Antiangiogenic Polyketides from Peperomia dindygulensis Miq.
Abstract
:1. Introduction

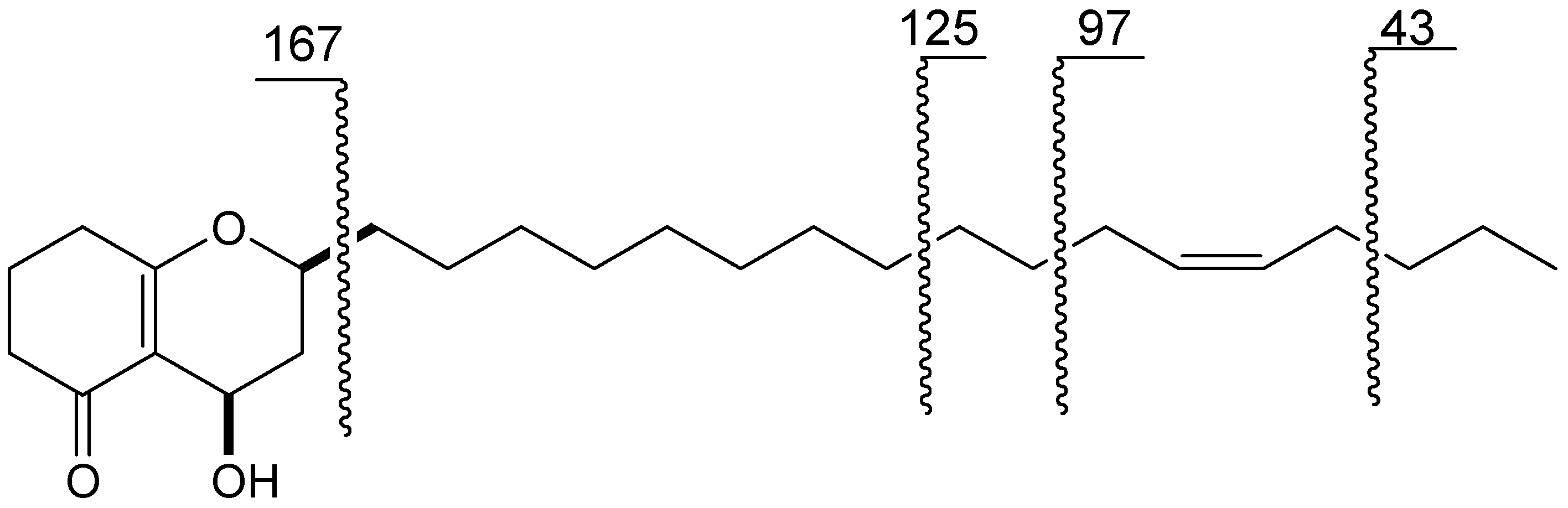
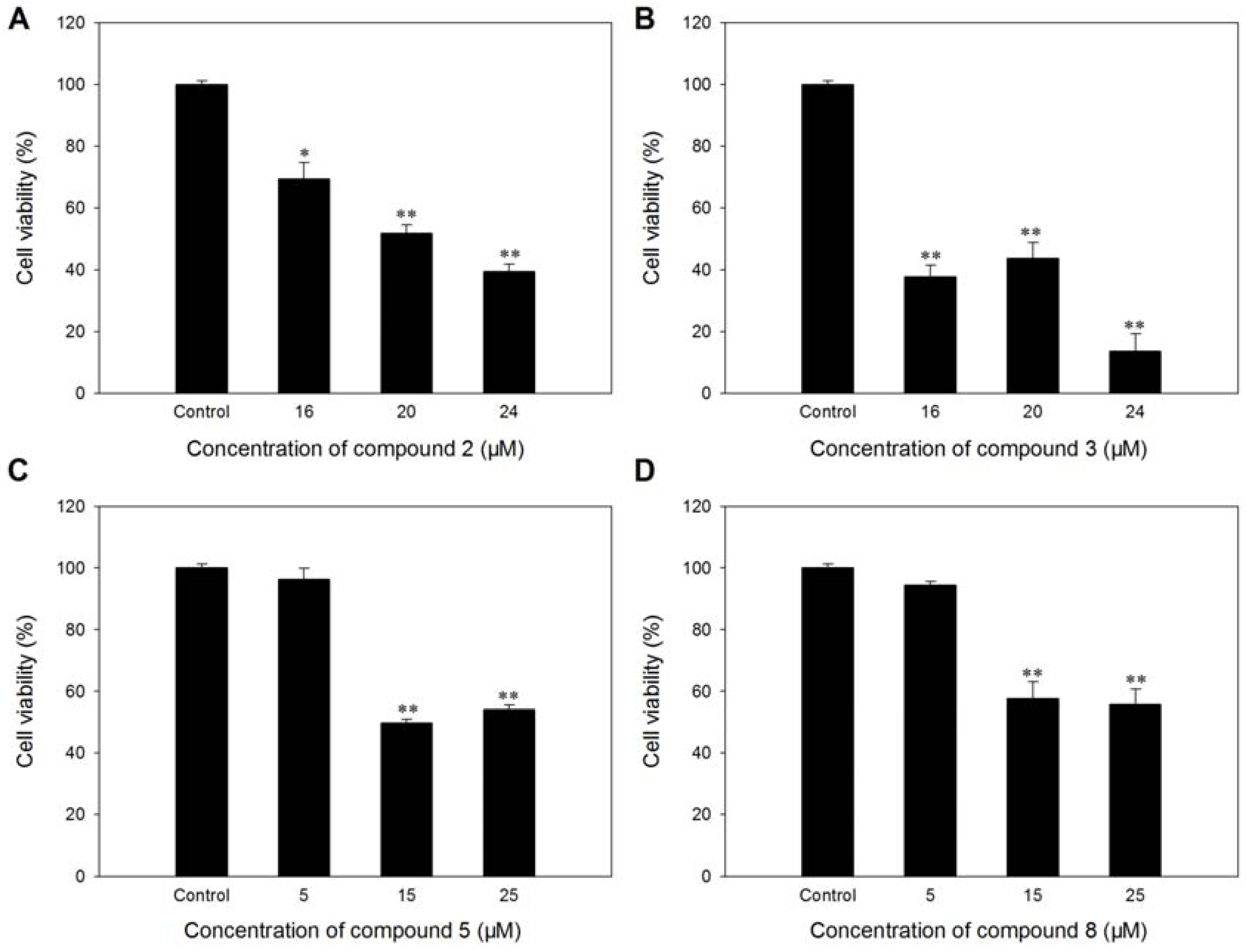
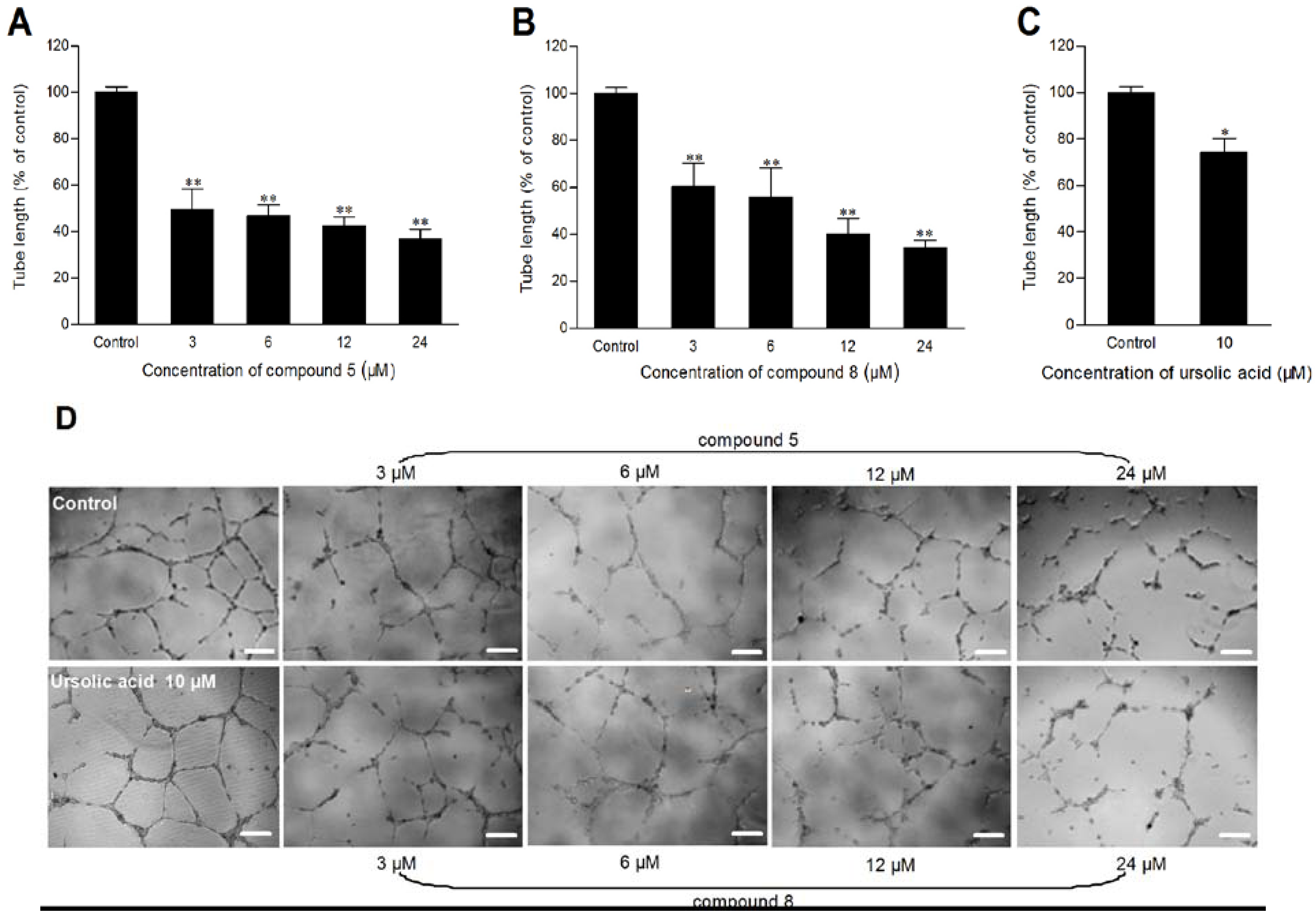
2. Results and Discussion
| Position | δC | δH |
|---|---|---|
| 2 | 77.1 | 4.00 (1H, dddd, 1.7, 5.4, 7.3,11.2) |
| 3 | 35.2 | 2.22 (1H, ddd,2.0, 6.7, 13.5); 1.70 (1H, m) |
| 4 | 62.1 | 4.75 (1H, dd, 7.0, 9.3) |
| 4a | 114.7 | |
| 5 | 200.6 | |
| 6 | 36.7 | 2.33 (1H, dd, 9.6, 16.8); 2.39 (1H, m) |
| 7 | 20.5 | 1.97 (1H, dd, 6.3, 12.9); 1.96 (1H, dd, 6.1, 12.9) |
| 8 | 28.4 | 2.40 (2H, m) |
| 8a | 173.4 | |
| 1' | 34.7 | 1.62 (1H, m); 1.73 (1H, m) |
| 2' | 25.0 | 1.46 (2H, m) |
| 3'-10' | 29.3–29.8 | 1.26–1.37 (16H, m) |
| 11' | 27.2 | 2.00 (2H, m) |
| 12', 13' | 129.8, 129.9 | 5.35 (2H, m) |
| 14' | 26.9 | 2.01 (2H, m) |
| 15' | 32.0 | 1.30 (2H, m) |
| 16' | 22.4 | 1.31 (2H, m) |
| 17' | 14.0 | 0.88 (3H, t, 7.0) |
| OH-4 | 4.63 ( brs) |

| Position | δC | δH |
|---|---|---|
| 2 | 169.3 | |
| 3 | 112.7 | 6.10 (1H, s) |
| 4 | 180.7 | |
| 4a | 123.2 | |
| 5 | 63.8 | 4.91 (1H, br t, 3.8) |
| 6 | 29.5 | 1.76 (1H, m); 1.96 (1H, m) |
| 7 | 18.1 | 1.74 (1H, m); 1.97 (1H, m) |
| 8 | 27.6 | 2.49 (1H, m); 2.57 (1H, m) |
| 8a | 165.0 | |
| 1' | 33.5 | 2.48 (2H, t, 7.6) |
| 2' | 26.8 | 1.61 (2H, m) |
| 3'–10' | 28.9–29.7 | 1.26–1.37 (16H, m) |
| 11' | 27.1 | 2.00 (2H, m) |
| 12', 13' | 129.8 | 5.35 (2H, m) |
| 14' | 26.9 | 2.01 (2H, m) |
| 15' | 31.9 | 1.30 (2H, m) |
| 16' | 22.3 | 1.31 (2H, m) |
| 17' | 14.0 | 0.89 (3H, t, 6.4) |
| 5-OH | 4.43 ( brs) |

3. Experimental
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Antiangiogenic Activity Assays
4. Conclusions
Acknowledgments
References and Notes
- Editorial Board of China HerbalState Administration of Traditional Chinese Medicine, ChinaChina Herbal; Shanghai Scientific and Technical Publishers: Shanghai, China, 1999; p. 421.
- Govindachari, T.R.; Kumari, K.G.N.; Partho, P.D. Two secolignans from Peperomia dindigulensis. Phytochemistry 1998, 49, 2129–2131. [Google Scholar]
- Wu, J.L.; Li, N.; Hasegawa, T.; Sakai, J.; Kakuta, S.; Tang, W.; Oka, S.; Kiuchi, M.; Ogura, H.; Kataoka, T.; et al. Bioactive tetrahydrofuran lignans from Peperomia dindygulensis. J. Nat. Prod. 2005, 68, 1656–1660. [Google Scholar]
- Wu, J.L.; Li, N.; Hasegawa, T.; Sakai, J.; Mitsui, T.; Ogura, H.; Kataoka, T.; Oka, S.; Kiuchi, M.; Tomida, A.; et al. Bioactive dibenzylbutyrolactone and dibenzylbutanediol lignans from Peperomia duclouxii. J. Nat. Prod. 2006, 69, 790–794. [Google Scholar] [CrossRef]
- Xu, S.; Li, N.; Ning, M.M.; Zhou, C.H.; Yang, Q.R.; Wang, M.W. Bioactive compounds from Peperomia pellucida. J. Nat. Prod. 2006, 69, 247–250. [Google Scholar] [CrossRef]
- Monache, F.D.; Compagnone, R.S. A secolignan from Peperomia glabella. Phytochemistry 1996, 43, 1097–1098. [Google Scholar]
- Mbah, J.A.; Tchuendem, M.H.K.; Tane, P.; Sterner, O. Two chromones from Peperomia vulcanica. Phytochemistry 2002, 60, 799–801. [Google Scholar]
- Zhang, G.L.; Li, N.; Wang, Y.H.; Zheng, Y.T.; Zhang, Z.; Wang, M.W. Bioactive lignans from Peperomia heyneana. J. Nat. Prod. 2007, 70, 662–664. [Google Scholar]
- 9. Lin, M.G.; Yu, D.H.; Wang, Q.W.; Lu, Q.; Zhu, W.J.; Bai, F.; Li, G.X.; Wang, X.W.; Yang, Y.F.; Qin, X.M.; et al. Secolignans with antiangiogenic activities from Peperomia dindygulensis. Chem. Biodiv. 2011, 8, 862–871. [Google Scholar]
- Li, N.; Wu, J.L.; Hasegawa, T.; Sakai, J.; Bai, L.M.; Wang, L.Y.; Kakuta, S.; Furuya, Y.; Ogura, H.; Kataoka, T.; et al. Bioactive polyketides from Peperomia duclouxii. J. Nat. Prod. 2007, 70, 998–1001. [Google Scholar] [CrossRef]
- Azevedo, N.R.; Santos, S.C.; De Miranda, E.G.; Ferri, P.H. A 2-Acylcyclohexane-1,3-dione from virola oleifera. Phytochemistry 1997, 46, 1375–1377. [Google Scholar]
- Kato, M.J.; Lopes, X.L.M.; Paulino Fo, H.F.; Yoshida, M.; Gottlieb, O.R. Acylresorcinols from Virola sebifera and Virola elongate. Phytochemistry 1985, 24, 533–536. [Google Scholar]
- Seeram, N.P.; Lewis, A.; Jacobs, H.; Nair, M.G.; McLean, S.; Reynolds, W.F. Proctoriones A–C: 2-Acylcyclohexane-1,3-dione derivatives from Peperomia proctorii. J. Nat. Prod. 2000, 63, 399–402. [Google Scholar] [CrossRef]
- Cheng, M.J.; Lee, S.J.; Chang, Y.Y.; Wu, S.H.; Tsai, I.L.; Jayaprakasam, B.; Chen, I.S. Chemical and cytotoxic constituents from Peperomia sui. Phytochemistry 2003, 63, 603–608. [Google Scholar]
- Chu, H.W.; Wu, H.T.; Lee, Y.J. Regioselective hydroxylation of 2-hydroxychalcones by dimethyldioxirane towards polymethoxylated flavonoids. Tetrahedron 2004, 60, 2647–2655. [Google Scholar]
- Shaw, S.C.; Azad, R.; Mandal, S.P.; Gandhi, R.S. Synthesis of 6-hydroxyluteolin and sinensetin by wessely-Moser rearrangement. J. Indian Chem. Soc. 1988, LXV, 107–109. [Google Scholar]
- Tang, J.; Li, H.L.; Li, Y.L.; Zhang, W.D. Flavonoids from rhizomes of Veratum dahuricum. Chem. Nat. Compd. 2007, 43, 696–697. [Google Scholar] [CrossRef]
- Araújo, J.X.; Chaves, M.C.O.; Da Cunha, E.V.L.; Gray, A.I. Cepharanone B from Piper tuberculatum. Biochem. Syst. Ecol. 1999, 27, 325–327. [Google Scholar] [CrossRef]
- Mizutani, K.; Fuknaga, Y.; Tanaka, O.; Takasugi, N.; Saruwatari, Y.I.; Fuwa, T.; Yamauchi, T.; Wang, J.; Jia, M.R.; Li, F.Y.; et al. Amides from Huajiao, Pericarps of Zanthoxylum bungeanum maxim. Chem. Pharm. Bull. 1988, 36, 2362–2365. [Google Scholar]
- Yoshii, E.; Koizumi, T.; Oribe, T. The structure of agarotetrol, a novel highly oxygenated chromone from agarwood (jinko). Tetrahedron Lett. 1978, 41, 3921–3924. [Google Scholar]
- El-Elimat, T.; Li, C.; Qandil, A.; Alkofahi, A.; Tawaha, K.; Burgess, J.P.; Nakanishi, Y.; Kroll, D.J.; Navarro, H.A. New colchicinoids from a native Jordanian meadow saffron, Colchicum brachyphyllum: Isolation of the first naturally occurring dextrorotatory colchicinoid. J. Nat. Prod. 2005, 68, 173–178. [Google Scholar] [CrossRef]
- Isaka, M.; Palasarn, S.; Auncharoen, P.; Kornmijit, S.; Jones, E.B.G. Acremoxanthones A and B, novel antibiotic polyketides from the fungus Acremonium sp. BCC 31806. Tetrahedron Lett. 2009, 50, 284–287. [Google Scholar]
- Liu, Y.B.; Mulabagal, V.; Bowen-Forbes, C.S.; Aviayan, R.; Nair, M.G. Inhibition of lipid peroxidation, cyclooxygenase enzyme and human tumor cell proliferation by compounds in herbal water. Mol. Nutr. Food Res. 2009, 53, 1177–1186. [Google Scholar]
- Dunlop, R.W.; Simon, A.; Sivasithamparam, K.; Ghisalberti, E.L. An antibiotic from Trichoderma koningii active against soilborne plant pathogens. J. Nat. Prod. 1989, 52, 67–74. [Google Scholar] [CrossRef]
- Cutler, H.G.; Himmelsbach, D.S.; Yagen, B.; Arrendale, R.F.; Jacyno, J.M.; Cole, P.D.; Cox, R.H. Koninginin B: A biologically active congener of koninginin A from Trichoderma koningii. J. Agric. Food Chem. 1991, 39, 977–980. [Google Scholar]
- Sample Availability: Not available.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, Q.-W.; Yu, D.-H.; Lin, M.-G.; Zhao, M.; Zhu, W.-J.; Lu, Q.; Li, G.-X.; Wang, C.; Yang, Y.-F.; Qin, X.-M.; et al. Antiangiogenic Polyketides from Peperomia dindygulensis Miq. Molecules 2012, 17, 4474-4483. https://doi.org/10.3390/molecules17044474
Wang Q-W, Yu D-H, Lin M-G, Zhao M, Zhu W-J, Lu Q, Li G-X, Wang C, Yang Y-F, Qin X-M, et al. Antiangiogenic Polyketides from Peperomia dindygulensis Miq. Molecules. 2012; 17(4):4474-4483. https://doi.org/10.3390/molecules17044474
Chicago/Turabian StyleWang, Qi-Wei, De-Hong Yu, Meng-Gan Lin, Mei Zhao, Wen-Jun Zhu, Qin Lu, Gui-Xiu Li, Chao Wang, Yi-Fang Yang, Xue-Mei Qin, and et al. 2012. "Antiangiogenic Polyketides from Peperomia dindygulensis Miq." Molecules 17, no. 4: 4474-4483. https://doi.org/10.3390/molecules17044474




