Aminodi(hetero)arylamines in the Thieno[3,2-b]pyridine Series: Synthesis, Effects in Human Tumor Cells Growth, Cell Cycle Analysis, Apoptosis and Evaluation of Toxicity Using Non-Tumor Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
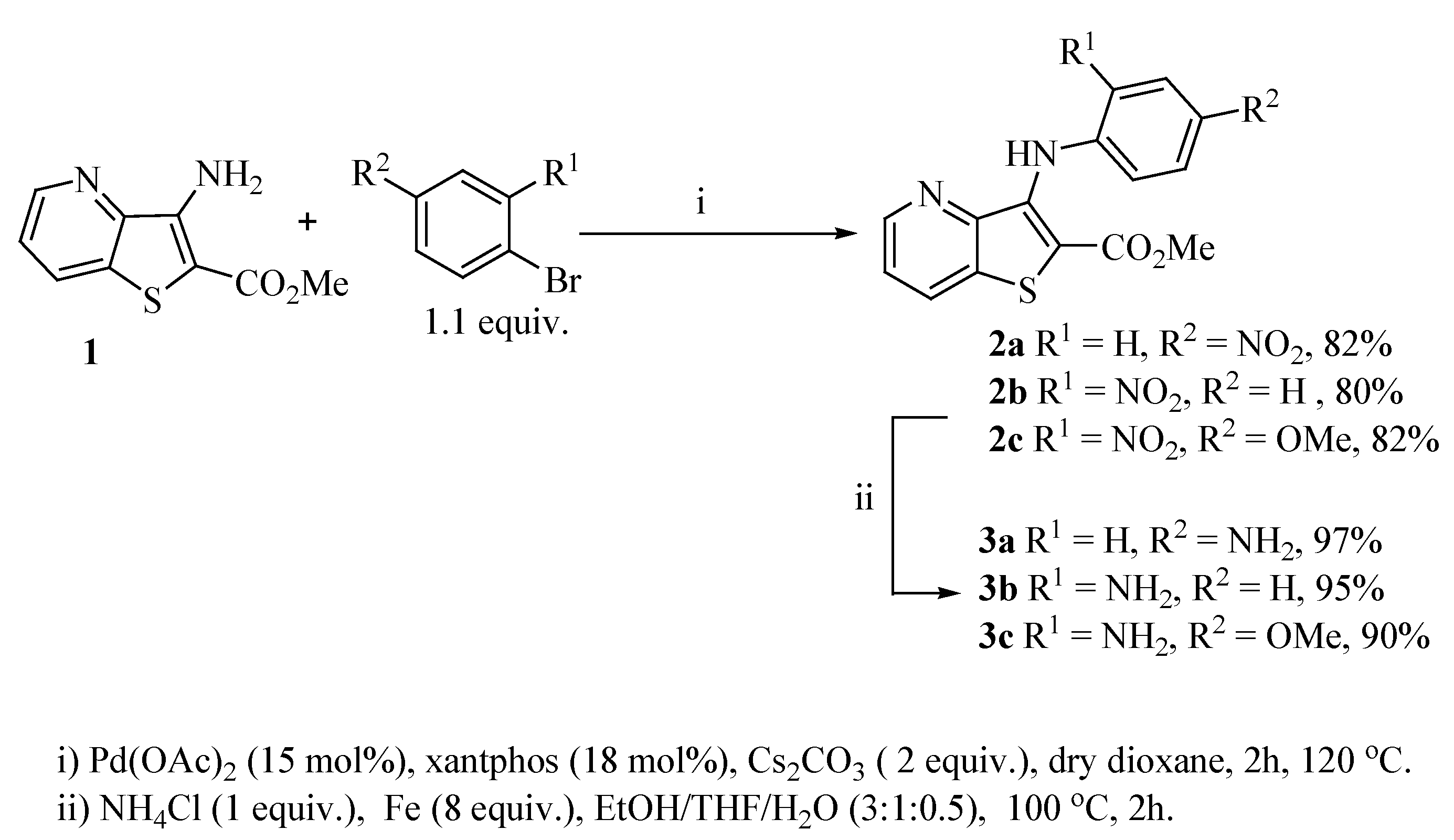
2.2. Cell Growth Inhibitory Activity and Toxicity to Non-Tumor Cells
| MCF-7 | A375-C5 | NCI-H460 | HepG2 | PLP1 | |
|---|---|---|---|---|---|
 | >125 | 111.80 ± 5.00 | >125 | >125 | >125 |
 | 33.80 ± 1.70 | 26.00 ± 2.30 | 31.30 ± 2.90 | 99.26 ± 0.92 | 61.27 ± 1.83 |
 | 1.40 ± 0.20 | 1.30 ± 0.10 | 1.40 ± 0.40 | 1.63 ± 0.09 | 12.49 ± 0.09 |
| Doxorubicin | 0.04 ± 0.01 | 0.13 ± 0.01 | 0.09 ± 0.01 | --- | --- |
| Ellipticine | --- | --- | --- | 0.80 ± 0.05 | 4.19 ± 0.08 |
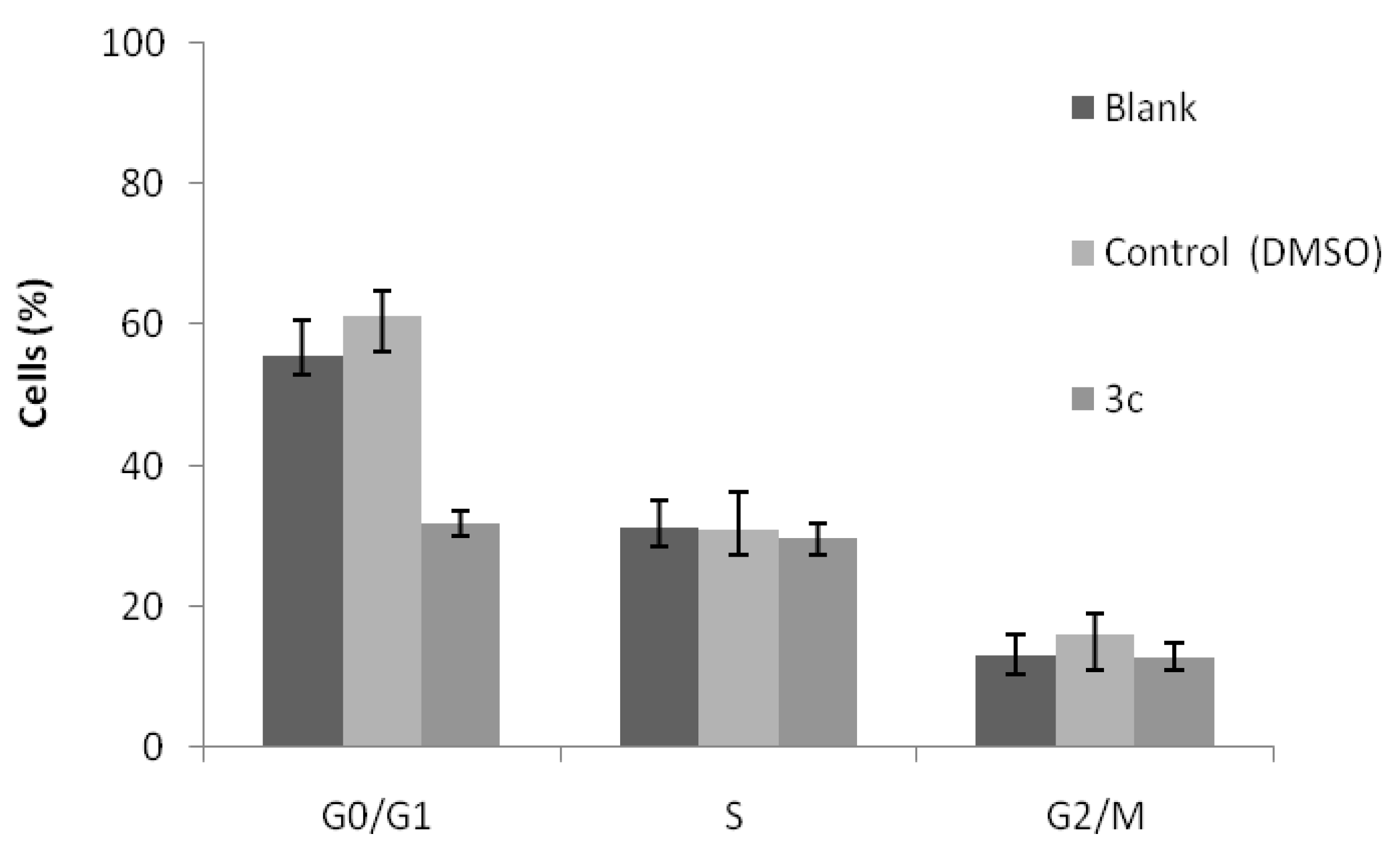
3. Experimental
3.1. Synthesis
3.1.1. General Procedure for the Synthesis of Nitrodiarylamines 2a–c
3.1.2. General Procedure for the Synthesis of Aminodiarylamines 3a–c
3.2. Cell Growth Inhibitory Activity and Toxicity to Non-Tumor Cells
3.2.1. Standards and Reagents
3.2.2. Solutions of the Compounds
3.2.3. Cell Culture of Cell Lines and of Porcine liver Primary Cells
3.2.4. Cell Growth Inhibition Assay in Tumour Cell Lines and Primary Porcine Liver Cells
3.2.5. Analysis of Cell Cycle Distribution Profile
3.2.6. Analysis of Apoptosis
4. Conclusions
Conflict of Interest
Acknowledgements
References and Notes
- Bray, F.; Møller, B. Predicting the future burden of cancer. Nat. Ver. Cancer 2006, 6, 63–74. [Google Scholar]
- Mimeault, M.; Batra, S.K. New promising drug targets in cancer- and metastasis-initiating cells. Drug Discov. Today 2010, 15, 354–364. [Google Scholar] [CrossRef]
- Munchhof, M.J.; Beebe, J.S.; Casavant, J.M.; Cooper, B.A.; Doty, J.L.; Higdon, R.C.; Hillerman, S.M.; Soderstrom, C.I.; Knauth, E.A.; Marx, M.A.; et al. Design and SAR of thienopyrimidine and thienopyridine inhibitors of VEGFR-2 kinase activity. Bioorg. Med. Chem. Lett. 2004, 14, 21–24. [Google Scholar]
- Zheng, R.-L.; Zhou, T.; He, H.-Y.; Liu, J.-Y.; Zheng, Y.; Tong, A.-P.; Xiang, M.-L.; Song, X.-R.; Yang, S.-Y.; Yu, L.-T.; et al. Novel thienopyridine derivatives as specific anti-hepatocellular carcinoma (HCC) agents: Synthesis, preliminary structure-activity relationships, and in vitro biological evaluation. Bioorg. Med. Chem. Lett. 2010, 20, 6282–6285. [Google Scholar]
- Queiroz, M.J.R.P.; Calhelha, R.C.; Vale-Silva, L.A.; Pinto, E.; Nascimento, M.S.-J. Efficient synthesis of new 6-(hetero)arylthieno[3,2-b]pyridines by Suzuki-Miyaura Coupling. Evaluation of growth inhibition on human tumor cell lines. Eur. J. Med. Chem. 2010, 45, 5628–5634. [Google Scholar] [CrossRef]
- Queiroz, M.J.R.P.; Calhelha, R.C.; Vale-Silva, L.A.; Pinto, E.; Lima, R.T.; Vasconcelos, M.H. New 6-[(hetero)arylamino]thieno[3,2-b]pyridines: Synthesis and antitumoral activities. Eur. J. Med. Chem. 2010, 45, 5732–5738. [Google Scholar]
- Queiroz, M.J.R.P.; Calhelha, R.C.; Vale-Silva, L.A.; Pinto, E.; Almeida, G.M.; Vasconcelos, M.H. Synthesis and evaluation of tumor cell growth inhibition of novel methyl 3-Amino-6-[(hetero)arylethynyl]thieno[3,2-b]pyridine-2-carboxylates. Structure-activity relationships and effects on the cell cycle and apoptosis. Eur. J. Med. Chem. 2011, 46, 236–240. [Google Scholar]
- Dunn, A.D.; Norrie, R.J.J. Nucleophilic displacement in pyridine ring. J. Heterocycl. Chem. 1987, 24, 85–89. [Google Scholar]
- Yin, J.; Zhao, M.M.; Hulfman, M.A.; McWamara, J.M. Pd-catalyzed N-arylation of heteroarylamines. Org. Lett. 2002, 4, 3481–3484. [Google Scholar]
- Queiroz, M.-J.R.P.; Calhelha, R.C.; Kirsch, G. Reactivity of several deactivated 3-aminobenzo[b]thiophenes in the Buchwald-Hartwig C-N coupling. Scope and limitations. Tetrahedron 2007, 63, 13000–13005. [Google Scholar]
- Dai, Y.; Guo, Y.; Frey, R.R.; Ji, Z.; Curtin, M.L.; Ahmed, A.A.; Albert, D.H.; Arnold, L.; Arries, S.S.; Barlozzari, T.; et al. Thienopyrimidine ureas as novel and potent multitargeted receptor tyrosine kinase inhibitors. J. Med. Chem. 2005, 48, 6066–6083. [Google Scholar]
- Queiroz, M.J.R.P.; Calhelha, R.C. Synthesis of new thieno[3,2-b]pyridine derivatives by palladium-catalyzed couplings and intramolecular cyclizations. Tetrahedron Lett. 2010, 51, 281–283. [Google Scholar] [CrossRef]
- Abreu, R.M.V.; Ferreira, I.C.F.R; Calhelha, R.C.; Lima, R.T.; Vasconcelos, M.H.; Adega, F.; Chaves, R.; Queiroz, M.J.R.P. Anti-hepatocellular carcinoma activity using HepG2 cells and hepatotoxicity of 6-substituted methyl 3-aminothieno[3,2-b]pyridine-2-carboxylate derivatives: In vitro evaluation, cell cycle analysis and QSAR studies. Eur. J. Med. Chem. 2011, 46, 5800–5806. [Google Scholar]
- Sample Availability: Contact the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Calhelha, R.C.; Ferreira, I.C.F.R.; Peixoto, D.; Abreu, R.M.V.; Vale-Silva, L.A.; Pinto, E.; Lima, R.T.; Alvelos, M.I.; Vasconcelos, M.H.; Queiroz, M.-J.R.P. Aminodi(hetero)arylamines in the Thieno[3,2-b]pyridine Series: Synthesis, Effects in Human Tumor Cells Growth, Cell Cycle Analysis, Apoptosis and Evaluation of Toxicity Using Non-Tumor Cells. Molecules 2012, 17, 3834-3843. https://doi.org/10.3390/molecules17043834
Calhelha RC, Ferreira ICFR, Peixoto D, Abreu RMV, Vale-Silva LA, Pinto E, Lima RT, Alvelos MI, Vasconcelos MH, Queiroz M-JRP. Aminodi(hetero)arylamines in the Thieno[3,2-b]pyridine Series: Synthesis, Effects in Human Tumor Cells Growth, Cell Cycle Analysis, Apoptosis and Evaluation of Toxicity Using Non-Tumor Cells. Molecules. 2012; 17(4):3834-3843. https://doi.org/10.3390/molecules17043834
Chicago/Turabian StyleCalhelha, Ricardo C., Isabel C. F. R. Ferreira, Daniela Peixoto, Rui M. V. Abreu, Luís A. Vale-Silva, Eugénia Pinto, Raquel T. Lima, M. Inês Alvelos, M. Helena Vasconcelos, and Maria-João R. P. Queiroz. 2012. "Aminodi(hetero)arylamines in the Thieno[3,2-b]pyridine Series: Synthesis, Effects in Human Tumor Cells Growth, Cell Cycle Analysis, Apoptosis and Evaluation of Toxicity Using Non-Tumor Cells" Molecules 17, no. 4: 3834-3843. https://doi.org/10.3390/molecules17043834






