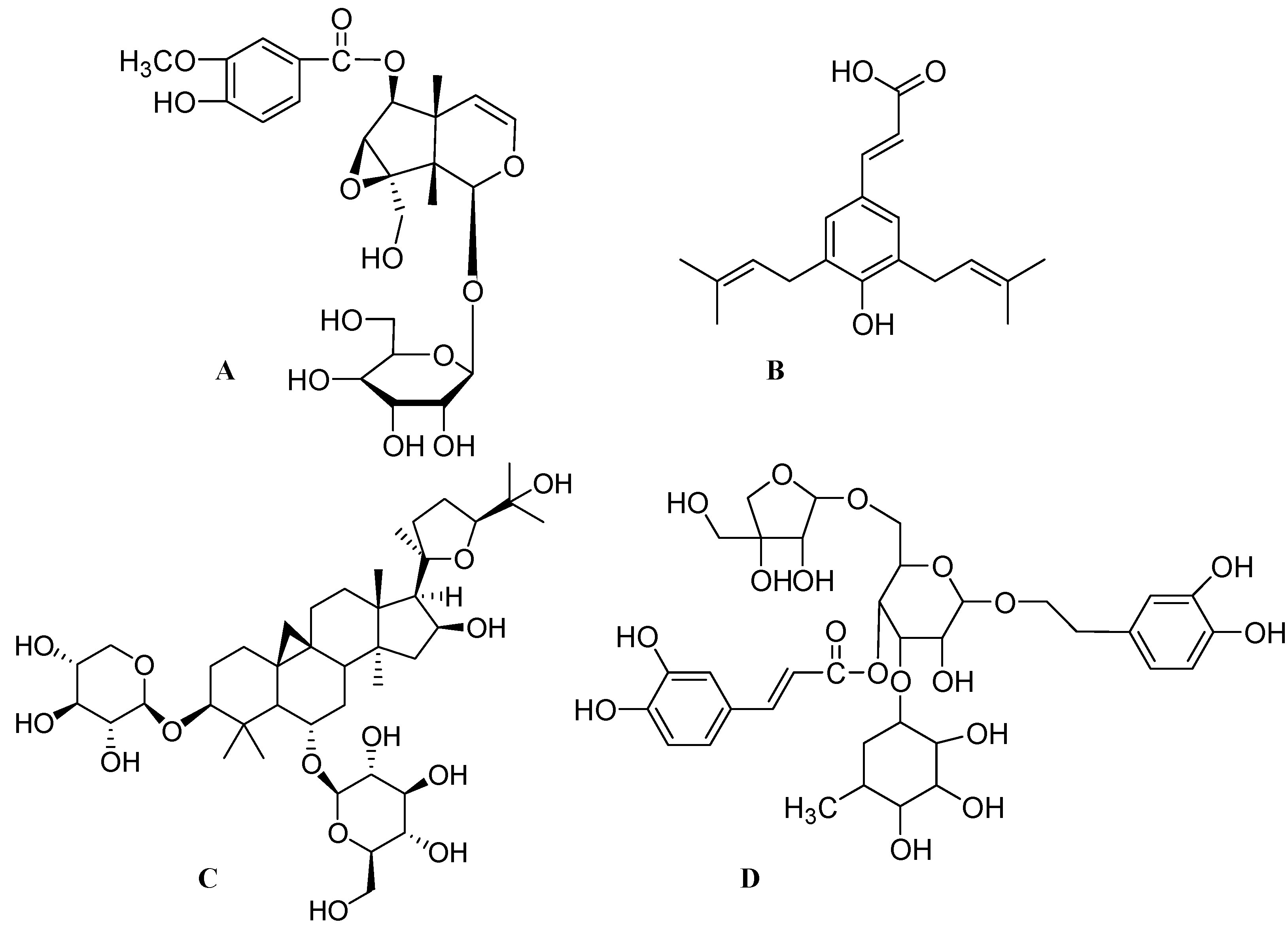
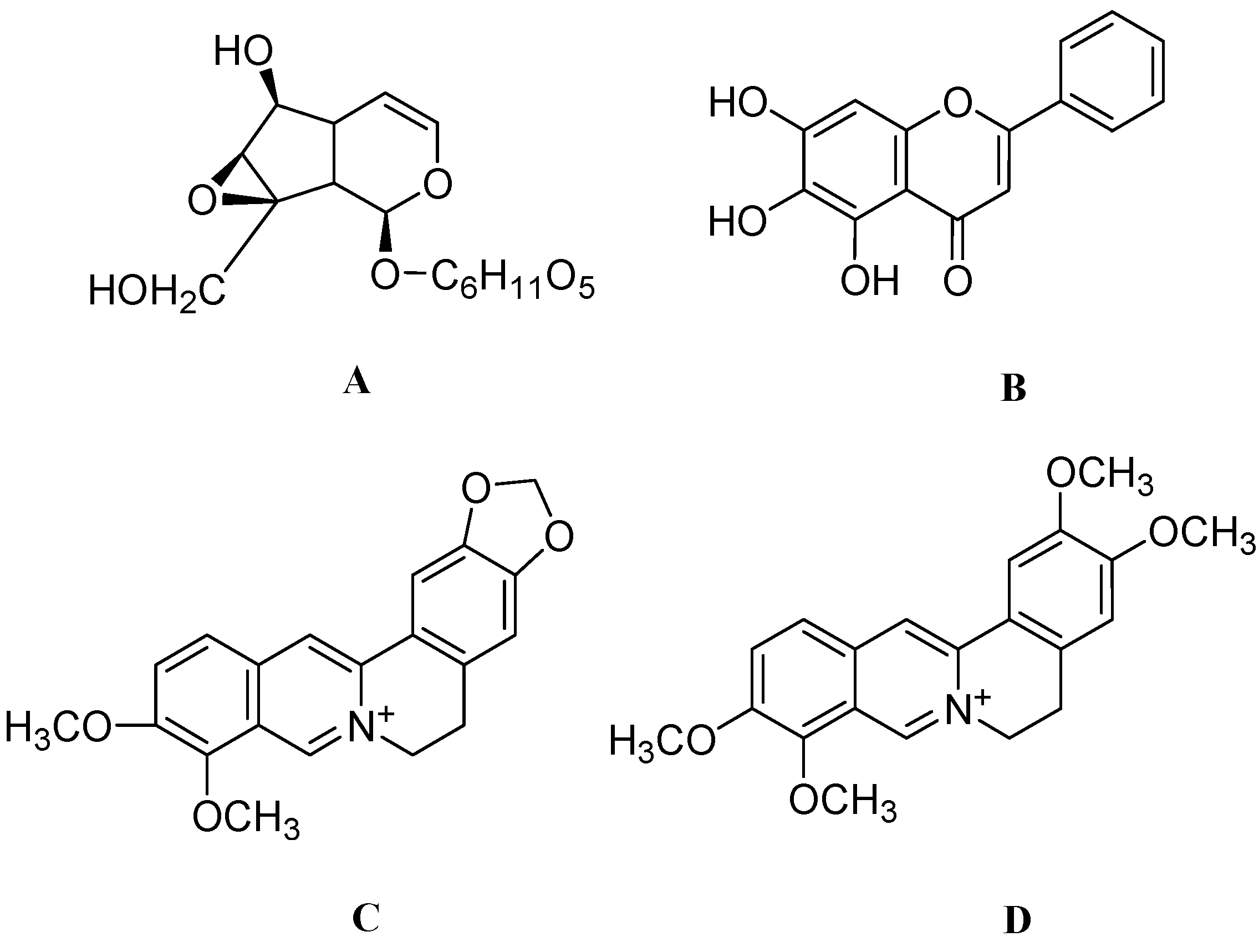
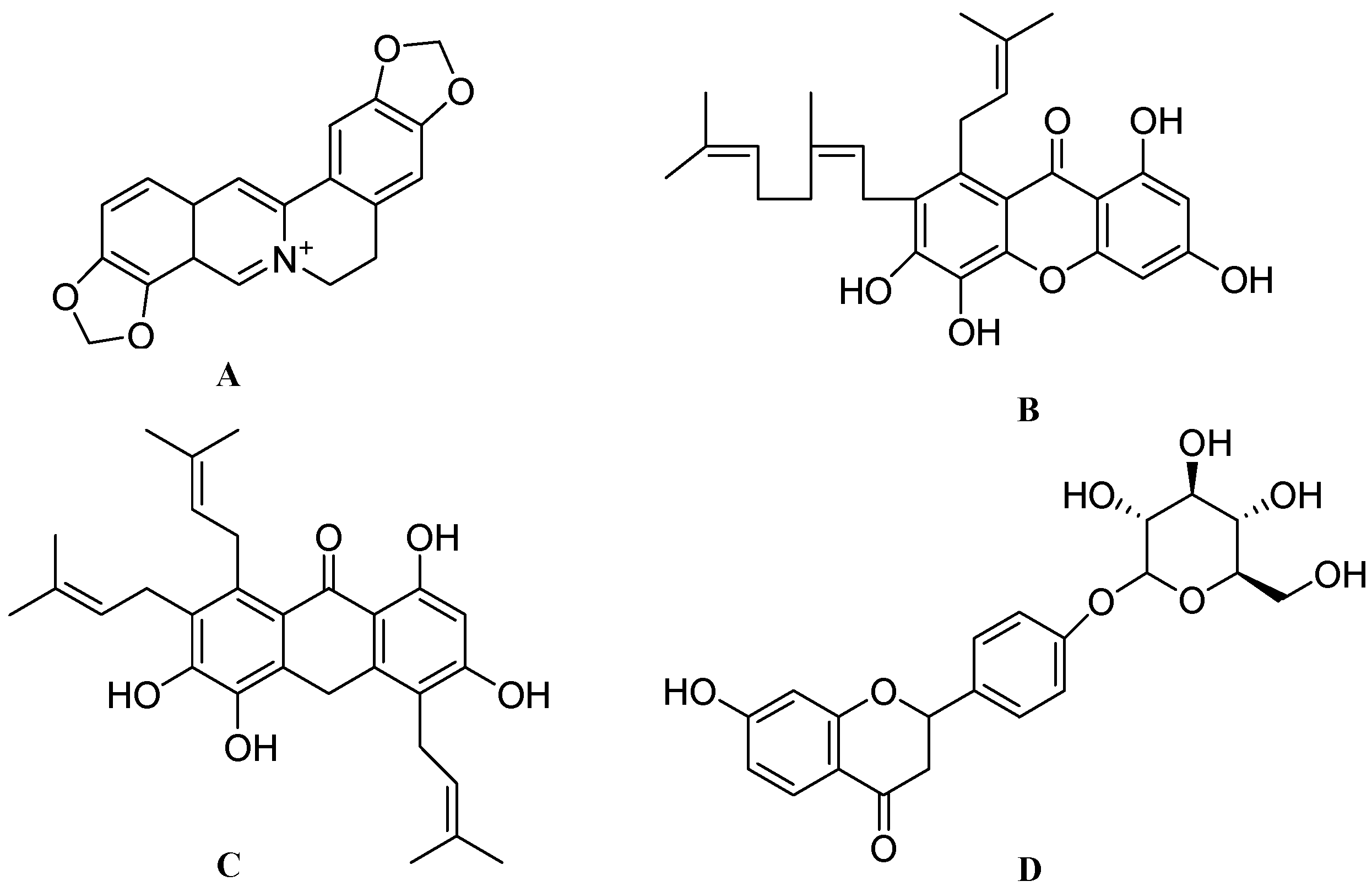



4. Conclusions
Acknowledgments
References
- Enciu, A.M.; Nicolescu, M.I.; Manole, C.G.; Muresanu, D.F.; Popescu, L.M.; Popescu, B.O. Neuroregeneration in neurodegenerative disorders. BMC Neurol. 2011, 11, 75. [Google Scholar] [CrossRef]
- Kiryushko, D.; Berezin, V.; Bock, E. Regulators of neurite outgrowth: Role of cell adhesion molecules. Ann. NY Acad. Sci. 2004, 1014, 140–154. [Google Scholar] [CrossRef]
- Hagg, T. From neurotransmitters to neurotrophic factors to neurogenesis. Neuroscientist 2009, 15, 20–27. [Google Scholar] [CrossRef]
- Tohda, C.; Kuboyama, T.; Komatsu, K. Search for natural products related to regeneration of the neuronal network. Neurosignals 2005, 14, 34–45. [Google Scholar] [CrossRef]
- Filbin, M.T. Axon regeneration: Vaccinating against spinal cord injury. Curr. Biol. 2000, 10, R100–R103. [Google Scholar] [CrossRef]
- Fournier, A.E.; Strittmatter, S.M. Repulsive factors and axon regeneration in the CNS. Curr. Opin. Neurobiol. 2001, 11, 89–94. [Google Scholar] [CrossRef]
- McKerracher, L. Spinal cord repair: Strategies to promote axon regeneration. Neurobiol. Dis. 2001, 8, 11–18. [Google Scholar] [CrossRef]
- Pesavento, E.; Capsoni, S.; Domenici, L.; Cattaneo, A. Acute cholinergic rescue of synaptic plasticity in the neurodegenerating cortex of anti-nerve-growth-factor mice. Eur. J. Neurosci. 2002, 15, 1030–1036. [Google Scholar] [CrossRef]
- Brinton, R.D.; Yamazaki, R.; Gonzalez, C.M.; O’Neill, K.; Schreiber, S.S. Vasopressin-induction of the immediate early gene, NGFI-A, in cultured hippocampal glial cells. Brain Res. Mol. Brain Res. 1998, 57, 73–85. [Google Scholar]
- Li, P.; Matsunaga, K.; Yamakuni, T.; Ohizumi, Y. Picrosides I and II, selective enhancers of the mitogen-activated protein kinase-dependent signaling pathway in the action of neuritogenic substances on PC12D cells. Life Sci. 2002, 71, 1821–1835. [Google Scholar] [CrossRef]
- Hur, J.Y.; Lee, P.; Kim, H.; Kang, I.; Lee, K.R.; Kim, S.Y. (−)-3,5-Dicaffeoyl-muco-quinic acid isolated from Aster scaber contributes to the differentiation of PC12 cells: Through tyrosine kinase cascade signaling. Biochem. Biophys. Res. Commun. 2004, 313, 948–953. [Google Scholar] [CrossRef]
- Nagao, H.; Matsuoka, I.; Kurihara, K. Effects of phorbol ester on expression of CNTF-mRNA in cultured astrocytes from rat olfactory bulb. Brain Res. 1996, 719, 23–28. [Google Scholar] [CrossRef]
- Li, P.; Matsunaga, K.; Yamakuni, T.; Ohizumi, Y. Nardosinone, the first enhancer of neurite outgrowth-promoting activity of staurosporine and dibutyryl cyclic AMP in PC12D cells. Brain Res. Dev. Brain Res. 2003, 145, 177–183. [Google Scholar]
- Sagara, Y.; Vanhnasy, J.; Maher, P. Induction of PC12 cell differentiation by flavonoids is dependent upon extracellular signal-regulated kinase activation. J. Neurochem. 2004, 90, 1144–1155. [Google Scholar] [CrossRef]
- Guo, Y.; Xu, J.; Li, Y.; Watanabe, R.; Oshima, Y.; Yamakuni, T.; Ohizumi, Y. Iridoids and sesquiterpenoids with NGF-potentiating activity from the rhizomes and roots of Valeriana fauriei. Chem. Pharm. Bull. (Tokyo) 2006, 54, 123–125. [Google Scholar] [CrossRef]
- Kano, Y.; Horie, N.; Doi, S.; Aramaki, F.; Maeda, H.; Hiragami, F.; Kawamura, K.; Motoda, H.; Koike, Y.; Akiyama, J.; et al. Artepillin C derived from propolis induces neurite outgrowth in PC12m3 cells via ERK and p38 MAPK pathways. Neurochem. Res. 2008, 33, 1795–1803. [Google Scholar] [CrossRef]
- Shibata, T.; Nakahara, H.; Kita, N.; Matsubara, Y.; Han, C.; Morimitsu, Y.; Iwamoto, N.; Kumagai, Y.; Nishida, M.; Kurose, H.; et al. A food-derived synergist of NGF signaling: Identification of protein tyrosine phosphatase 1B as a key regulator of NGF receptor-initiated signal transduction. J. Neurochem. 2008, 107, 1248–1260. [Google Scholar] [CrossRef]
- Connor, B.; Dragunow, M. The role of neuronal growth factors in neurodegenerative disorders of the human brain. Brain Res. Brain Res. Rev. 1998, 27, 1–39. [Google Scholar] [CrossRef]
- Siegel, G.J.; Chauhan, N.B. Neurotrophic factors in Alzheimer’s and Parkinson’s disease brain. Brain Res. Brain Res. Rev. 2000, 33, 199–227. [Google Scholar]
- Kaneko, N.; Sawamoto, K. Adult neurogenesis and its alteration under pathological conditions. Neurosci. Res. 2009, 63, 155–164. [Google Scholar] [CrossRef]
- Gao, X.Q.; Yang, C.X.; Chen, G.J.; Wang, G.Y.; Chen, B.; Tan, S.K.; Liu, J.; Yuan, Q.L. Ginsenoside Rb1 regulates the expressions of brain-derived neurotrophic factor and caspase-3 and induces neurogenesis in rats with experimental cerebral ischemia. J. Ethnopharmacol. 2010, 132, 393–399. [Google Scholar] [CrossRef]
- Radad, K.; Gille, G.; Moldzio, R.; Saito, H.; Ishige, K.; Rausch, W.D. Ginsenosides Rb1 and Rg1 effects on survival and neurite growth of MPP+-affected mesencephalic dopaminergic cells. J. Neural Transm. 2004, 111, 37–45. [Google Scholar] [CrossRef]
- Liao, K.K.; Wu, M.J.; Chen, P.Y.; Huang, S.W.; Chiu, S.J.; Ho, C.T.; Yen, J.H. Curcuminoids Promote Neurite Outgrowth in PC12 Cells through MAPK/ERK- and PKC-Dependent Pathways. J. Agric. Food Chem. 2012, 60, 433–443. [Google Scholar] [CrossRef]
- Haughey, N.J.; Nath, A.; Chan, S.L.; Borchard, A.C.; Rao, M.S.; Mattson, M.P. Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer’s disease. J. Neurochem. 2002, 83, 1509–1524. [Google Scholar] [CrossRef]
- Reznichenko, L.; Amit, T.; Youdim, M.B.; Mandel, S. Green tea polyphenol (−)-epigallocatechin-3-gallate induces neurorescue of long-term serum-deprived PC12 cells and promotes neurite outgrowth. J. Neurochem. 2005, 93, 1157–1167. [Google Scholar] [CrossRef]
- Li, P.; Matsunaga, K.; Yamakuni, T.; Ohizumi, Y. Potentiation of nerve growth factor-action by picrosides I and II, natural iridoids, in PC12D cells. Eur. J. Pharmacol. 2000, 406, 203–208. [Google Scholar] [CrossRef]
- Xu, G.; Xiong, Z.; Yong, Y.; Wang, Z.; Ke, Z.; Xia, Z.; Hu, Y. Catalpol attenuates MPTP induced neuronal degeneration of nigral-striatal dopaminergic pathway in mice through elevating glial cell derived neurotrophic factor in striatum. Neuroscience 2010, 167, 174–184. [Google Scholar] [CrossRef]
- Nagase, H.; Yamakuni, T.; Matsuzaki, K.; Maruyama, Y.; Kasahara, J.; Hinohara, Y.; Kondo, S.; Mimaki, Y.; Sashida, Y.; Tank, A.W.; et al. Mechanism of neurotrophic action of nobiletin in PC12D cells. Biochemistry 2005, 44, 13683–13691. [Google Scholar]
- Tsang, C.K.; Kamei, Y. Sargaquinoic acid supports the survival of neuronal PC12D cells in a nerve growth factor-independent manner. Eur. J. Pharmacol. 2004, 488, 11–18. [Google Scholar] [CrossRef]
- Li, F.Q.; Cheng, X.X.; Liang, X.B.; Wang, X.H.; Xue, B.; He, Q.H.; Wang, X.M.; Han, J.S. Neurotrophic and neuroprotective effects of tripchlorolide, an extract of Chinese herb Tripterygium wilfordii Hook F, on dopaminergic neurons. Exp. Neurol. 2003, 179, 28–37. [Google Scholar] [CrossRef]
- Mu, X.; He, G.; Cheng, Y.; Li, X.; Xu, B.; Du, G. Baicalein exerts neuroprotective effects in 6-hydroxydopamine-induced experimental parkinsonism in vivo and in vitro. Pharmacol. Biochem. Behav. 2009, 92, 642–648. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, Y.K.; Il Park, N.; Kim, C.S.; Lee, C.Y.; Park, S.U. Chemical constituents and biological activities of the berry of Panax ginseng. J. Med. Plants Res. 2010, 4, 349–353. [Google Scholar]
- Yamahara, J.; Kubomura, Y.; Miki, K.; Fujimura, H. Anti-ulcer action of Panax japonicus rhizome. J. Ethnopharmacol. 1987, 19, 95–101. [Google Scholar] [CrossRef]
- Park, S.; Yeo, M.; Jin, J.H.; Lee, K.M.; Jung, J.Y.; Choue, R.; Cho, S.W.; Hahm, K.B. Rescue of Helicobacter pylori-induced cytotoxicity by red ginseng. Dig. Dis. Sci. 2005, 50, 1218–1227. [Google Scholar] [CrossRef]
- Sathishkumar, N.; Sathiyamoorthy, S.; Ramya, M.; Yang, D.U.; Lee, H.N.; Yang, D.C. Molecular docking studies of anti-apoptotic BCL-2, BCL-XL, and MCL-1 proteins with ginsenosides from Panax ginseng. J. Enzyme Inhib. Med. Chem. [CrossRef]
- Hong, C.E.; Lyu, S.Y. Anti-inflammatory and Anti-oxidative Effects of Korean Red Ginseng Extract in Human Keratinocytes. Immune Netw. 2011, 11, 42–49. [Google Scholar] [CrossRef]
- Ni, W.; Zhang, X.; Wang, B.; Chen, Y.; Han, H.; Fan, Y.; Zhou, Y.; Tai, G. Antitumor activities and immunomodulatory effects of ginseng neutral polysaccharides in combination with 5-fluorouracil. J. Med. Food 2010, 13, 270–277. [Google Scholar] [CrossRef]
- Lee, K.T.; Jung, T.W.; Lee, H.J.; Kim, S.G.; Shin, Y.S.; Whang, W.K. The antidiabetic effect of ginsenoside Rb2 via activation of AMPK. Arch. Pharm. Res. 2011, 34, 1201–1208. [Google Scholar] [CrossRef]
- Lo, Y.T.; Tsai, Y.H.; Wu, S.J.; Chen, J.R.; Chao, J.C. Ginsenoside Rb1 inhibits cell activation and liver fibrosis in rat hepatic stellate cells. J. Med. Food 2011, 14, 1135–1143. [Google Scholar] [CrossRef]
- Karmazyn, M.; Moey, M.; Gan, X.T. Therapeutic potential of ginseng in the management of cardiovascular disorders. Drugs 2011, 71, 1989–2008. [Google Scholar] [CrossRef]
- Yang, J.H.; Han, S.J.; Ryu, J.H.; Jang, I.S.; Kim, D.H. Ginsenoside Rh2 ameliorates scopolamine-induced learning deficit in mice. Biol. Pharm. Bull. 2009, 32, 1710–1715. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, Y.; Wu, L.; Lu, T.; Xu, G.; Liu, X. Suppression of local inflammation contributes to the neuroprotective effect of ginsenoside Rb1 in rats with cerebral ischemia. Neuroscience 2011, 202, 342–351. [Google Scholar]
- Xiang, Y.Z.; Shang, H.C.; Gao, X.M.; Zhang, B.L. A comparison of the ancient use of ginseng in traditional Chinese medicine with modern pharmacological experiments and clinical trials. Phytother. Res. 2008, 22, 851–858. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.R.; Bae, C.S.; Kim, D.; Hong, H.; Nah, S. Protective effect of ginsenosides, active ingredients of Panax ginseng, on kainic acid-induced neurotoxicity in rat hippocampus. Neurosci. Lett. 2002, 325, 129–133. [Google Scholar] [CrossRef]
- Zou, K.; Zhu, S.; Meselhy, M.R.; Tohda, C.; Cai, S.; Komatsu, K. Dammarane-type Saponins from Panax japonicus and their neurite outgrowth activity in SK-N-SH cells. J. Nat. Prod. 2002, 65, 1288–1292. [Google Scholar] [CrossRef]
- Sugaya, A.; Yuzurihara, M.; Tsuda, T.; Yasuda, K.; Kajiwara, K.; Sugaya, E. Proliferative effect of ginseng saponin on neurite extension of primary cultured neurons of the rat cerebral cortex. J. Ethnopharmacol. 1988, 22, 173–181. [Google Scholar] [CrossRef]
- Saito, H.; Suda, K.; Schwab, M.; Thoenen, H. Potentiation of the NGF-mediated nerve fiber outgrowth by ginsenoside Rb1 in organ cultures of chicken dorsal root ganglia. Jpn. J. Pharmacol. 1977, 27, 445–451. [Google Scholar] [CrossRef]
- Nishiyama, N.; Cho, S.I.; Kitagawa, I.; Saito, H. Malonylginsenoside Rb1 potentiates nerve growth factor (NGF)-induced neurite outgrowth of cultured chick embryonic dorsal root ganglia. Biol. Pharm. Bull. 1994, 17, 509–513. [Google Scholar] [CrossRef]
- Maheshwari, R.K.; Singh, A.K.; Gaddipati, J.; Srimal, R.C. Multiple biological activities of curcumin: A short review. Life Sci. 2006, 78, 2081–2087. [Google Scholar] [CrossRef]
- Olivera, A.; Moore, T.W.; Hu, F.; Brown, A.P.; Sun, A.; Liotta, D.C.; Snyder, J.P.; Yoon, Y.; Shim, H.; Marcus, A.I.; et al. Inhibition of the NF-kappaB signaling pathway by the curcumin analog, 3,5-Bis(2-pyridinylmethylidene)-4-piperidone (EF31): Anti-inflammatory and anti-cancer properties. Int. Immunopharmacol. 2011, 12, 368–377. [Google Scholar]
- Nakmareong, S.; Kukongviriyapan, U.; Pakdeechote, P.; Donpunha, W.; Kukongviriyapan, V.; Kongyingyoes, B.; Sompamit, K.; Phisalaphong, C. Antioxidant and vascular protective effects of curcumin and tetrahydrocurcumin in rats with L-NAME-induced hypertension. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 519–529. [Google Scholar] [CrossRef]
- Park, S.H.; Jang, J.H.; Chen, C.Y.; Na, H.K.; Surh, Y.J. A formulated red ginseng extract rescues PC12 cells from PCB-induced oxidative cell death through Nrf2-mediated upregulation of heme oxygenase-1 and glutamate cysteine ligase. Toxicology 2010, 278, 131–139. [Google Scholar] [CrossRef]
- Jackson-Guilford, J.; Leander, J.D.; Nisenbaum, L.K. The effect of streptozotocin-induced diabetes on cell proliferation in the rat dentate gyrus. Neurosci. Lett. 2000, 293, 91–94. [Google Scholar] [CrossRef]
- Wilken, R.; Veena, M.S.; Wang, M.B.; Srivatsan, E.S. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer 2011, 10, 12. [Google Scholar] [CrossRef]
- El-Azab, M.; Hishe, H.; Moustafa, Y.; El-Awady, S. Anti-angiogenic effect of resveratrol or curcumin in Ehrlich ascites carcinoma-bearing mice. Eur. J. Pharmacol. 2011, 652, 7–14. [Google Scholar] [CrossRef]
- Singh, R.K.; Rai, D.; Yadav, D.; Bhargava, A.; Balzarini, J.; De Clercq, E. Synthesis, antibacterial and antiviral properties of curcumin bioconjugates bearing dipeptide, fatty acids and folic acid. Eur. J. Med. Chem. 2010, 45, 1078–1086. [Google Scholar] [CrossRef]
- Srivastava, R.M.; Singh, S.; Dubey, S.K.; Misra, K.; Khar, A. Immunomodulatory and therapeutic activity of curcumin. Int. Immunopharmacol. 2011, 11, 331–341. [Google Scholar] [CrossRef]
- Madhyastha, R.; Madhyastha, H.; Nakajima, Y.; Omura, S.; Maruyama, M. Curcumin facilitates fibrinolysis and cellular migration during wound healing by modulating urokinase plasminogen activator expression. Pathophysiol. Haemost. Thromb. 2010, 37, 59–66. [Google Scholar]
- Kuo, C.P.; Lu, C.H.; Wen, L.L.; Cherng, C.H.; Wong, C.S.; Borel, C.O.; Ju, D.T.; Chen, C.M.; Wu, C.T. Neuroprotective effect of curcumin in an experimental rat model of subarachnoid hemorrhage. Anesthesiology 2011, 115, 1229–1238. [Google Scholar]
- Hatcher, H.; Planalp, R.; Cho, J.; Torti, F.M.; Torti, S.V. Curcumin: From ancient medicine to current clinical trials. Cell Mol. Life Sci. 2008, 65, 1631–1652. [Google Scholar] [CrossRef]
- Xu, Y.; Ku, B.; Cui, L.; Li, X.; Barish, P.A.; Foster, T.C.; Ogle, W.O. Curcumin reverses impaired hippocampal neurogenesis and increases serotonin receptor 1A mRNA and brain-derived neurotrophic factor expression in chronically stressed rats. Brain Res. 2007, 1162, 9–18. [Google Scholar] [CrossRef]
- Cameron, H.A.; McKay, R.D. Restoring production of hippocampal neurons in old age. Nat. Neurosci. 1999, 2, 894–897. [Google Scholar] [CrossRef]
- Harikrishnan, R.; Balasundaram, C.; Jawahar, S.; Heo, M.S. Immunomodulatory effect of Withania somnifera supplementation diet in the giant freshwater prawn Macrobrachium rosenbergii (de Man) against Aeromonas hydrophila. Fish Shellfish Immunol. 2012, 32, 94–100. [Google Scholar] [CrossRef]
- Konar, A.; Shah, N.; Singh, R.; Saxena, N.; Kaul, S.C.; Wadhwa, R.; Thakur, M.K. Protective role of Ashwagandha leaf extract and its component withanone on scopolamine-induced changes in the brain and brain-derived cells. PLoS One 2011, 6, e27265. [Google Scholar]
- El Bouzidi, L.; Larhsini, M.; Markouk, M.; Abbad, A.; Hassani, L.; Bekkouche, K. Antioxidant and antimicrobial activities of Withania frutescens. Nat. Prod. Commun. 2011, 6, 1447–1450. [Google Scholar]
- Javaid, A.; Shafique, S. Herbicidal activity of Withania somnifera against Phalaris minor. Nat. Prod. Res. 2010, 24, 1457–1468. [Google Scholar] [CrossRef]
- Khanna, D.; Sethi, G.; Ahn, K.S.; Pandey, M.K.; Kunnumakkara, A.B.; Sung, B.; Aggarwal, A.; Aggarwal, B.B. Natural products as a gold mine for arthritis treatment. Curr. Opin. Pharmacol. 2007, 7, 344–351. [Google Scholar] [CrossRef]
- Zhao, J.; Nakamura, N.; Hattori, M.; Kuboyama, T.; Tohda, C.; Komatsu, K. Withanolide derivatives from the roots of Withania somnifera and their neurite outgrowth activities. Chem. Pharm. Bull. (Tokyo) 2002, 50, 760–765. [Google Scholar] [CrossRef]
- Kuboyama, T.; Tohda, C.; Zhao, J.; Nakamura, N.; Hattori, M.; Komatsu, K. Axon- or dendrite-predominant outgrowth induced by constituents from Ashwagandha. Neuroreport 2002, 13, 1715–1720. [Google Scholar] [CrossRef]
- Choi, D.K.; Koppula, S.; Suk, K. Inhibitors of microglial neurotoxicity: Focus on natural products. Molecules 2011, 16, 1021–1043. [Google Scholar] [CrossRef]
- Butterfield, D.; Castegna, A.; Pocernich, C.; Drake, J.; Scapagnini, G.; Calabrese, V. Nutritional approaches to combat oxidative stress in Alzheimer’s disease. J. Nutr. Biochem. 2002, 13, 444. [Google Scholar] [CrossRef]
- Chan, E.W.; Soh, E.Y.; Tie, P.P.; Law, Y.P. Antioxidant and antibacterial properties of green, black, and herbal teas of Camellia sinensis. Pharmacognosy Res. 2011, 3, 266–272. [Google Scholar] [CrossRef]
- Park, H.J.; Lee, J.Y.; Chung, M.Y.; Park, Y.K.; Bower, A.M.; Koo, S.I.; Giardina, C.; Bruno, R.S. Green Tea Extract Suppresses NFkappaB Activation and Inflammatory Responses in Diet-Induced Obese Rats with Nonalcoholic Steatohepatitis. J. Nutr. 2012, 142, 57–63. [Google Scholar] [CrossRef]
- Wang, H.; Bian, S.; Yang, C.S. Green tea polyphenol EGCG suppresses lung cancer cell growth through upregulating miR-210 expression caused by stabilizing HIF-1alpha. Carcinogenesis 2011, 32, 1881–1889. [Google Scholar] [CrossRef]
- Fu, Z.; Zhen, W.; Yuskavage, J.; Liu, D. Epigallocatechin gallate delays the onset of type 1 diabetes in spontaneous non-obese diabetic mice. Br. J. Nutr. 2011, 105, 1218–1225. [Google Scholar]
- Potenza, M.A.; Marasciulo, F.L.; Tarquinio, M.; Tiravanti, E.; Colantuono, G.; Federici, A.; Kim, J.A.; Quon, M.J.; Montagnani, M. EGCG, a green tea polyphenol, improves endothelial function and insulin sensitivity, reduces blood pressure, and protects against myocardial I/R injury in SHR. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1378–E1387. [Google Scholar] [CrossRef]
- Mandel, S.A.; Weinreb, O.; Amit, T.; Youdim, M.B. The importance of the multiple target action of green tea polyphenols for neuroprotection. Front. Biosci. (Schol. Ed.) 2012, 4, 581–598. [Google Scholar]
- Mereles, D.; Hunstein, W. Epigallocatechin-3-gallate (EGCG) for Clinical Trials: More Pitfalls than Promises? Int. J. Mol. Sci. 2011, 12, 5592–5603. [Google Scholar] [CrossRef]
- Gundimeda, U.; McNeill, T.H.; Schiffman, J.E.; Hinton, D.R.; Gopalakrishna, R. Green tea polyphenols potentiate the action of nerve growth factor to induce neuritogenesis: Possible role of reactive oxygen species. J. Neurosci. Res. 2010, 88, 3644–3655. [Google Scholar] [CrossRef]
- Zou, L.C.; Zhu, T.F.; Xiang, H.; Yu, L.; Yan, Z.H.; Gan, S.C.; Wang, D.C.; Zeng, S.; Deng, X.M. New secoiridoid glycosides from the roots of Picrorhiza scrophulariiflora. Molecules 2008, 13, 2049–2057. [Google Scholar] [CrossRef]
- He, L.J.; Liang, M.; Hou, F.F.; Guo, Z.J.; Xie, D.; Zhang, X. Ethanol extraction of Picrorhiza scrophulariiflora prevents renal injury in experimental diabetes via anti-inflammation action. J. Endocrinol. 2009, 200, 347–355. [Google Scholar]
- Zeng, S.; Wang, D.; Cao, Y.; An, N.; Zeng, F.; Han, C.; Song, Y.; Deng, X. Immunopotentiation of Caffeoyl Glycoside from Picrorhiza scrophulariiflora on activation and cytokines secretion of immunocyte in vitro. Int. Immunopharmacol. 2008, 8, 1707–1712. [Google Scholar] [CrossRef]
- Vaidya, A.B.; Antarkar, D.S.; Doshi, J.C.; Bhatt, A.D.; Ramesh, V.; Vora, P.V.; Perissond, D.; Baxi, A.J.; Kale, P.M. Picrorhiza kurroa (Kutaki) Royle ex Benth as a hepatoprotective agent—Experimental & clinical studies. J. Postgrad. Med. 1996, 42, 105–108. [Google Scholar]
- Rajkumar, V.; Guha, G.; Kumar, R.A. Antioxidant and anti-neoplastic activities of Picrorhiza kurroa extracts. Food Chem. Toxicol. 2011, 49, 363–369. [Google Scholar] [CrossRef]
- Shukla, B.; Visen, P.K.; Patnaik, G.K.; Dhawan, B.N. Choleretic effect of picroliv, the hepatoprotective principle of Picrorhiza kurroa. Planta Med. 1991, 57, 29–33. [Google Scholar] [CrossRef]
- Vivekanandan, P.; Gobianand, K.; Priya, S.; Vijayalakshmi, P.; Karthikeyan, S. Protective effect of picroliv against hydrazine-induced hyperlipidemia and hepatic steatosis in rats. Drug Chem. Toxicol. 2007, 30, 241–252. [Google Scholar] [CrossRef]
- Lee, H.S.; Ku, S.K. Effect of Picrorrhiza rhizoma extracts on early diabetic nephropathy in streptozotocin-induced diabetic rats. J. Med. Food 2008, 11, 294–301. [Google Scholar] [CrossRef]
- da Rocha, M.D.; Viegas, F.P.; Campos, H.C.; Nicastro, P.C.; Fossaluzza, P.C.; Fraga, C.A.; Barreiro, E.J.; Viegas, C., Jr. The role of natural products in the discovery of new drug candidates for the treatment of neurodegenerative disorders II: Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 2011, 10, 251–270. [Google Scholar]
- Shimazawa, M.; Chikamatsu, S.; Morimoto, N.; Mishima, S.; Nagai, H.; Hara, H. Neuroprotection by Brazilian Green Propolis against In vitro and In vivo Ischemic Neuronal Damage. Evid. Based Complement. Alternat. Med. 2005, 2, 201–207. [Google Scholar] [CrossRef]
- Amodio, R.; De Ruvo, C.; Sacchetti, A.; Di Santo, A.; Martelli, N.; Di Matteo, V.; Lorenzet, R.; Poggi, A.; Rotilio, D.; Cacchio, M.; et al. Caffeic acid phenethyl ester blocks apoptosis induced by low potassium in cerebellar granule cells. Int. J. Dev. Neurosci. 2003, 21, 379–389. [Google Scholar] [CrossRef]
- Wei, X.; Zhao, L.; Ma, Z.; Holtzman, D.M.; Yan, C.; Dodel, R.C.; Hampel, H.; Oertel, W.; Farlow, M.R.; Du, Y. Caffeic acid phenethyl ester prevents neonatal hypoxic-ischaemic brain injury. Brain 2004, 127, 2629–2635. [Google Scholar] [CrossRef]
- Ilhan, A.; Iraz, M.; Gurel, A.; Armutcu, F.; Akyol, O. Caffeic acid phenethyl ester exerts a neuroprotective effect on CNS against pentylenetetrazol-induced seizures in mice. Neurochem. Res. 2004, 29, 2287–2292. [Google Scholar] [CrossRef]
- Isla, M.I.; Nieva Moreno, M.I.; Sampietro, A.R.; Vattuone, M.A. Antioxidant activity of Argentine propolis extracts. J. Ethnopharmacol. 2001, 76, 165–170. [Google Scholar] [CrossRef]
- Pereira, E.M.; da Silva, J.L.; Silva, F.F.; De Luca, M.P.; Ferreira, E.F.; Lorentz, T.C.; Santos, V.R. Clinical Evidence of the Efficacy of a Mouthwash Containing Propolis for the Control of Plaque and Gingivitis: A Phase II Study. Evid. Based Complement. Alternat. Med. 2011, 2011, 750249. [Google Scholar]
- Kimoto, T.; Koya-Miyata, S.; Hino, K.; Micallef, M.J.; Hanaya, T.; Arai, S.; Ikeda, M.; Kurimoto, M. Pulmonary carcinogenesis induced by ferric nitrilotriacetate in mice and protection from it by Brazilian propolis and artepillin C. Virchows Arch. 2001, 438, 259–270. [Google Scholar] [CrossRef]
- Matsuno, T.; Jung, S.K.; Matsumoto, Y.; Saito, M.; Morikawa, J. Preferential cytotoxicity to tumor cells of 3,5-diprenyl-4-hydroxycinnamic acid (artepillin C) isolated from propolis. Anticancer Res. 1997, 17, 3565–3568. [Google Scholar]
- Rios, J.L.; Waterman, P.G. A review of the pharmacology and toxicology of Astragalus. Phytother. Res. 1997, 11, 411–418. [Google Scholar] [CrossRef]
- Chen, C.C.; Lee, H.C.; Chang, J.H.; Chen, S.S.; Li, T.C.; Tsai, C.H.; Cho, D.Y.; Hsieh, C.L. Chinese Herb Astragalus membranaceus Enhances Recovery of Hemorrhagic Stroke: Double-Blind, Placebo-Controlled, Randomized Study. Evid. Based Complement. Alternat. Med. 2012, 2012, 708452. [Google Scholar]
- Ko, J.K.; Lam, F.Y.; Cheung, A.P. Amelioration of experimental colitis by Astragalus membranaceus through anti-oxidation and inhibition of adhesion molecule synthesis. World J. Gastroenterol. 2005, 11, 5787–5794. [Google Scholar]
- Tohda, C.; Tamura, T.; Matsuyama, S.; Komatsu, K. Promotion of axonal maturation and prevention of memory loss in mice by extracts of Astragalus mongholicus. Br. J. Pharmacol. 2006, 149, 532–541. [Google Scholar] [CrossRef]
- Chan, W.S.; Durairajan, S.S.; Lu, J.H.; Wang, Y.; Xie, L.X.; Kum, W.F.; Koo, I.; Yung, K.K.; Li, M. Neuroprotective effects of Astragaloside IV in 6-hydroxydopamine-treated primary nigral cell culture. Neurochem. Int. 2009, 55, 414–422. [Google Scholar] [CrossRef]
- Lu, J.; Pu, X.; Li, Y.; Zhao, Y.; Tu, G. Z. Nat. Forsch. 2005, 60b, 211–214.
- Li, Y.Y.; Lu, J.H.; Li, Q.; Zhao, Y.Y.; Pu, X.P. Pedicularioside A from Buddleia lindleyana inhibits cell death induced by 1-methyl-4-phenylpyridinium ions (MPP+) in primary cultures of rat mesencephalic neurons. Eur. J. Pharmacol. 2008, 579, 134–140. [Google Scholar] [CrossRef]
- Cai, Q.Y.; Chen, X.S.; Zhan, X.L.; Yao, Z.X. Protective effects of catalpol on oligodendrocyte death and myelin breakdown in a rat model of chronic cerebral hypoperfusion. Neurosci. Lett. 2011, 497, 22–26. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Q.; Zhang, R.; Liu, S.; Xia, Z.; Hu, Y. Catalpol ameliorates beta amyloid-induced degeneration of cholinergic neurons by elevating brain-derived neurotrophic factors. Neuroscience 2009, 163, 1363–1372. [Google Scholar] [CrossRef]
- van Leyen, K.; Kim, H.Y.; Lee, S.R.; Jin, G.; Arai, K.; Lo, E.H. Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke 2006, 37, 3014–3018. [Google Scholar] [CrossRef]
- Hsieh, C.J.; Hall, K.; Ha, T.; Li, C.; Krishnaswamy, G.; Chi, D.S. Baicalein inhibits IL-1beta- and TNF-alpha-induced inflammatory cytokine production from human mast cells via regulation of the NF-kappaB pathway. Clin. Mol. Allergy 2007, 5, 5. [Google Scholar] [CrossRef]
- Schmeller, T.; Latz-Bruning, B.; Wink, M. Biochemical activities of berberine, palmatine and sanguinarine mediating chemical defence against microorganisms and herbivores. Phytochemistry 1997, 44, 257–266. [Google Scholar]
- Iwasa, K.; Kamigauchi, M.; Ueki, M.; Taniguchi, M. Antibacterial activity and structure-activity relationships of berberine analogs. Eur. J. Med. Chem. 1996, 31, 469–478. [Google Scholar] [CrossRef]
- Cao, H.; Ren, M.; Guo, L.; Shang, H.; Zhang, J.; Song, Y.; Wang, H.; Wang, B.; Li, X.; Hu, J.; et al. JinQi-Jiangtang tablet, a Chinese patent medicine, for pre-diabetes: A randomized controlled trial. Trials 2010, 11, 27. [Google Scholar] [CrossRef]
- Shigeta, K.; Ootaki, K.; Tatemoto, H.; Nakanishi, T.; Inada, A.; Muto, N. Potentiation of nerve growth factor-induced neurite outgrowth in PC12 cells by a Coptidis Rhizoma extract and protoberberine alkaloids. Biosci. Biotechnol. Biochem. 2002, 66, 2491–2494. [Google Scholar] [CrossRef]
- Niu, S.L.; Li, Z.L.; Ji, F.; Liu, G.Y.; Zhao, N.; Liu, X.Q.; Jing, Y.K.; Hua, H.M. Xanthones from the stem bark of Garcinia bracteata with growth inhibitory effects against HL-60 cells. Phytochemistry 2012, 77, 280–286. [Google Scholar] [CrossRef]
- Rukachaisirikul, V.; Phainuphong, P.; Sukpondma, Y.; Phongpaichit, S.; Taylor, W.C. Antibacterial caged-tetraprenylated xanthones from the stem bark of Garcinia scortechinii. Planta Med. 2005, 71, 165–170. [Google Scholar] [CrossRef]
- Likhitwitayawuid, K.; Chanmahasathien, W.; Ruangrungsi, N.; Krungkrai, J. Xanthones with antimalarial activity from Garcinia dulcis. Planta Med. 1998, 64, 281–282. [Google Scholar] [CrossRef]
- Nakatani, K.; Nakahata, N.; Arakawa, T.; Yasuda, H.; Ohizumi, Y. Inhibition of cyclooxygenase and prostaglandin E2 synthesis by gamma-mangostin, a xanthone derivative in mangosteen, in C6 rat glioma cells. Biochem. Pharmacol. 2002, 63, 73–79. [Google Scholar]
- Chanmahasathien, W.; Li, Y.; Satake, M.; Oshima, Y.; Ishibashi, M.; Ruangrungsi, N.; Ohizumi, Y. Prenylated xanthones from Garcinia xanthochymus. Chem. Pharm. Bull. (Tokyo) 2003, 51, 1332–1334. [Google Scholar] [CrossRef]
- Chen, Z.A.; Wang, J.L.; Liu, R.T.; Ren, J.P.; Wen, L.Q.; Chen, X.J.; Bian, G.X. Liquiritin potentiate neurite outgrowth induced by nerve growth factor in PC12 cells. Cytotechnology 2009, 60, 125–132. [Google Scholar]
- Hibasami, H.; Iwase, H.; Yoshioka, K.; Takahashi, H. Glycyrrhizin induces apoptosis in human stomach cancer KATO III and human promyelotic leukemia HL-60 cells. Int. J. Mol. Med. 2005, 16, 233–236. [Google Scholar]
- Amer, M.; Metwalli, M. Topical liquiritin improves melasma. Int. J. Dermatol. 2000, 39, 299–301. [Google Scholar] [CrossRef]
- Whitman, S.C.; Kurowska, E.M.; Manthey, J.A.; Daugherty, A. Nobiletin, a citrus flavonoid isolated from tangerines, selectively inhibits class A scavenger receptor-mediated metabolism of acetylated LDL by mouse macrophages. Atherosclerosis 2005, 178, 25–32. [Google Scholar] [CrossRef]
- Bagchi, A.; Oshima, Y.; Hikino, H. Planta Med. 1988, 54, 87–88.
- Li, P.; Matsunaga, K.; Yamamoto, K.; Yoshikawa, R.; Kawashima, K.; Ohizumi, Y. Nardosinone, a novel enhancer of nerve growth factor in neurite outgrowth from PC12D cells. Neurosci. Lett. 1999, 273, 53–56. [Google Scholar] [CrossRef]
- Hur, S.; Lee, H.; Kim, Y.; Lee, B.H.; Shin, J.; Kim, T.Y. Sargaquinoic acid and sargachromenol, extracts of Sargassum sagamianum, induce apoptosis in HaCaT cells and mice skin: Its potentiation of UVB-induced apoptosis. Eur. J. Pharmacol. 2008, 582, 1–11. [Google Scholar] [CrossRef]
- Kamei, Y.; Tsang, C.K. Sargaquinoic acid promotes neurite outgrowth via protein kinase A and MAP kinases-mediated signaling pathways in PC12D cells. Int. J. Dev. Neurosci. 2003, 21, 255–262. [Google Scholar] [CrossRef]
- Nagao, T.; Okabe, H. Studies on the constituents of Aster scaber Thunb. III. Structures of scaberosides B7, B8 and B9, minor oleanolic acid glycosides isolated from the root. Chem. Pharm. Bull. (Tokyo) 1992, 40, 886–888. [Google Scholar] [CrossRef]
- Nagao, T.; Tanaka, R.; Iwase, Y.; Okabe, H. Studies on the constituents of Aster scaber Thunb. IV. Structures of four new echinocystic acid glycosides isolated from the herb. Chem. Pharm. Bull. (Tokyo) 1993, 41, 659–665. [Google Scholar]
- van Dijk, A.E.; Olthof, M.R.; Meeuse, J.C.; Seebus, E.; Heine, R.J.; van Dam, R.M. Acute effects of decaffeinated coffee and the major coffee components chlorogenic acid and trigonelline on glucose tolerance. Diabetes Care 2009, 32, 1023–1025. [Google Scholar] [CrossRef]
- Tohda, C.; Nakamura, N.; Komatsu, K.; Hattori, M. Trigonelline-induced neurite outgrowth in human neuroblastoma SK-N-SH cells. Biol. Pharm. Bull. 1999, 22, 679–682. [Google Scholar] [CrossRef]
- Ishiguro, K.; Yamaki, M.; Takagi, S. Studies on iridoid-related compounds, II. The structure and antimicrobial activity of aglucones of galioside and gardenoside. J. Nat. Prod. 1983, 46, 532–526. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Yang, J.J.; Lin, S.Y.; Lin, C.C. Comparisons of geniposidic acid and geniposide on antitumor and radioprotection after sublethal irradiation. Cancer Lett. 1997, 113, 31–37. [Google Scholar] [CrossRef]
- Circosta, C.; Occhiuto, F.; Ragusa, S.; Trovato, A.; Tumino, G.; Briguglio, F.; de Pasquale, A. A drug used in traditional medicine: Harpagophytum procumbens DC. II. Cardiovascular activity. J. Ethnopharmacol. 1984, 11, 259–274. [Google Scholar] [CrossRef]
- Miyagoshi, M.; Amagaya, S.; Ogihara, Y. Choleretic actions of iridoid compounds. J. Pharmacobiodyn. 1988, 11, 186–190. [Google Scholar] [CrossRef]
- Chang, I.M. Liver-protective activities of aucubin derived from traditional oriental medicine. Res. Commun. Mol. Pathol. Pharmacol. 1998, 102, 189–204. [Google Scholar]
- Yamazaki, M.; Chiba, K. Genipin exhibits neurotrophic effects through a common signaling pathway in nitric oxide synthase-expressing cells. Eur. J. Pharmacol. 2008, 581, 255–261. [Google Scholar] [CrossRef]
- Tao, X.L. Mechanism of treating rheumatoid arthritis with Tripterygium wilfordii hook. II. Effect on PGE2 secretion. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 1989, 11, 36–40. [Google Scholar]
- Tao, X.; Younger, J.; Fan, F.Z.; Wang, B.; Lipsky, P.E. Benefit of an extract of Tripterygium Wilfordii Hook F in patients with rheumatoid arthritis: A double-blind, placebo-controlled study. Arthritis Rheum. 2002, 46, 1735–1743. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Zhao, C.C.; Wang, Y. Pregnane glycosides and steroid saponins from Smilax bockii Warb. and their NGF-potentiating activity. Nat. Prod. Res. 2008, 22, 884–889. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Zhang, P.; Li, Z.L.; Wang, Y. Antiinflammatory constituents from the roots of Smilax bockii warb. Arch. Pharm. Res. 2005, 28, 395–399. [Google Scholar] [CrossRef]
- Castro-Gamboa, I.; Castro, O. Iridoids from the aerial parts of Verbena littoralis (Verbenaceae). Phytochemistry 2004, 65, 2369–2372. [Google Scholar] [CrossRef]
- Li, Y.; Ishibashi, M.; Chen, X.; Ohizumi, Y. Littorachalcone, a new enhancer of NGF-mediated neurite outgrowth, from Verbena littoralis. Chem. Pharm. Bull. (Tokyo) 2003, 51, 872–874. [Google Scholar] [CrossRef]
- Gao, L.; Xiang, L.; Luo, Y.; Wang, G.; Li, J.; Qi, J. Gentisides C-K: Nine new neuritogenic compounds from the traditional Chinese medicine Gentiana rigescens Franch. Bioorg. Med. Chem. 2010, 18, 6995–7000. [Google Scholar]
- Gao, L.; Li, J.; Qi, J. Gentisides A and B, two new neuritogenic compounds from the traditional Chinese medicine Gentiana rigescens Franch. Bioorg. Med. Chem. 2010, 18, 2131–2134. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Korman, N.J.; Mukhtar, H.; Agarwal, R. Protective effects of silymarin against photocarcinogenesis in a mouse skin model. J. Natl. Cancer Inst. 1997, 89, 556–566. [Google Scholar] [CrossRef]
- Ferenci, P.; Dragosics, B.; Dittrich, H.; Frank, H.; Benda, L.; Lochs, H.; Meryn, S.; Base, W.; Schneider, B. Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J. Hepatol. 1989, 9, 105–113. [Google Scholar]
- Tager, M.; Dietzmann, J.; Thiel, U.; Hinrich Neumann, K.; Ansorge, S. Restoration of the cellular thiol status of peritoneal macrophages from CAPD patients by the flavonoids silibinin and silymarin. Free Radic. Res. 2001, 34, 137–151. [Google Scholar] [CrossRef]
- Manna, S.K.; Mukhopadhyay, A.; Van, N.T.; Aggarwal, B.B. Silymarin suppresses TNF-induced activation of NF-kappa B, c-Jun N-terminal kinase, and apoptosis. J. Immunol. 1999, 163, 6800–6809. [Google Scholar]
- Pradhan, S.C.; Girish, C. Hepatoprotective herbal drug, silymarin from experimental pharmacology to clinical medicine. Indian J. Med. Res. 2006, 124, 491–504. [Google Scholar]
- Kittur, S.; Wilasrusmee, S.; Pedersen, W.A.; Mattson, M.P.; Straube-West, K.; Wilasrusmee, C.; Lubelt, B.; Kittur, D.S. Neurotrophic and neuroprotective effects of milk thistle (Silybum marianum) on neurons in culture. J. Mol. Neurosci. 2002, 18, 265–269. [Google Scholar] [CrossRef]
- Lieu, C.W.; Lee, S.S.; Wang, S.Y. Anticancer Res. 1992, 12, 1211–1215.
- Wang, G.; Zhang, J.; Mizuno, T.; Zhuang, C.; Ito, H.; Mayuzumi, H.; Okamoto, H.; Li, J. Antitumor active polysaccharides from the Chinese mushroom Songshan lingzhi, the fruiting body of Ganoderma tsugae. Biosci. Biotechnol. Biochem. 1993, 57, 894–900. [Google Scholar] [CrossRef]
- Kim, R.S.; Kim, H.W.; Kim, B.K. Mol. Cells 1997, 7, 52–57.
- van der Hem, L.G.; van der Vliet, J.A.; Bocken, C.F.; Kino, K.; Hoitsma, A.J.; Tax, W.J. Ling Zhi-8: Studies of a new immunomodulating agent. Transplantation 1995, 60, 438–443. [Google Scholar] [CrossRef]
- Cheung, W.M.; Hui, W.S.; Chu, P.W.; Chiu, S.W.; Ip, N.Y. Ganoderma extract activates MAP kinases and induces the neuronal differentiation of rat pheochromocytoma PC12 cells. FEBS Lett. 2000, 486, 291–296. [Google Scholar] [CrossRef]
- Shi, Y.L.; Chen, W.Y. Effect of Toosendanin on acetylcholine level of rat brain, a microdialysis study. Brain Res. 1999, 850, 173–178. [Google Scholar] [CrossRef]
- Yu, J.C.; Min, Z.D.; Ip, N.Y. Melia toosendan regulates PC12 Cell differentiation via the activation of protein kinase A and extracellular signal-regulated kinases. Neurosignals 2004, 13, 248–257. [Google Scholar] [CrossRef]
- Cheon, E.W.; Saito, T. Choline acetyltransferase and acetylcholinesterase in the normal, developing and regenerating newt retinas. Brain Res. Dev. Brain Res. 1999, 116, 97–109. [Google Scholar] [CrossRef]
- Liu, J.-H.; Bo, J.; Bao, Y.-M.; An, L.-J. Int. J. Dev. Neurosci. 2003, 21, 277–281. [CrossRef]
- Schachter, S.C. Botanicals and herbs: A traditional approach to treating epilepsy. Neurotherapeutics 2009, 6, 415–420. [Google Scholar] [CrossRef]
- Rates, S.M. Plants as source of drugs. Toxicon 2001, 39, 603–613. [Google Scholar] [CrossRef]
- Guo, Z.R. Modification of natural products for drug discovery. Yao Xue Xue Bao 2012, 47, 144–157. [Google Scholar]
- Rishton, G.M. Natural products as a robust source of new drugs and drug leads: Past successes and present day issues. Am. J. Cardiol. 2008, 101, 43–49. [Google Scholar] [CrossRef]
- Hamburger, M.; Hostettmann, K. Bioactivity in plants: The link between phytochemistry and medicine. Phytochemistry 1991, 30, 3864–3874. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
More, S.V.; Koppula, S.; Kim, I.-S.; Kumar, H.; Kim, B.-W.; Choi, D.-K. The Role of Bioactive Compounds on the Promotion of Neurite Outgrowth. Molecules 2012, 17, 6728-6753. https://doi.org/10.3390/molecules17066728
More SV, Koppula S, Kim I-S, Kumar H, Kim B-W, Choi D-K. The Role of Bioactive Compounds on the Promotion of Neurite Outgrowth. Molecules. 2012; 17(6):6728-6753. https://doi.org/10.3390/molecules17066728
Chicago/Turabian StyleMore, Sandeep Vasant, Sushruta Koppula, In-Su Kim, Hemant Kumar, Byung-Wook Kim, and Dong-Kug Choi. 2012. "The Role of Bioactive Compounds on the Promotion of Neurite Outgrowth" Molecules 17, no. 6: 6728-6753. https://doi.org/10.3390/molecules17066728
APA StyleMore, S. V., Koppula, S., Kim, I.-S., Kumar, H., Kim, B.-W., & Choi, D.-K. (2012). The Role of Bioactive Compounds on the Promotion of Neurite Outgrowth. Molecules, 17(6), 6728-6753. https://doi.org/10.3390/molecules17066728





