Synthesis of 2RS,4RS-1-[2-Phenyl-4-[2-(2-trifluromethoxy-phenoxy)-ethyl]-1,3-dioxolan-2-yl-methyl]-1H-1,2,4-triazole Derivatives as Potent Inhibitors of Brassinosteroid Biosynthesis
Abstract
:1. Introduction
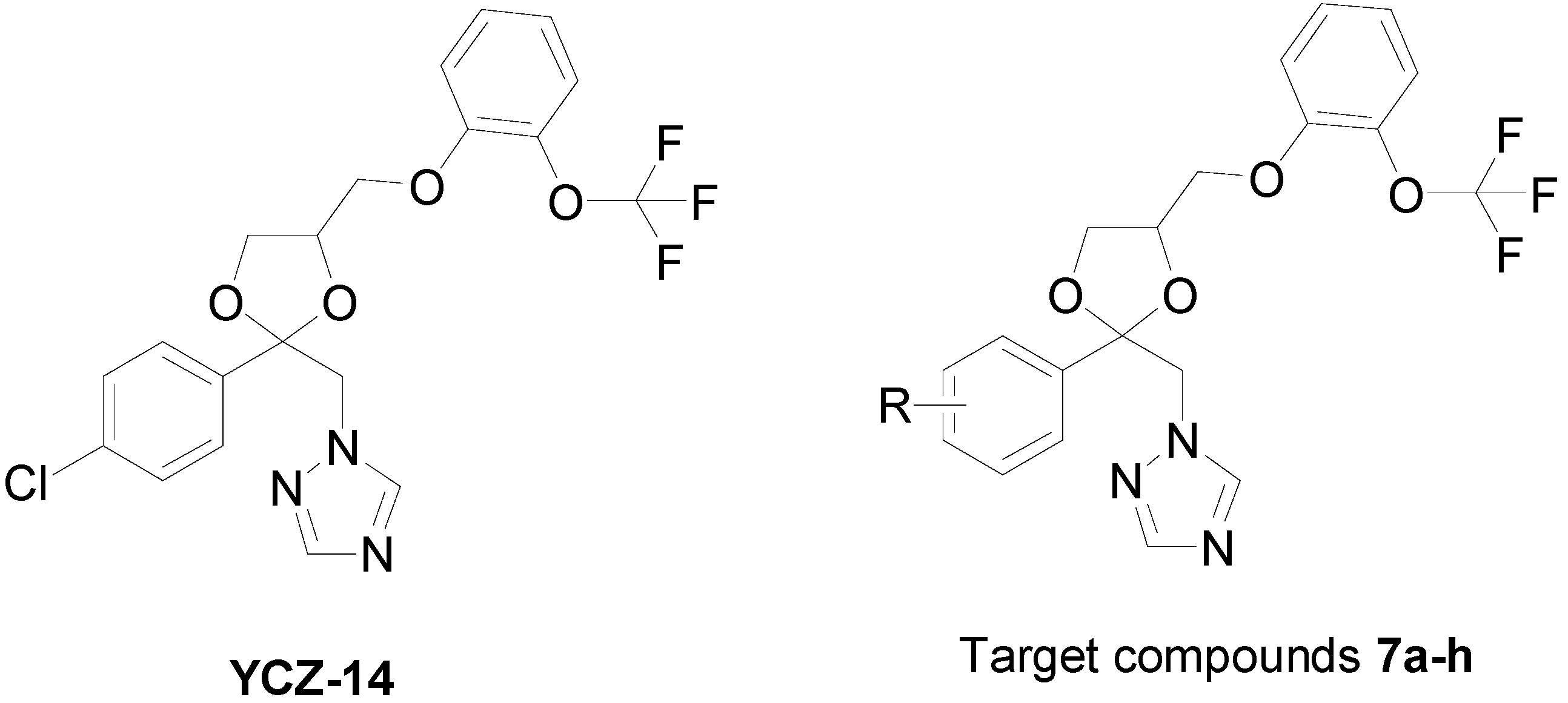
2. Results and Discussion
2.1. Chemistry
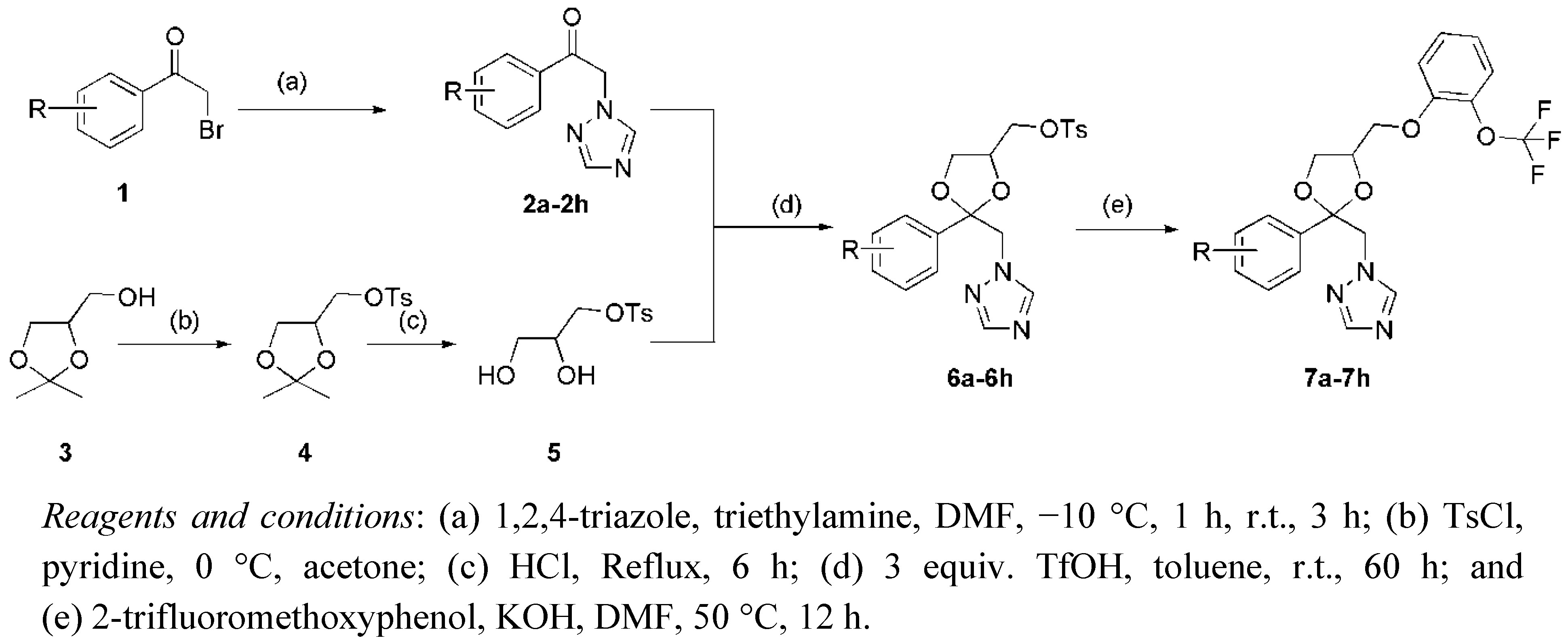
2.2. Bioassay Methods
2.3. Biological Activities of Newly Synthesized Brassinosteroid Biosynthesis Inhibitors
| No. | -R | Inhibition of Arabidopsisstem elongation (IC50, μM) a |
|---|---|---|
| 7a |  | 0.46 ± 0.04 |
| 7b |  | 0.26 ± 0.05 |
| 7c |  | 0.21 ± 0.01 |
| 7d |  | 0.73 ± 0.06 |
| 7e |  | >10 |
| 7f |  | 2.63 ± 0.39 |
| 7g |  | 0.19 ± 0.05 |
| 7h |  | 2.40 ± 0.22 |
| YCZ-14 |  | 0.12 ± 0.04 |
| Brz | ‒ | 0.73 ± 0.13 |
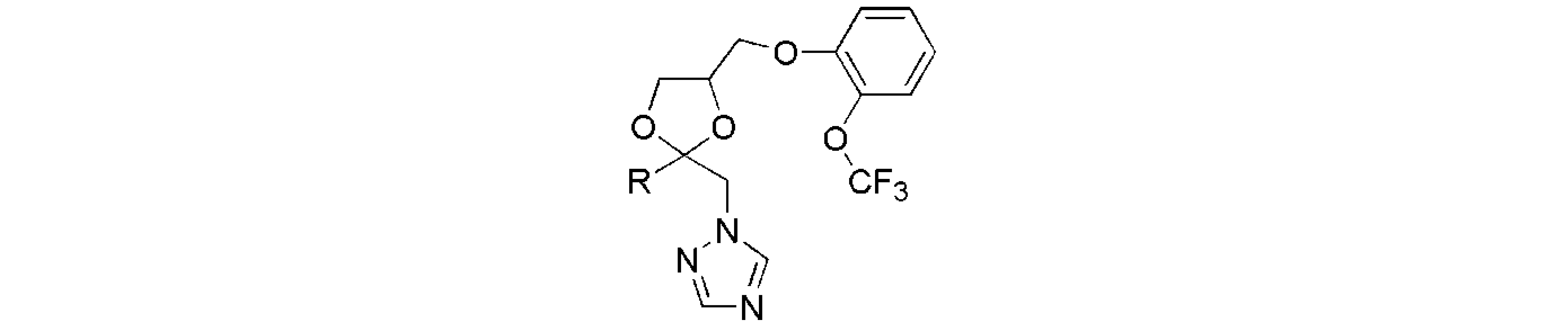
| No. | -R | Hypocotyl length, % relative to untreated Arabidopsis seedlings (%) | ||
|---|---|---|---|---|
| Chem *. | Chem. + BL (10 nM) | Chem. + GA (1 µM) | ||
| Control | - | 100 | 114.3 ± 8.0 | 104.3 ± 5.4 |
| 7a |  | 45.9 ± 2.5 | 99.8 ± 4.4 | 48.0 ± 3.7 |
| 7b |  | 37.6 ± 3.2 | 95.4 ± 4.9 | 43.8 ± 3.7 |
| 7c |  | 32.5 ± 3.2 | 101.2 ± 4.5 | 39.7 ± 2.5 |
| 7d |  | 56.7 ± 1.1 | 103.3 ± 4.5 | 51.8 ± 2.8 |
| 7g |  | 21.2 ± 2.3 | 104.1 ± 3.3 | 31.5 ± 3.7 |
| YCZ-14 |  | 18.2 ± 2.0 | 97.0 ± 3.9 | 25.5 ± 3.0 |
| Brz | ‒ | 56.0 ± 3.3 | 63.0 ± 6.1 | 61.8 ± 2.3 |
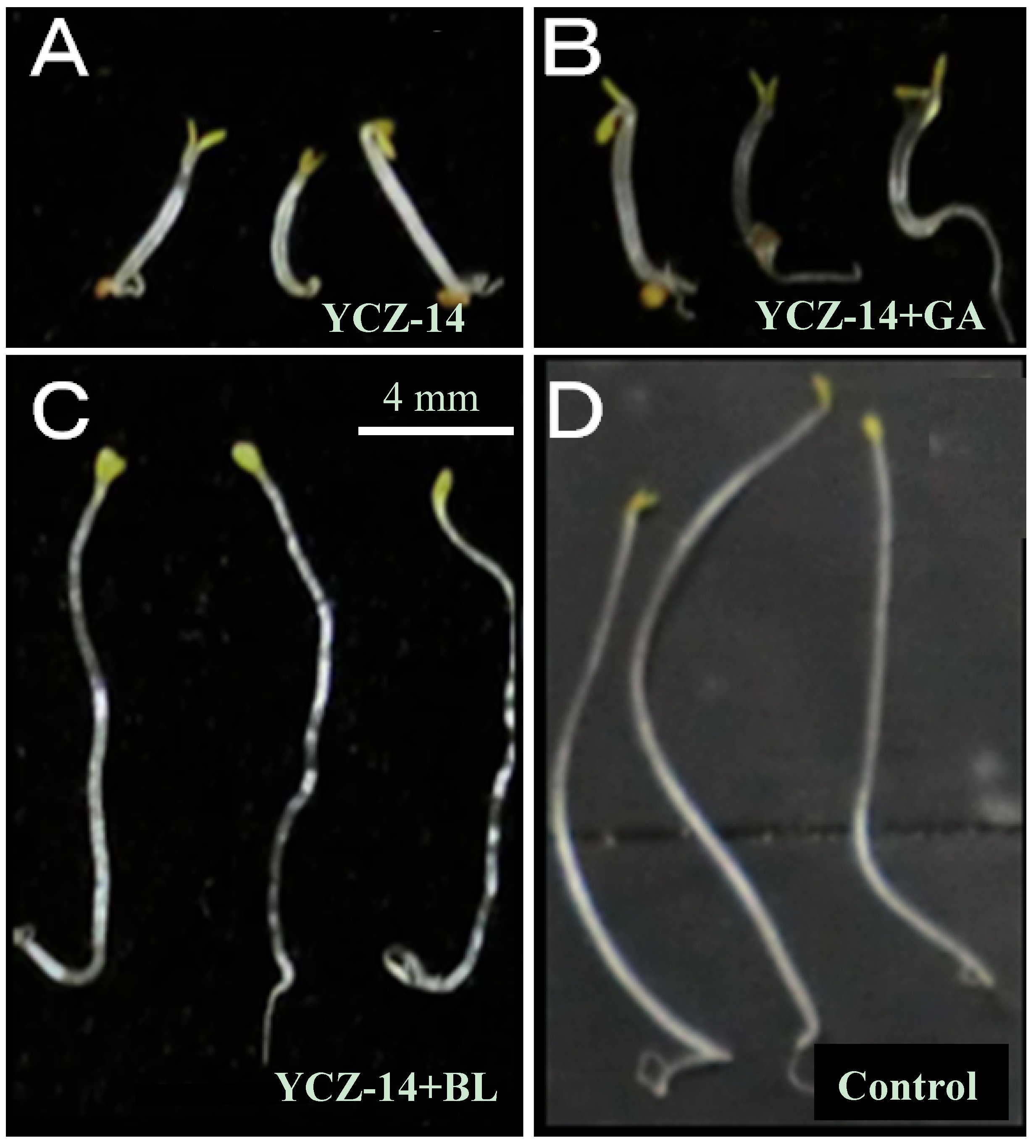
3. Experimental
3.1. General
3.2. Synthesis
3.3. Bioassay Methods for Evaluation Brassinosteroid Biosynthesis Inhibitor
4. Conclusions
Acknowledgments
References and Notes
- Clouse, S.D.; Sasse, J.M. Brassinosteroids: Essential regulators of plant growth and development. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 427–451. [Google Scholar]
- Sasse, J. Physiological actions of brassinosteroids: An update. J. Plant Growth Regul. 2003, 22, 276–288. [Google Scholar]
- Krishna, P. Brassinosteroid-mediated stress responses. J. Plant Growth Regul. 2003, 22, 289–297. [Google Scholar]
- Choe, S.; Dilkes, B.P.; Gregory, B.D.; Ross, A.S.; Yuan, H.; Noguchi, T.; Fujioka, S.; Takatsuto, S.; Tanaka, A.; Yoshida, S.; et al. The Arabidopsis dwarf1 mutant is defective in the conversion of 24-methylenecholesterol to campesterol in brassinosteroid biosynthesis. Plant Physiol. 1999, 119, 897–907. [Google Scholar]
- Bishop, G.J.; Nomura, T.; Yokota, T.; Harrison, K.; Noguchi, T.; Fujioka, S.; Takatsuto, S.; Jones, J.D.; Kamiya, Y. The tomato DWARF enzyme catalyses C-6 oxidation in brassinosteroid biosynthesis. Proc. Natl. Acad. Sci. USA 1999, 96, 1761–1766. [Google Scholar]
- Hong, Z.; Ueguchi-Tanaka, M.; Umemura, K.; Uozu, S.; Fujioka, S.; Takatsuto, S.; Yoshida, S.; Ashikari, M.; Kitano, H.; Matsuoka, M. A rice brassinosteroid-deficient mutant, ebisu dwarf (d2), is caused by a loss of function of a new member of cytochrome P450. Pant Cell 2003, 15, 2900–2910. [Google Scholar]
- Nomura, T.; Nakayama, M.; Reid, J.B.; Takeuchi, Y.; Yokota, T. Blockage of Brassinosteroid Biosynthesis and Sensitivity Causes Dwarfism in Garden Pea. Plant Physiol. 1997, 113, 31–37. [Google Scholar]
- Wu, C.Y.; Trieu, A.; Radhakrishnan, P.; Kwok, S.F.; Harris, S.; Zhang, K.; Wang, J.; Wan, J.; Zhai, H.; Takatsuto, S.; et al. Brassinosteroids regulate grain filling in rice. Plant Cell 2008, 20, 2130–2145. [Google Scholar]
- Sakamoto, T.; Morinaka, Y.; Ohnishi, T.; Sunohara, H.; Fujioka, S.; Ueguchi-Tanaka, M.; Mizutani, M.; Sakata, K.; Takatsuto, S.; Yoshida, S.; et al. Erect leaves caused by brassinosteroid deficiency increase biomass production and grain yield in rice. Nat. Biotechnol. 2006, 24, 105–109. [Google Scholar] [CrossRef]
- Min, Y.K.; Asami, T.; Fujioka, S.; Murofushi, N.; Yamaguchi, I.; Yoshida, S. New lead compounds for brassinosteroid biosynthesis inhibitors. Bioorg. Med. Chem. Lett. 1999, 9, 425–430. [Google Scholar] [CrossRef]
- Asami, T.; Min, Y.K.; Nagata, N.; Yamagishi, K.; Takatsuto, S.; Fujioka, S.; Murofushi, N.; Yamaguchi, I.; Yoshida, S. Characterization of brassinazole, a triazole-type brassinosteroid biosynthesis inhibitor. Plant Physiol. 2000, 123, 93–100. [Google Scholar]
- Wang, Z.Y.; Nakano, T.; Gendron, J.; He, J.; Chen, M.; Vafeados, D.; Yang, Y.; Fujioka, S.; Yoshida, S.; Asami, T.; et al. Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev. Cell 2002, 4, 505–513. [Google Scholar]
- Mathur, J.; Molnár, G.; Fujioka, S.; Takatsuto, S.; Sakurai, A.; Yokota, T.; Adam, G.; Voigt, B.; Nagy, F.; Maas, C.; et al. Transcription of the Arabidopsis CPD gene, encoding a steroidogenic cytochrome P450, is negatively controlled by brassinosteroids. Plant J. 1998, 14, 593–602. [Google Scholar] [CrossRef]
- Kim, T.W.; Chang, S.C.; Lee, J.S.; Hwang, B.; Takatsuto, S.; Yokota, T.; Kim, S.K. Cytochrome P450-catalyzed brassinosteroid pathway activation through synthesis of castasterone and brassinolide in Phaseolus vulgaris. Phytochemistry 2004, 65, 679–689. [Google Scholar]
- Kim, T.W.; Hwang, J.Y.; Kim, Y.S.; Joo, S.H.; Chang, S.C.; Lee, J.S.; Takatsuto, S.; Kim, S.K. Arabidopsis CYP85A2, a cytochrome P450, mediates the Baeyer-Villiger oxidation of castasterone to brassinolide in brassinosteroid biosynthesis. Plant Cell 2005, 17, 2397–2412. [Google Scholar]
- Testa, B.; Jenner, P. Inhibitors of Cytochrome P-450s and their mechanism of action. Drug Metab. Rev. 1981, 12, 1–117. [Google Scholar]
- Rogerson, T.D.; Wilkinson, C.F.; Hetarski, K. Steric factors in the inhibitory interaction of imidazoles with microsomal enzymes. Biochem. Pharmacol. 1977, 26, 1039–1042. [Google Scholar]
- Oh, K. Jasmonic acid biosynthesis inhibitor. Regul. Plant Growth Devel. 2009, 44, 166–169. [Google Scholar]
- Oh, K.; Murofushi, N. Design and synthesis of novel imidazole derivatives as potent inhibitors of allene oxide synthase (CYP74). Bioorg. Med. Chem. 2002, 10, 3707–3711. [Google Scholar]
- Oh, K.; Seki, Y.; Murofushi, N.; Yoshizawa, Y. Effect of miconazole, an antifungal agent, on allene oxide synthase: Inhibition, kinetics, and binding. Pestic. Biochem. Physiol. 2009, 94, 107–111. [Google Scholar] [CrossRef]
- Oh, K.; Asami, T.; Matsui, K.; Howe, G.A.; Murofushi, N. Characterization of novel imidazole derivative, JM-8686, a potent inhibitor of allene oxide synthase. FEBS Lett. 2006, 580, 5791–5796. [Google Scholar] [CrossRef]
- Oh, K.; Yamada, K.; Asami, T.; Yoshizawa, Y. Synthesis of novel brassinosteroid biosynthesis inhibitors based on the ketoconazole scaffold. Bioorg. Med. Chem. Lett. 2012, 22, 1625–1628. [Google Scholar]
- Oh, K.; Shimura, Y.; Ishikawa, K.; Ito, Y.; Asami, T.; Murofushi, N.; Yoshizawa, Y. Asymmetric synthesis and stereochemical structure-activity relationship of (R)- and (S)-8-[1-(2,4-dichlorophenyl)-2-imidazol-1-yl-ethoxy] octanoic acid heptyl ester, a potent inhibitor of allene oxide synthase. Bioorg. Med. Chem. 2008, 16, 1090–1095. [Google Scholar] [CrossRef]
- Sekimata, K.; Ohnishi, T.; Mizutani, M.; Todoroki, Y.; Han, S.Y.; Uzawa, J.; Fujioka, S.; Yoneyama, K.; Takeuchi, Y.; Takatsuto, S.; et al. Brz220 interacts with DWF4, a cytochrome P450 monooxygenase in brassinosteroid biosynthesis, and exerts biological activity. Biosci. Biotechnol. Biochem. 2008, 72, 7–12. [Google Scholar] [CrossRef]
- Sekimata, K.; Han, S.Y.; Yoneyama, K.; Takeuchi, Y.; Yoshida, S.; Asami, T. A specific and potent inhibitor of brassinosteroid biosynthesis possessing a dioxolane ring. J. Agric. Food Chem. 2002, 50, 3486–3490. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yamada, K.; Yoshizawa, Y.; Oh, K. Synthesis of 2RS,4RS-1-[2-Phenyl-4-[2-(2-trifluromethoxy-phenoxy)-ethyl]-1,3-dioxolan-2-yl-methyl]-1H-1,2,4-triazole Derivatives as Potent Inhibitors of Brassinosteroid Biosynthesis. Molecules 2012, 17, 4460-4473. https://doi.org/10.3390/molecules17044460
Yamada K, Yoshizawa Y, Oh K. Synthesis of 2RS,4RS-1-[2-Phenyl-4-[2-(2-trifluromethoxy-phenoxy)-ethyl]-1,3-dioxolan-2-yl-methyl]-1H-1,2,4-triazole Derivatives as Potent Inhibitors of Brassinosteroid Biosynthesis. Molecules. 2012; 17(4):4460-4473. https://doi.org/10.3390/molecules17044460
Chicago/Turabian StyleYamada, Kazuhiro, Yuko Yoshizawa, and Keimei Oh. 2012. "Synthesis of 2RS,4RS-1-[2-Phenyl-4-[2-(2-trifluromethoxy-phenoxy)-ethyl]-1,3-dioxolan-2-yl-methyl]-1H-1,2,4-triazole Derivatives as Potent Inhibitors of Brassinosteroid Biosynthesis" Molecules 17, no. 4: 4460-4473. https://doi.org/10.3390/molecules17044460
APA StyleYamada, K., Yoshizawa, Y., & Oh, K. (2012). Synthesis of 2RS,4RS-1-[2-Phenyl-4-[2-(2-trifluromethoxy-phenoxy)-ethyl]-1,3-dioxolan-2-yl-methyl]-1H-1,2,4-triazole Derivatives as Potent Inhibitors of Brassinosteroid Biosynthesis. Molecules, 17(4), 4460-4473. https://doi.org/10.3390/molecules17044460





