Synthesis and Antiangiogenic Activity of Novel Gambogic Acid Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry




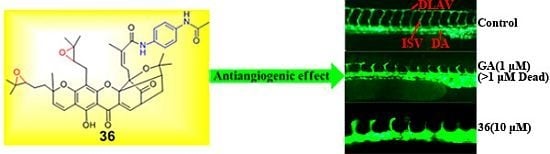
2.2. Antiangiogenic Activity and Toxicity in the Transgenic Zebrafish Model
| Compounds | Anti-angiogenic phenotype a | ||
|---|---|---|---|
| 1 μM | 2.5 μM | 10 μM | |
| 4 | ++ | +++ | ++++ |
| 9 | + | ++ | ++ |
| 16 | О | О | ++ |
| 20 | О | О | ++ |
| 22 | О | О | ++ |
| 23 | О | О | ++ |
| 26 | ++ | ++ | ++ |
| 31 | О | + | ++ |
| 32 | ++ | +++ | ++++ |
| 35 | ++ | +++ | ++++ |
| 36 | ++ | +++ | ++++ |
| GA | ++ | Dead b | Dead |


| Compounds | Heart rates of zebrafish(beats/min) a | ||
|---|---|---|---|
| 1 μM | 2.5 μM | 10 μM | |
| 4 | 84 ± 3.22 *** | 80 ± 5.31 | 54 ± 4.00 |
| 32 | 72 ± 6.13 ** | 60 ± 4.36 | 54 ± 3.68 |
| 35 | 87 ± 8.99 ** | 57 ± 2.61 | 45 ± 4.95 |
| 36 | 84 ± 3.24 *** | 60 ± 5.2 | 60 ± 2.86 |
| GA | 36 ± 3.32 | Dead b | Dead |
| Control | 117 | ||
| Compounds | Mortality rates (%) a | ||
|---|---|---|---|
| 1 μM | 2.5 μM | 10 μM | |
| 4 | 0 | 6.7 ± 5.77 ** | 20 ± 0.00 *** |
| 32 | 0 | 6.7 ± 5.77 ** | 16.7 ± 5.77 *** |
| 35 | 3.3 ± 5.77 * | 10 ± 0.00 *** | 16.7 ± 5.77 *** |
| 36 | 0 | 3.3 ± 5.77** | 16.7 ± 5.77 *** |
| A | 20 ± 0.00 | 73.3 ± 5.77 | 100 |
| Control | 0 | ||
2.3. Effects on the HUVECs Migration

2.4. Effects on the HUVECs Tube Formation
2.5. Antiproliferative and Anticancer Activity

| Compound | Cell lines (IC50, μM) a | ||||||
|---|---|---|---|---|---|---|---|
| A549 | HepG2 | HCT116 | K562 | HeLa | HUVEC | LO2 | |
| 1 | 43.50 ± 3.92 | 30.83 ± 2.76 | 25.53 ± 1.79 | 10.25 ± 0.48 | 32.89 ± 1.69 | 45.20 ± 2.64 | ND |
| 2 | 49.15 ± 5.16 | 41.43 ± 3.52 | 45.27 ± 4.20 | 16.10 ± 1.74 | 57.00 ± 0.62 | 31.90 ± 1.27 | ND |
| 3 | 38.03 ± 3.10 | 28.83 ± 2.84 | 18.68 ± 0.97 | 8.18 ± 1.22 | 13.07 ± 0.77 | 52.67 ± 1.15 | ND |
| 4 | 0.95 ± 0.09 | 1.10 ± 0.06 | 0.48 ± 0.04 | 0.41 ± 0.02 * | 1.34 ± 0.04 | 1.17 ± 0.12 | 0.83 ± 0.03 |
| 5 | 5.53 ± 0.40 | 9.45 ± 1.42 | 5.47 ± 0.24 | 2.03 ± 0.20 | 6.37 ± 0.11 | 4.39 ± 0.27 | 4.03 ± 0.34 ## |
| 6 | 19.70 ± 2.62 | 25.77 ± 1.76 | 14.53 ± 1.22 | 5.47 ± 0.15 | 33.30 ± 1.83 | 14.60 ± 0.34 | >20 |
| 7 | 15.43 ± 2.57 | 10.77 ± 1.10 | 6.00 ± 0.40 | 2.50 ± 0.12 | 16.10 ± 0.55 | 10.90 ± 0.72 | 8.28 ± 0.33 ### |
| 8 | >80 | >80 | 66.40 ± 1.10 | 17.95 ± 1.38 | >80 | 77.50 ± 3.65 | ND |
| 9 | 3.75 ± 0.35 | 5.16 ± 0.55 | 2.05 ± 0.41 | 1.14 ± 0.03 | 3.42 ± 0.19 | 4.27 ± 0.25 | 1.00 ± 0.10 |
| 10 | >80 | >80 | >80 | >80 | >80 | >80 | ND |
| 11 | 3.31 ± 0.45 | 3.64 ± 0.53 | 3.13 ± 0.30 | 2.09 ± 0.19 | 3.31 ± 0.24 | 3.48 ± 0.18 | 3.94 ± 0.19 ### |
| 12 | 10.70 ± 0.26 | 7.80 ± 0.11 | 5.57 ± 0.26 | 1.79 ± 0.08 | 7.10 ± 1.43 | 7.58 ± 1.36 | 5.15 ± 1.19 ### |
| 13 | 66.75 ± 4.59 | 60.50 ± 4.24 | 17.47 ± 1.59 | 8.05 ± 0.28 | >80 | 27.67 ± 2.77 | ND |
| 14 | >80 | 40.63 ± 2.75 | 34.80 ± 1.84 | 22.60 ± 0.53 | >80 | 41.95 ± 2.76 | 70.83 ± 2.47 ### |
| 15 | 32.95 ± 1.50 | 10.85 ± 0.06 | 6.80 ± 0.78 | 6.11 ± 0.39 | 62.57 ± 3.12 | 13.64 ± 1.16 | 14.83 ± 1.19 ### |
| 16 | >80 | 4.51 ± 0.29 | 5.08 ± 0.77 | 3.46 ± 0.34 | 8.98 ± 0.58 | 11.93 ± 1.36 | 5.25 ± 0.51 ### |
| 17 | >80 | 61.90 ± 2.69 | 33.00 ± 1.27 | 27.00 ± 1.41 | >80 | 29.90 ± 1.44 | 72.83 ± 2.84 ### |
| 18 | 66.15 ± 1.65 | 6.45 ± 0.37 | 2.45 ± 0.15 | 7.52 ± 0.83 | >80 | 10.37 ± 1.08 | 15.20 ± 0.72 ### |
| 19 | >80 | >80 | 70.25 ± 3.06 | >80 | >80 | 68.00 ± 4.00 | >80 |
| 20 | 31.20 ± 2.29 | 5.86 ± 0.40 | 7.90 ± 0.14 | 4.52 ± 0.43 | 24.33 ± 2.13 | 7.70 ± 0.32 | 9.92 ± 0.52 ### |
| 21 | >80 | >80 | >80 | >80 | >80 | 70.5 ± 4.86 | >80 |
| 22 | >80 | 63.25 ± 3.88 | 36.90 ± 0.64 | 36.90 ± 0.56 | >80 | 51.30 ± 1.10 | >80 |
| 23 | 73.50 ± 3.40 | 19.95 ± 2.89 | 15.93 ± 1.46 | 15.45 ± 1.35 | 61.65 ± 5.86 | 31.97 ± 1.76 | 31.70 ± 1.94 ### |
| 24 | >80 | >80 | >80 | >80 | >80 | >80 | >80 |
| 25 | >80 | >80 | >80 | >80 | >80 | >80 | >80 |
| 26 | 4.00 ± 0.80 | 1.72 ± 0.12 | 1.73 ± 0.44 | 0.77 ± 0.03 | 4.46 ± 0.57 | 2.98 ± 0.10 | 4.22 ± 0.50 ## |
| 27 | 1.22 ± 0.25 | 0.69 ± 0.08 * | 1.05 ± 0.07 | 0.52 ± 0.02 | 3.51 ± 0.15 | 0.50 ± 0.03 | 1.52 ± 0.12 # |
| 28 | 0.91 ± 0.03 | 0.66 ± 0.02 * | 0.83 ± 0.04 | 0.50 ± 0.06 | 1.70 ± 0.09 | 0.62 ± 0.03 | 1.61 ± 0.15 # |
| 29 | 0.63 ± 0.01 ** | 0.85 ± 0.15 | 1.63 ± 0.15 | 0.79 ± 0.06 | 1.45 ± 0.07 | 6.64 ± 0.11 | 4.28 ± 0.33 ### |
| 30 | 1.24 ± 0.09 | 1.72 ± 0.03 | 1.33 ± 0.07 | 0.44 ± 0.02 | 3.43 ± 0.28 | 1.03 ± 0.07 | 2.84 ± 0.34 # |
| 31 | 61.60 ± 1.73 | 49.17 ± 4.31 | 18.85 ± 1.34 | 13.73 ± 0.56 | 57.93 ± 2.60 | 30.23 ± 2.04 | ND |
| 32 | 0.74 ± 0.04 * | 0.81 ± 0.06 | 0.45 ± 0.01 * | 0.40 ± 0.02 | 1.08 ± 0.10 | 1.09 ± 0.09 | 1.70 ± 0.48 |
| 33 | 1.30 ± 0.08 | 1.00 ± 0.06 | 1.20 ± 0.09 | 0.53 ± 0.05 | 4.23 ± 0.39 | 0.57 ± 0.03 | 1.75 ± 0.09 # |
| 34 | 21.40 ± 3.71 | 8.65 ± 0.46 | 7.74 ± 1.43 | >20 | >80 | 5.63 ± 0.20 | 7.30 ± 1.20 # |
| 35 | 0.50 ± 0.05 ** | 0.27 ± 0.05 ** | 0.40 ± 0.02 ** | 0.26 ± 0.01 ** | 0.49 ± 0.02 *** | 0.20 ± 0.01 *** | 1.17 ± 0.41 |
| 36 | 1.29 ± 0.14 | 1.85 ± 0.08 | 0.56 ± 0.01 | 0.28 ± 0.03* | 4.96 ± 0.88 | 2.45 ± 0.09 | 1.62 ± 0.08 # |
| GA | 0.94 ± 0.03 | 0.84 ± 0.04 | 0.59 ± 0.03 | 0.49 ± 0.03 | 1.14 ± 0.06 | 0.27 ± 0.01 | 0.89 ± 0.08 |
2.6. Structure-Activity Relationship
3. Experimental
3.1. Chemistry
3.2. Biological General Methods
3.2.1. Zebrafish Embryo Assay
3.2.2. Cell Culture
3.2.3. Wound-Healing Assay
3.2.4. Tube Formation Assay
3.2.5. MTT Assay
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Harris, A.L. Angiogenesis as a new target for cancer control. Eur. J. Cancer Suppl. 2003, 1, 1–12. [Google Scholar] [CrossRef]
- Schmidt, J.M.; Tremblay, G.B.; Pagé, M.; Mercure, J.; Feher, M.; Dunn-Dufault, R.; Peter, M.G.; Redden, P.R. Synthesis and Evaluation of a Novel Nonsteroidal-Specific Endothelial Cell Proliferation Inhibitor. J. Med. Chem. 2003, 46, 1289–1292. [Google Scholar] [CrossRef]
- Wong, M.L.H.; Prawira, A.; Kaye, A.H.; Hovens, C.M. Tumour angiogenesis Its mechanism and therapeutic implications in malignant gliomas. J. Clin. Neurosci. 2009, 16, 1119–1130. [Google Scholar] [CrossRef]
- Noguer, O.; Villena, J.; Lorita, J.; Vilaro, S.; Reina, M. Syndecan-2 downregulation impairs angiogenesis in human microvascular endothelial cells. Exp. Cell. Res. 2009, 315, 795–808. [Google Scholar] [CrossRef]
- Kondo, T.; Ohta, T.; Igura, K.; Hara, Y.; Kaji, K. Tea catechins inhibit angiogenesis in vitro, measured by human endothelial cell growth, migration and tube formation, through inhibition of VEGF receptor binding. Cancer Lett. 2002, 180, 139–144. [Google Scholar] [CrossRef]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef]
- Jansen, M.; Hamer, P.C.D.W.; Witmer, A.N.; Troost, D.; Noorden, C.J.F.V. Current perspectives on antiangiogenesis strategies in the treatment of malignant gliomas. Brain Res. Rev. 2004, 45, 143–163. [Google Scholar] [CrossRef]
- Farinelle, S.; Malonne, H.; Chaboteaux, C.; Decaestecker, C.; Dedecker, R.; Gras, T.; Darro, F.; Fontaine, J.; Atassi, G.; Kiss, R. Characterization of TNP-470-induced modifications to cell functions in HUVEC and cancer cells. J. Pharmacol. Toxicol. 2000, 43, 15–24. [Google Scholar] [CrossRef]
- Baldessari, D.; Mione, M. How to create the vascular tree (Latest) help from the zebrafish. Pharmaco. Therapeut. 2008, 118, 206–230. [Google Scholar] [CrossRef]
- Sumanas, S.; Lin, S. Zebrafish as a model system for drug target screening and validation. Drug Disc Today: TARGETS 2004, 3, 89–96. [Google Scholar] [CrossRef]
- Ma, L.; Chen, J.Y.; Wang, X.W.; Liang, X.L.; Luo, Y.F.; Zhu, W.; Wang, T.E.; Peng, M.; Li, S.C.; Shi, J.; Peng, A.H.; Wei, Y.Q.; Chen, L.J. Structural Modification of Honokiol, a Biphenyl Occurring in Magnolia officinalis the Evaluation of Honokiol Analogues as Inhibitors of Angiogenesis and for Their Cytotoxicity and Structure Activity Relationship. J. Med. Chem. 2011, 54, 6469–6481. [Google Scholar]
- Lele, Z.; Krone, P.H. The zebrafish as a model system in developmental, toxicological and transgenic research. Biotechnol. Adv. 1996, 14, 57–72. [Google Scholar] [CrossRef]
- Xie, H.; Qin, Y.X.; Zhou, Y.L.; Tong, L.J.; Lin, L.P.; Geng, M.Y.; Duan, W.H.; Ding, J. GA3, a new gambogic acid derivative, exhibits potent antitumor activities in vitro via apoptosis-involved mechanisms. Acta Pharmacol. Sin. 2009, 30, 346–354. [Google Scholar] [CrossRef]
- Qiang, L.; Yang, Y.; You, Q.D.; Ma, Y.J.; Yang, L.; Nie, F.F.; Gu, H.Y.; Zhao, L.; Lu, N.; Qi, Q.; Liu, W.; Wang, X.T.; Guo, Q.L. Inhibition of glioblastoma growth and angiogenesis by gambogic acid An in vitro and in vivo study. Biol. Pharm. 2008, 75, 1083–1092. [Google Scholar]
- Lu, N.; Yang, Y.; You, Q.D.; Ling, Y.; Gao, Y.; Gu, H.Y.; Zhao, L.; Wang, X.T.; Guo, Q.L. Gambogic acid inhibits angiogenesis through suppressing vascular endothelial growth factor-induced tyrosine phosphorylation of KDRFlk-1. Cancer Lett. 2007, 258, 80–89. [Google Scholar] [CrossRef]
- Yi, T.F.; Yi, Z.F.; Cho, S.G.; Luo, J.; Pandey, M.K.; Aggarwal, B.B.; Liu, M.Y. Gambogic Acid Inhibits Angiogenesis and Prostate Tumor Growth by Suppressing Vascular Endothelial Growth Factor Receptor 2 Signaling. Cancer Res. 2008, 68, 1843–1850. [Google Scholar]
- Guo, Q.L.; Qi, Q.; You, Q.D.; Gu, H.Y.; Zhao, L.; Wu, Z.Q. Toxicological Studies of Gambogic Acid and its Potential Targets in Experimental Animals. Basic Clin. Pharmacol. Toxicol. 2006, 99, 178–184. [Google Scholar] [CrossRef]
- Qi, Q.; You, Q.D.; Gu, H.Y.; Zhao, L.; Liu, W.; Lu, N.; Guo, Q.L. Studies on the toxicity of gambogic acid in rats. J. Ethnopharmacol. 2008, 117, 433–438. [Google Scholar] [CrossRef]
- Jang, S.W.; Okada, M.; Sayeed, I.; Xiao, G.; Stein, D.; Jin, P.; Ye, K.Q. Gambogic amide, a selective agonist for TrkA receptor that possesses robust neurotrophic activity, prevents neuronal cell death-2007. Proc. Natl. Acad. Sci. USA 2007, 104, 16329–16334. [Google Scholar]
- Guo, Q.L.; Zhao, L.; You, Q.D. Gambogic Acid Inducing Apoptosis in Human Gastric Adenocarcinom SGC-7901 Cells. Chin. J. Nat. Med. 2004, 2, 106–110. [Google Scholar]
- Wang, J.X.; Zhao, L.; Hu, Y.; Guo, Q.L.; Zhang, L.; Wang, X.J.; Li, N.G.; You, Q.D. Studies on chemical structure modification and biology of a natural product, Gambogic acid (I) Synthesis and biological evaluation of oxidized analogues of gambogic acid. Eur. J. Med. Chem. 2009, 44, 2611–2620. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Kasibhatla, S.; Wang, Y.; Herich, J.; Guastella, J.; Tseng, B.; Drewe, J.; Cai, S.X. Discovery, characterization and SAR of gambogic acid as a potent apoptosis inducer by a HTS assay. Bioorg. Med. Chem. 2004, 12, 309–317. [Google Scholar] [CrossRef]
- Udvadia, A.J.; Linney, E. Windows into development historic, current, and future perspectives on transgenic zebrafish. Dev. Biol. 2003, 256, 1–17. [Google Scholar] [CrossRef]
- Park, Y.J.; Lee, T.; Ha, J.; Jung, I.M.; Chung, J.K.; Kim, S.J. Effect of nicotine on human umbilical vein endothelial cells (HUVECs) migration and angiogenesis. Vasc. Pharmacol. 2008, 49, 32–36. [Google Scholar] [CrossRef]
- Yang, S.P.; Cai, Y.J.; Zhang, B.L.; Tong, L.J.; Xie, H.; Wu, Y.; Lin, L.P.; Ding, J.; Yue, J.M. Structural Modification of an Angiogenesis Inhibitor Discovered from Traditional Chinese Medicine and a Structure–Activity Relationship Study. J. Med. Chem. 2008, 51, 77–85. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–36 are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, T.; Zhang, R.-H.; He, S.-C.; Xu, Q.-Y.; Ma, L.; Wang, G.-C.; Qiu, N.; Peng, F.; Chen, J.-Y.; Qiu, J.-X.; et al. Synthesis and Antiangiogenic Activity of Novel Gambogic Acid Derivatives. Molecules 2012, 17, 6249-6268. https://doi.org/10.3390/molecules17066249
Chen T, Zhang R-H, He S-C, Xu Q-Y, Ma L, Wang G-C, Qiu N, Peng F, Chen J-Y, Qiu J-X, et al. Synthesis and Antiangiogenic Activity of Novel Gambogic Acid Derivatives. Molecules. 2012; 17(6):6249-6268. https://doi.org/10.3390/molecules17066249
Chicago/Turabian StyleChen, Tao, Rong-Hong Zhang, Shi-Chao He, Qin-Yuan Xu, Liang Ma, Guang-Cheng Wang, Neng Qiu, Fei Peng, Jin-Ying Chen, Jing-Xiang Qiu, and et al. 2012. "Synthesis and Antiangiogenic Activity of Novel Gambogic Acid Derivatives" Molecules 17, no. 6: 6249-6268. https://doi.org/10.3390/molecules17066249