1H-Nuclear Magnetic Resonance Analysis of the Triacylglyceride Composition of Cold-Pressed Oil from Camellia japonica
Abstract
:1. Introduction
2. Results and Discussion
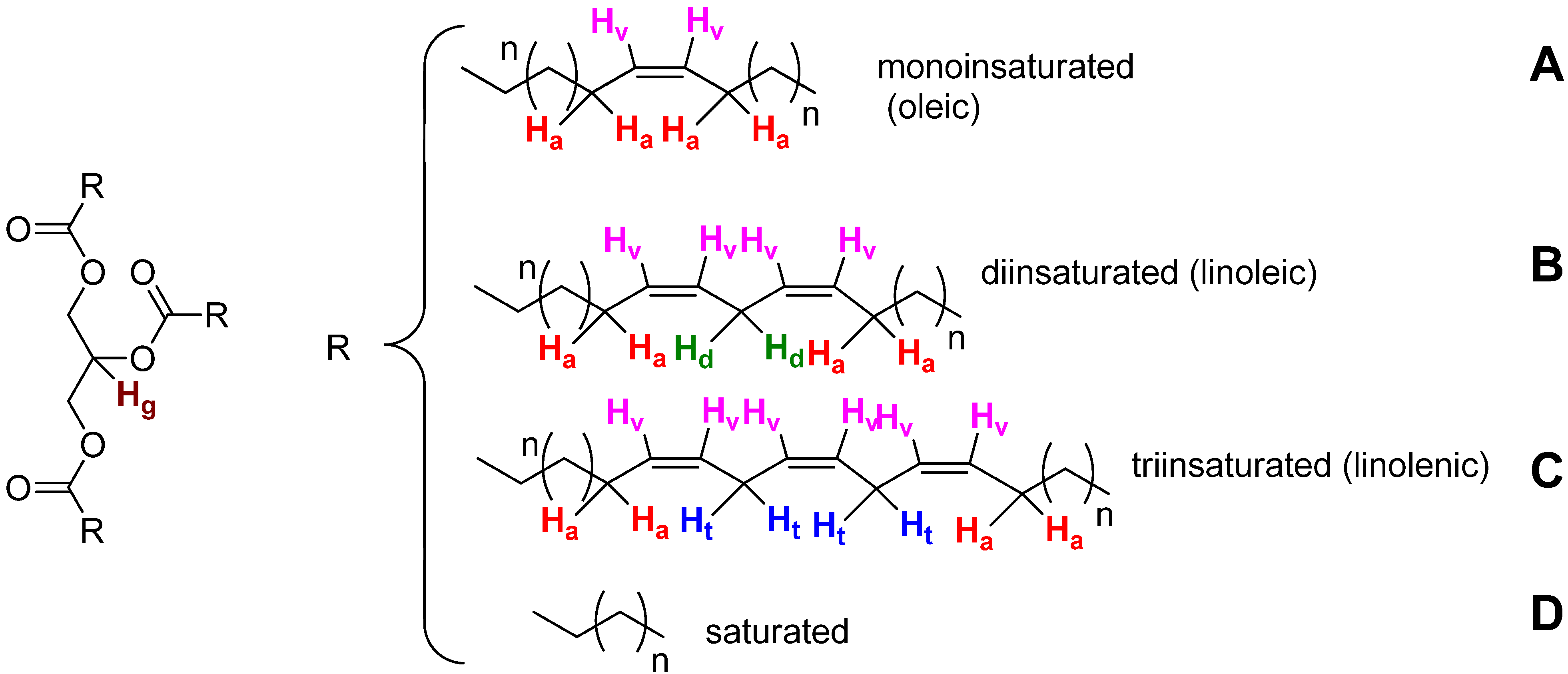
| Signal | Functional group | Multiplicity | Chemical shift (ppm) |
|---|---|---|---|
| 1 | I (dd) –CH3 | dd | 0.96–0.82 |
| 2 | H (m) –CH2– | m | 1.43–1.16 |
| 3 | G (m) –CH2–C–CO2 | m | 1.70–1.51 |
| 4 | F (m) –CH2–CO2– | m | 2.11–1.91 |
| 5 | E (m) –C–CH2–C=C– | m | 2.38–2.21 |
| 6 | D (t) –C=C–CH2–C=C––C=C–CH2–C=C–CH2–C=C | t | 2.83–2.73 |
| 7 | C (dd) –C–CH2–O–CO–C | dd | 4.21–4.08 |
| 8 | B (dd) –C–CH2–O–CO–C | dd | 4.36–4.22 |
| 9 | A (m) CH(–C–O–CO–C–)2+ C–HC=CH–C | m | 5.43–5.13 |
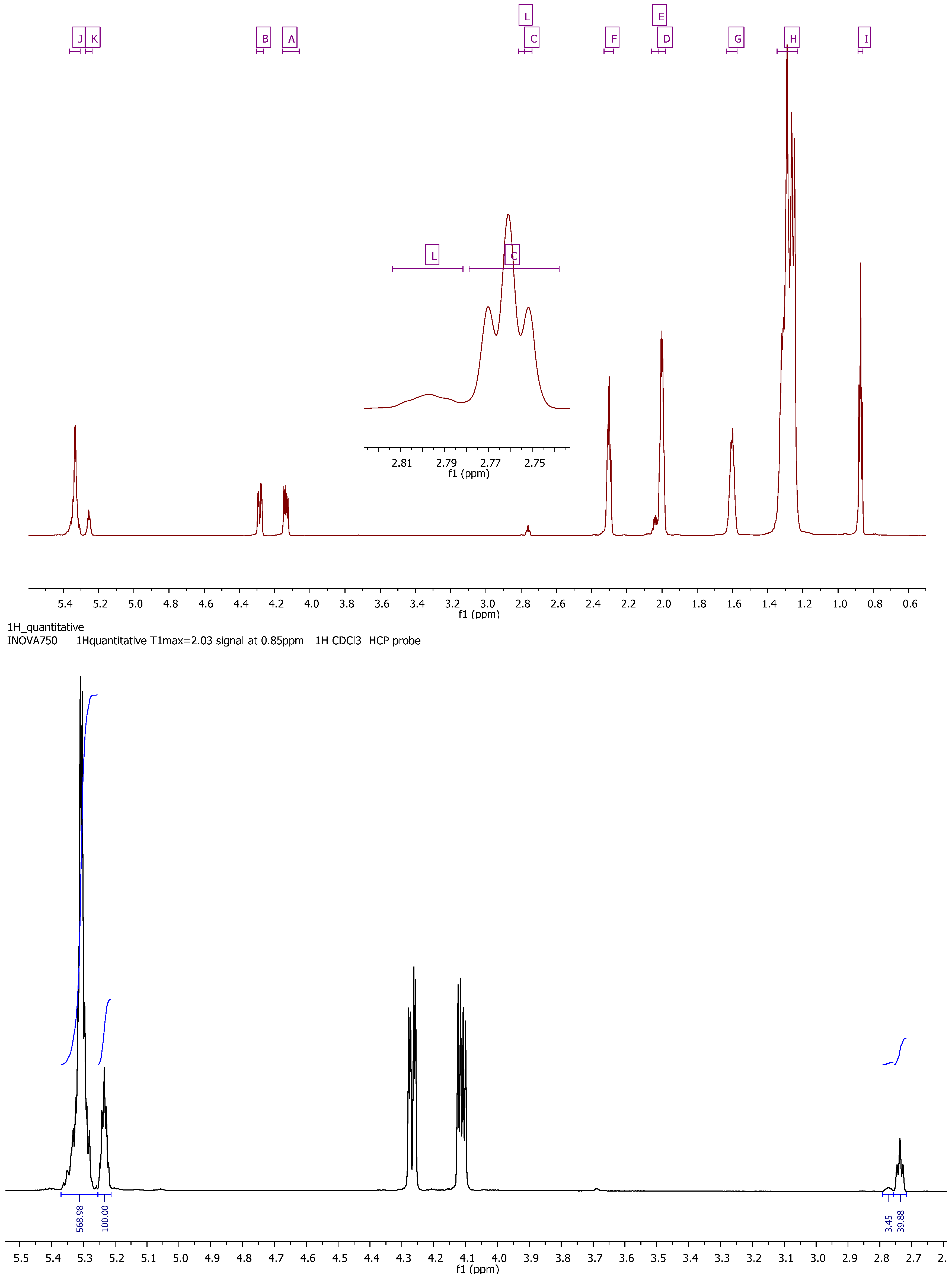
| Signal | Functional group | Multiplicity | Chemical shift (ppm) |
|---|---|---|---|
| 1 | I (t) –CH3 | t | 0.89–0.86 |
| 2 | H (m) –CH2– | m | 1.35–1.23 |
| 3 | G (m) –CH2–C–CO2– | m | 1.64–1.57 |
| 4 | D (m) –CH2–CO2– | m | 2.02–1.98 |
| 5 | E (m) –CH2–CO2– | m | 2.06–2.02 |
| 6 | F (dt) –C–CH2–C=C– | dt | 2.33–2.28 |
| 7 | C (t) –C=C–CH2–C=C– | t | 2.78–2.74 |
| 8 | L (m) –C=C–CH2–C=C–CH2–C=C | m | 2.81–2.78 |
| 9 | A (dd) –C–CH2–O–CO–C | dd | 4.15–4.06 |
| 10 | B (dd) –C–CH2–O–CO–C | dd | 4.30–4.26 |
| 11 | K (m) CH(–C–O–CO–C–)2 | m | 5.27–5.24 |
| 12 | J (m) C–HC=CH–C | m | 5.37–5.30 |


| Oil | Linoleic | Linolelic | Oleic | Saturated |
|---|---|---|---|---|
| Virgin olive a | 6.0 | 0.7 | 81.3 | 12 |
| Hazelnut a | 13.5 | 0.0 | 77.9 | 8.6 |
| Peanut a | 21.6 | 0.0 | 61.8 | 16.6 |
| Virgin olive b | 5.9 | 0.7 | 80.0 | 13.4 |
| Olive b | 7.4 | 0.7 | 77.5 | 14.4 |
| Hazelnut b | 10.7 | 0.0 | 81.0 | 8.3 |
| Corn b | 51.0 | 0.7 | 33.0 | 15.3 |
| Sunflower b | 58.8 | 0.0 | 29.2 | 12.0 |
| Soybean b | 54.2 | 10.4 | 20.4 | 15.0 |
| Linseed b | 17.1 | 54.2 | 20.0 | 8.7 |
| Avocado c | 10 | 1 | 65 | 20 |
| Tea seed oil c,* | 10 | <1 | 80 | 10 |
| Pumpkin c | 40 | <1 | 40 | 10 |
| Soybean c | 50 | 7 | 25 | 15 |
| Canola c | 20 | 10 | 60 | 7 |
| Olive c | 8 | <1 | 75 | 14 |
| Camellia japonica ** | 6.65 | 0.29 | 80.67 | 12.39 |
3. Experimental
3.1. Oil Obtention
4.2. 1H-NMR Analysis
4.3. 13C-NMR Analysis
4. Conclusions
Acknowledgements
- Sample Availability: Samples of the Camellia japonica oil used are available from the authors.
References and notes
- Ming, T.L.; Bartholomew, B. Theaceae. In Flora of China; Wu, Z.Y., Raven, P.H., Hong, D.Y., Eds.; Science Press: Beijing, China, and Missouri Botanical Garden: St. Louis, MO, USA, 2007; Volume 12, pp. 366–478. [Google Scholar]
- Gao, J.Y.; Parks, C.R.; Du, Y.Q. Collected Species of the Genus Camellia-An Illustrated Outline; Zhejiang Scientific & Technology: Hangzhou, China, 2005. [Google Scholar]
- Savige, T.J. The International Camellia Register; The International Camellia Society: Wirlinga, NSW, Australia, 1993. [Google Scholar]
- Yoshikawa, M.; Morikawa, T.; Asao, Y.; Fujiwara, E.; Nakamura, S.; Matsuda, H. Medicinal Flowers. XV. The Structures of Noroleanane- and Oleanane-Type Triterpene Oligoglycosides with Gastroprotective and Platelet Aggregation Activities from Flower Buds of Camellia Japonica. Chem. Pharm. Bull. 2007, 55, 606–612. [Google Scholar]
- Han, L.; Hatano, T.; Yoshida, T.; Okuda, T. Tannins of Theaceous Plants. V. Camelliatannins F, G and H, Three New Tannins from Camellia Jjaponica L. Chem. Pharm. Bull. 1994, 42, 1399–1409. [Google Scholar]
- Saito, N.; Yokoi, M.; Yamaji, M.; Honda, T. Cyanidin 3-p-Coumaroylglucoside in Camellia Species and Cultivars. Phytochemistry 1987, 26, 2761–2762. [Google Scholar]
- Thao, N.T.P.; Hung, T.M.; Lee, M.K.; Kim, J.C.; Min, B.S.; Bae, K. Triterpenoids from Camellia Japonica and their Cytotoxic Activity. Chem. Pharm. Bull. 2010, 58, 121–124. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Morikawa, T.; Fujiwara, E.; Ohgushi, T.; Asao, Y.; Matsuda, H. New Noroleanane-Type Triterpene Saponins with Gastroprotective Effect and Platelet Aggregation Activity from the Flowers of Camellia Japonica: Revised Structures of Camellenodiol and Camelledionol. Heterocycles 2001, 55, 1653–1657. [Google Scholar] [CrossRef]
- Hatano, T.; Han, L.; Taniguchi, S.; Shingu, T.; Okuda, T.; Yoshida, T. Tannins and Related Polyphenols of Theaceous Plants. VIII. Camelliatannins C and E, New Complex Tannins from Camellia Japonica Leaves. Chem. Pharm. Bull. 1995, 43, 1629–1633. [Google Scholar]
- Azuma, C.M.; dos Santos, F.C.S.; Lago, J.H.G. Flavonoids and Fatty Acids of Camellia Japonica Leaves Extract. Braz. J. Pharamacogn. 2011, 21, 1159–1162. [Google Scholar]
- Cho, J.; Ji, S.; Moon, J.; Lee, K.; Jung, K.; Park, K. A Novel Benzoyl Glucoside and Phenolic Compounds from the Leaves of Camellia Japonica. Food Sci. Biotechnol. 2008, 17, 1060–1065. [Google Scholar]
- Zhang, Y.; Yin, C.; Kong, L.; Jiang, D. Extraction Optimisation, Purification and Major Antioxidant Component of Red Pigments Extracted from Camellia Japonica. Food Chem. 2011, 129, 660–664. [Google Scholar] [CrossRef]
- Kim, K.Y.; Davidson, P.M.; Chung, H.J. Antibacterial Activity in Extracts of Camellia Japonica L. Petals and its Application to a Model Food System. J. Food Protect. 2001, 64, 1255–1260. [Google Scholar]
- Onodera, K.; Hanashiro, K.; Yasumoto, T. Camellianoside, a Novel Antioxidant Glycoside from the Leaves of Camellia Japonica. Biosci. Biotechnol. Biochem. 2006, 70, 1995–1998. [Google Scholar] [CrossRef]
- Piao, M.J.; Yoo, E.S.; Koh, Y.S.; Kang, H.K.; Kim, J.; Kim, Y.J.; Kang, H.H.; Hyun, J.W. Antioxidant Effects of the Ethanol Extract from Flower of Camellia japonica via Scavenging of Reactive Oxygen Species and Induction of Antioxidant Enzymes. Int. J. Mol. Sci. 2011, 12, 2618–2630. [Google Scholar] [CrossRef]
- Thao, N.T.P.; Hung, T.M.; Lee, M.K.; Kim, J.C.; Min, B.S.; Bae, K. Triterpenoids from Camellia Japonica and their Cytotoxic Activity. Chem. Pharm. Bull. 2010, 58, 121–124. [Google Scholar] [CrossRef]
- Jeong, C.; Kim, J.H.; Choi, G.N.; Kwak, J.H.; Kim, D.; Heo, H.J. Protective Effects of Extract with Phenolics from Camellia (Camellia Japonica) Leaf Against Oxidative Stress-Induced Neurotoxicity. Food Sci. Biotechnol. 2010, 19, 1347–1353. [Google Scholar] [CrossRef]
- Onodera, K.I.; Tsuha, K.; Yasumoto-Hirose, M.; Tsuha, K.; Hanashiro, K.; Naoki, H.; Yasumoto, T. Okicamelliaside, an Extraordinarily Potent Anti-Degranulation Glucoside Isolated from Leaves of Camellia Japonica. Biosci. Biotechnol. Biochem. 2010, 74, 2532–2534. [Google Scholar] [CrossRef]
- Kuba, M.; Tsuha, K.; Tsuha, K.; Matsuzaki, G.; Yasumoto, T. In Vivo Analysis of the Anti-Allergic Activities of Camellia Japonica Extract and Okicamelliaside, a Degranulation Inhibitor. J. Health Sci. 2008, 54, 584–588. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Ko, N.; Mun, S.; Kim, D.; Kim, J.; Kim, H.; Lee, K.; Kim, Y.; Radinger, M.; et al. Camellia Japonica Suppresses Immunoglobulin E-Mediated Allergic Response by the Inhibition of Syk Kinase Activation in Mast Cells. Clin. Exp. Allergy 2008, 38, 794–804. [Google Scholar] [CrossRef]
- Park, J.C.; Hur, J.M.; Park, J.G.; Hatano, T.; Yoshida, T.; Miyashiro, H.; Min, B.S.; Hattori, M. Inhibitory Effects of Korean Medicinal Plants and Camelliatannin H from Camellia Japonica on Human Immunodeficiency Virus Type 1 Protease. Phytother. Res. 2002, 16, 422–426. [Google Scholar] [CrossRef]
- Akihisa, T.; Tokuda, H.; Ukiya, M.; Suzuki, T.; Enjo, F.; Koike, K.; Nikaido, T.; Nishino, H. 3-Epicabraleahydroxylactone and Other Triterpenoids from Camellia Oil and their Inhibitory Effects on Epstein-Barr Virus Activation. Chem. Pharm. Bull. 2004, 52, 153–156. [Google Scholar] [CrossRef]
- Somasundaram, A. Antidiabetic Activity of Methanolic Extract of Camellia japonica Leaves against Alloxan Induced Diabetes in Rats. Int. J. Pharm. BioSci. 2011, 1, 43–46. [Google Scholar]
- Yoshikawa, M.; Harada, E.; Murakami, T.; Matsuda, H.; Yamahara, J.; Murakami, N. Camelliasaponins B1, B2, C1 and C2, New Type Inhibitors of Ethanol Absorption in Rats from the Seeds of Camellia Japonica. Chem. Pharm. Bull. 1994, 42, 742–744. [Google Scholar] [CrossRef]
- Jung, E.; Lee, J.; Baek, J.; Jung, K.; Lee, J.; Huh, S.; Kim, S.; Koh, J.; Park, D. Effect of Camellia Japonica Oil on Human Type I Procollagen Production and Skin Barrier Function. J. Ethnopharmacol. 2007, 112, 127–131. [Google Scholar] [CrossRef]
- Ming, T.L. Monograph of the Genus Camellia; Yunnan Science and Technology Press: Kunming, China, 2000. [Google Scholar]
- Mondal, T.K. Camellia. In Wild Crop Relatives: Genomic and Breeding Resources; Kole, C., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2011; pp. 15–39. [Google Scholar]
- Wang, L.; Lee, F.S.C.; Wang, X.; He, Y. Feasibility Study of Quantifying and Discriminating Soybean Oil Adulteration in Camellia Oils by Attenuated Total Reflectance MIR and Fiber Optic Diffuse Reflectance NIR. Food Chem. 2006, 95, 529–536. [Google Scholar] [CrossRef]
- Buchgraber, M.; Ulberth, F.; Emons, H.; Anklam, E. Triacylglycerol Profiling by Using Chromatographic Techniques. Eur. J. Lipid Sci. Technol. 2004, 106, 621–648. [Google Scholar] [CrossRef]
- Laakso, P. Analysis of Triacylglycerols-Approaching the Molecular Composition of Natural Mixtures. Food Rev. Int. 1996, 12, 199–250. [Google Scholar] [CrossRef]
- Ribeiro, A.P.B.; Basso, R.C.; Grimaldi, R.; Gioielli, L.A.; Guaraldo, L.A. Instrumental Methods for the Evaluation of Interesterified Fats. Food Anal. Methods 2009, 2, 282–302. [Google Scholar] [CrossRef]
- Martinez, I.; Aursand, M.; Erikson, U.; Singstad, T.E.; Veliyulin, E.; Van Der Zwaag, C. Destructive and Non-Destructive Analytical Techniques for Authentication and Composition Analyses of Foodstuffs. Trends Food Sci. Technol. 2003, 14, 489–498. [Google Scholar] [CrossRef]
- Beyer, T.; Diehl, B.; Holzgrabe, U. Quantitative NMR Spectroscopy of Biologically Active Substances and Excipients. Bioanal. Rev. 2010, 2, 1–22. [Google Scholar]
- Guillén, M.D.; Ruiz, A. High Resolution 1H Nuclear Magnetic Resonance in the Study of Edible Oils and Fats. Trends Food Sci. Technol. 2001, 12, 328–338. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. 1H nuclear magnetic resonance as a fast tool for determining the composition of acyl chains in acylglycerol mixtures. Eur. J. Lipid Sci. Technol. 2003, 105, 502–507. [Google Scholar] [CrossRef]
- Guillen, M.D.; Ruiz, A. Rapid simultaneous determination by proton NMR of unsaturation and composition of acyl groups in vegetable oils. Eur. J. Lipid Sci. Technol. 2003, 105, 688–696. [Google Scholar] [CrossRef]
- Lie Ken Jie, M.S.F.; Mustafa, J. High-Resolution Nuclear Magnetic Resonance Spectroscopy-Applications to Fatty Acids and Triacylglycerols. Lipids 1997, 32, 1019–1034. [Google Scholar] [CrossRef]
- Vlahov, G. Application of NMR to the Study of Olive Oils. Prog. Nucl. Magn. Reson. Spectrosc. 1999, 35, 341–357. [Google Scholar]
- Mannina, L.; Luchinat, C.; Patumi, M.; Emanuele, M.C.; Rossi, E.; Segre, A.L. Concentration Dependence of 13C-NMR Spectra of Triglycerides: Implications for the NMR Analysis of Olive Oil. Magn. Reson. Chem. 2000, 38, 886–890. [Google Scholar] [CrossRef]
- The acquisition of the data was carried out with a relaxation delay of 14 s and a pulse of 20° which ensures quantitative integrals for al protons with T1 < 3s.
- Guillén, M.D.; Ruiz, A. Study by Means of Proton Nuclear Magnetic Resonance of the Thermal Oxidation Process Undergone by Oils Rich in Oleic Acyl Groups. J. Am. Oil Chem. Soc. 2005, 82, 349–355. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Monitoring of Heat-Induced Degradation of Edible Oils by Proton NMR. Eur. J. Lipid Sci. Technol. 2008, 110, 52–60. [Google Scholar] [CrossRef]
- Haiyan, Z.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Endogenous biophenol, fatty acid and volatile profiles of selected oils. Food Chem. 2006, 100, 1544–1551. [Google Scholar]
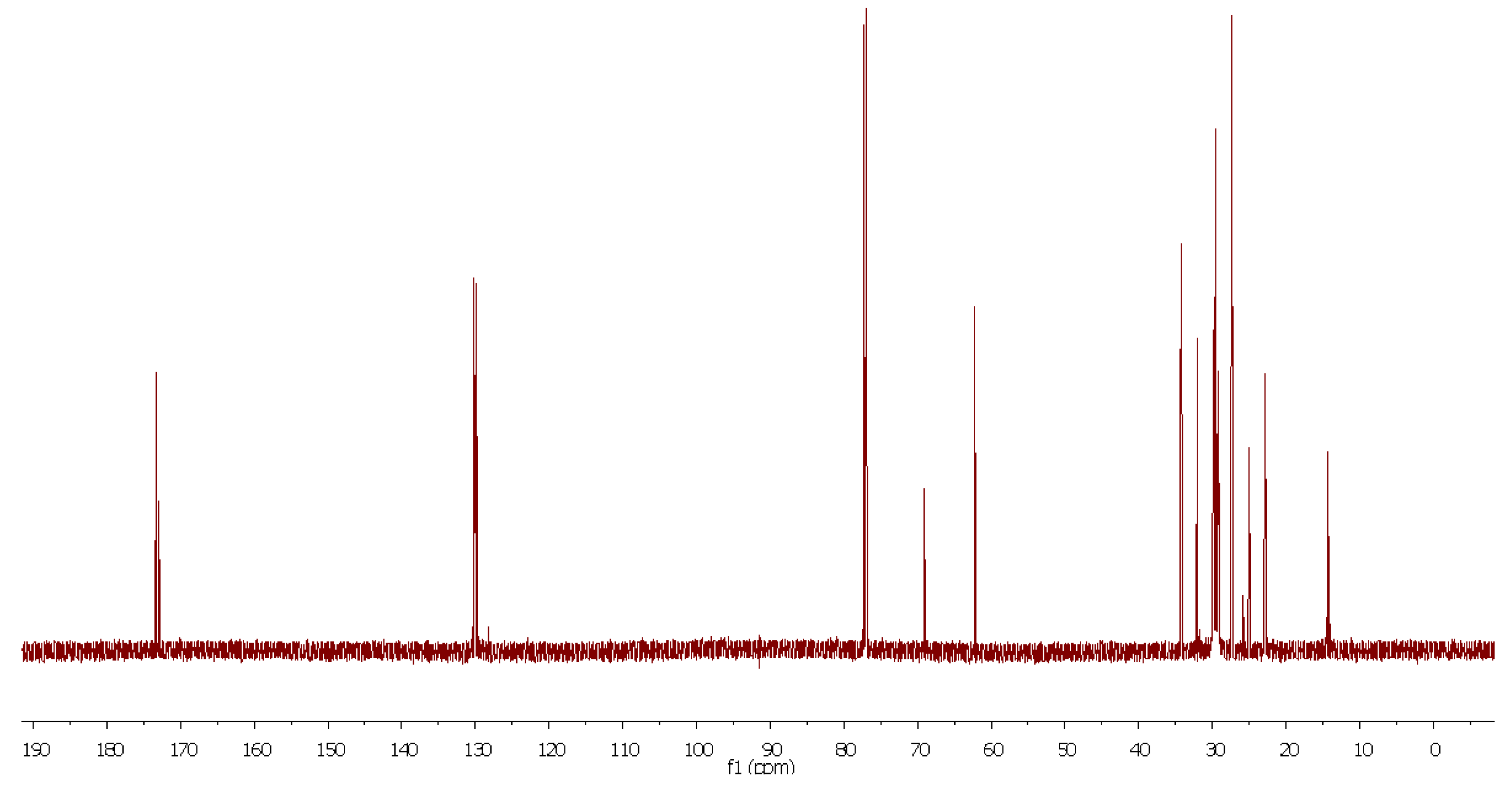
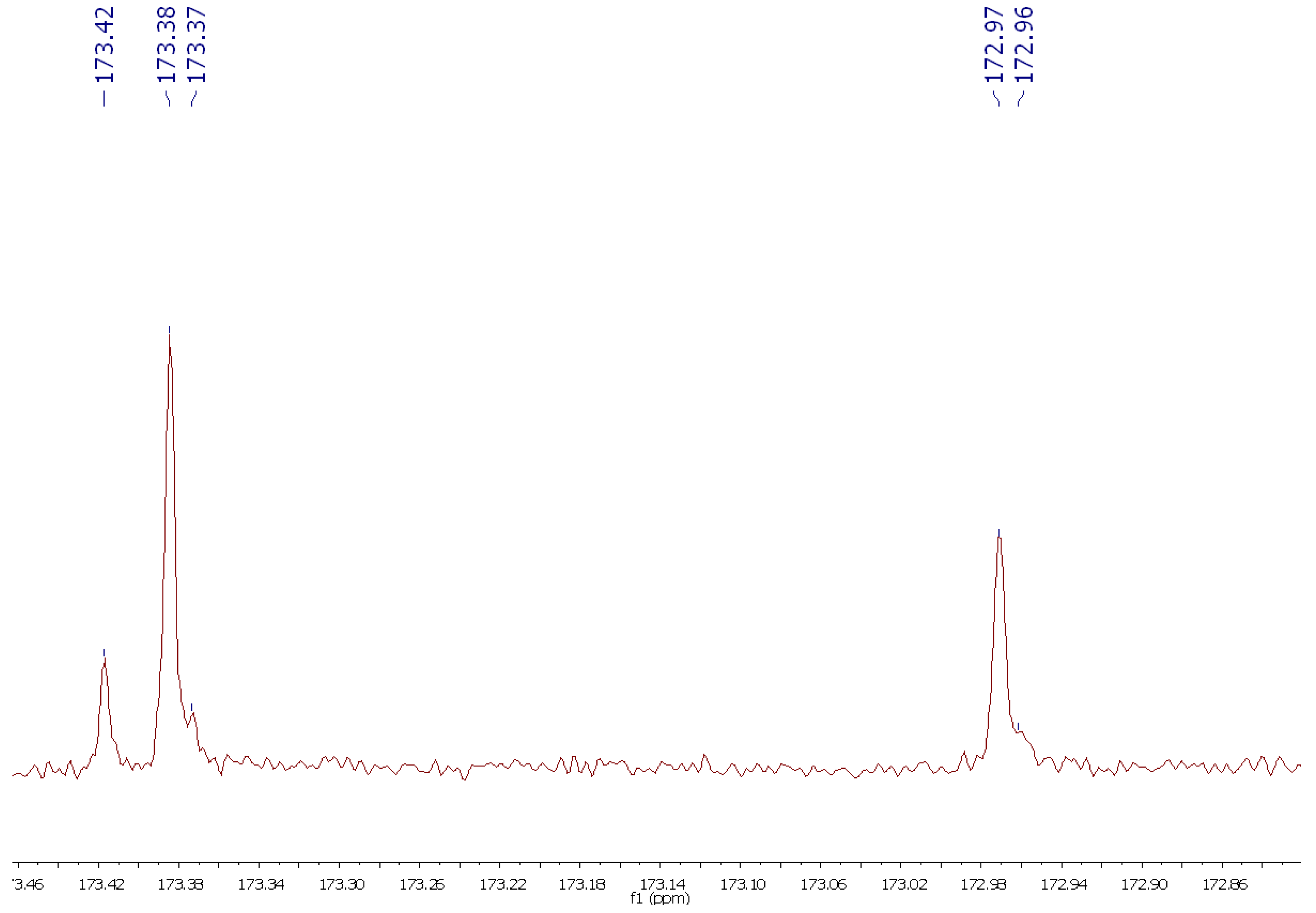
- 13C-NMR (189 MHz, CDCl3) δ: 173.42, 173.38, 173.37, 172.97, 172.96, 130.35, 130.16, 130.14, 130.11, 129.84, 129.81, 128.20, 128.02, 69.02, 62.24, 34.34, 34.20, 34.17, 32.05, 29.91, 29.85, 29.81, 29.77, 29.68, 29.47, 29.35, 29.33, 29.26, 29.24, 29.20, 27.37, 27.32, 25.77, 25.03, 24.99, 22.83, 20.75, 14.26 ppm.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Salinero, C.; Feás, X.; Mansilla, J.P.; Seijas, J.A.; Vázquez-Tato, M.P.; Vela, P.; Sainz, M.J. 1H-Nuclear Magnetic Resonance Analysis of the Triacylglyceride Composition of Cold-Pressed Oil from Camellia japonica. Molecules 2012, 17, 6716-6727. https://doi.org/10.3390/molecules17066716
Salinero C, Feás X, Mansilla JP, Seijas JA, Vázquez-Tato MP, Vela P, Sainz MJ. 1H-Nuclear Magnetic Resonance Analysis of the Triacylglyceride Composition of Cold-Pressed Oil from Camellia japonica. Molecules. 2012; 17(6):6716-6727. https://doi.org/10.3390/molecules17066716
Chicago/Turabian StyleSalinero, Carmen, Xesús Feás, J. Pedro Mansilla, Julio A. Seijas, M. Pilar Vázquez-Tato, Pilar Vela, and María J. Sainz. 2012. "1H-Nuclear Magnetic Resonance Analysis of the Triacylglyceride Composition of Cold-Pressed Oil from Camellia japonica" Molecules 17, no. 6: 6716-6727. https://doi.org/10.3390/molecules17066716
APA StyleSalinero, C., Feás, X., Mansilla, J. P., Seijas, J. A., Vázquez-Tato, M. P., Vela, P., & Sainz, M. J. (2012). 1H-Nuclear Magnetic Resonance Analysis of the Triacylglyceride Composition of Cold-Pressed Oil from Camellia japonica. Molecules, 17(6), 6716-6727. https://doi.org/10.3390/molecules17066716







