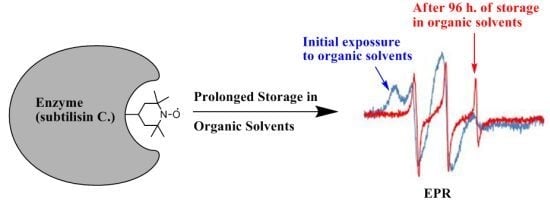
Low Operational Stability of Enzymes in Dry Organic Solvents: Changes in the Active Site Might Affect Catalysis
Abstract
:1. Introduction
2. Results and Discussion
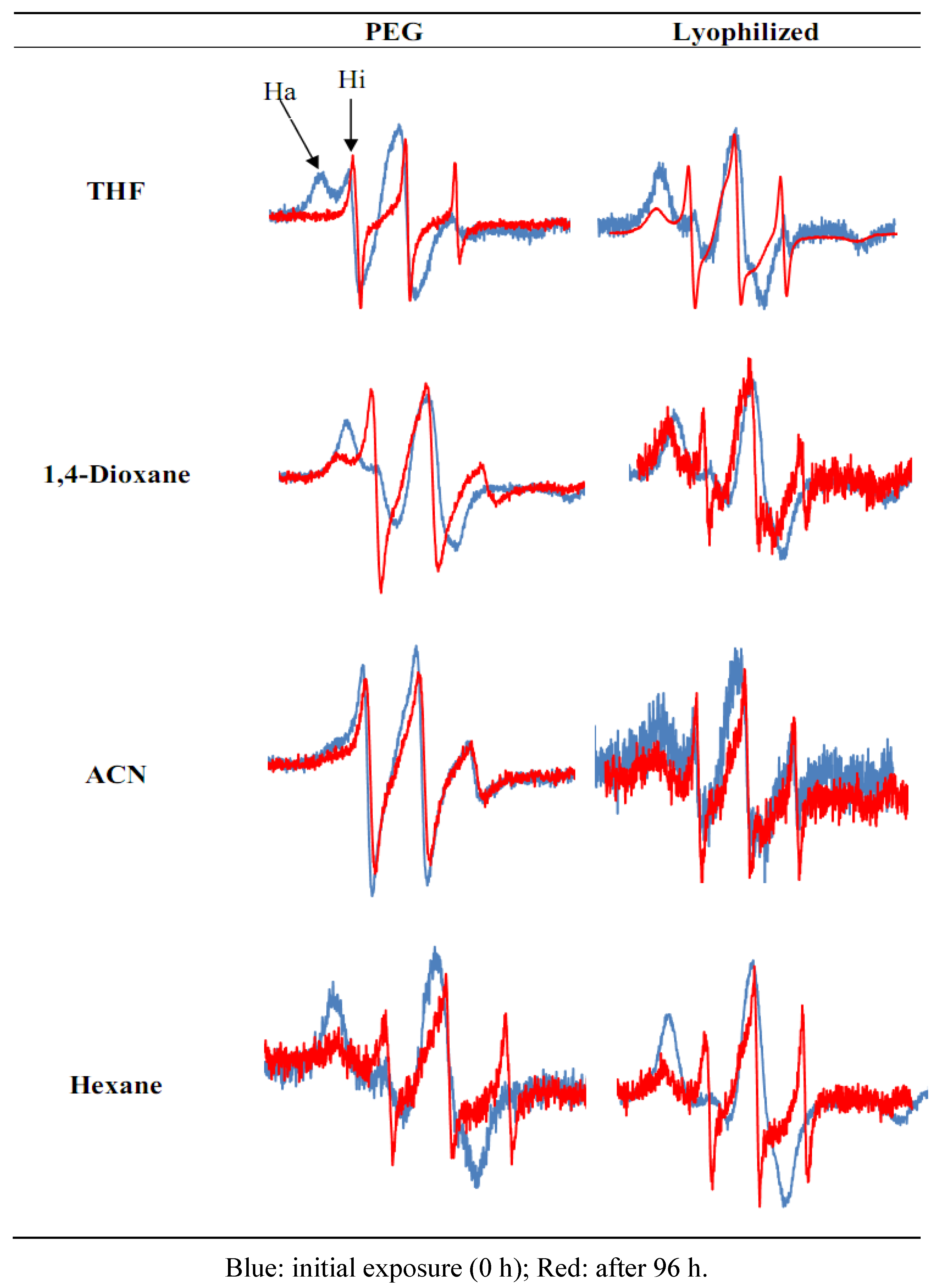
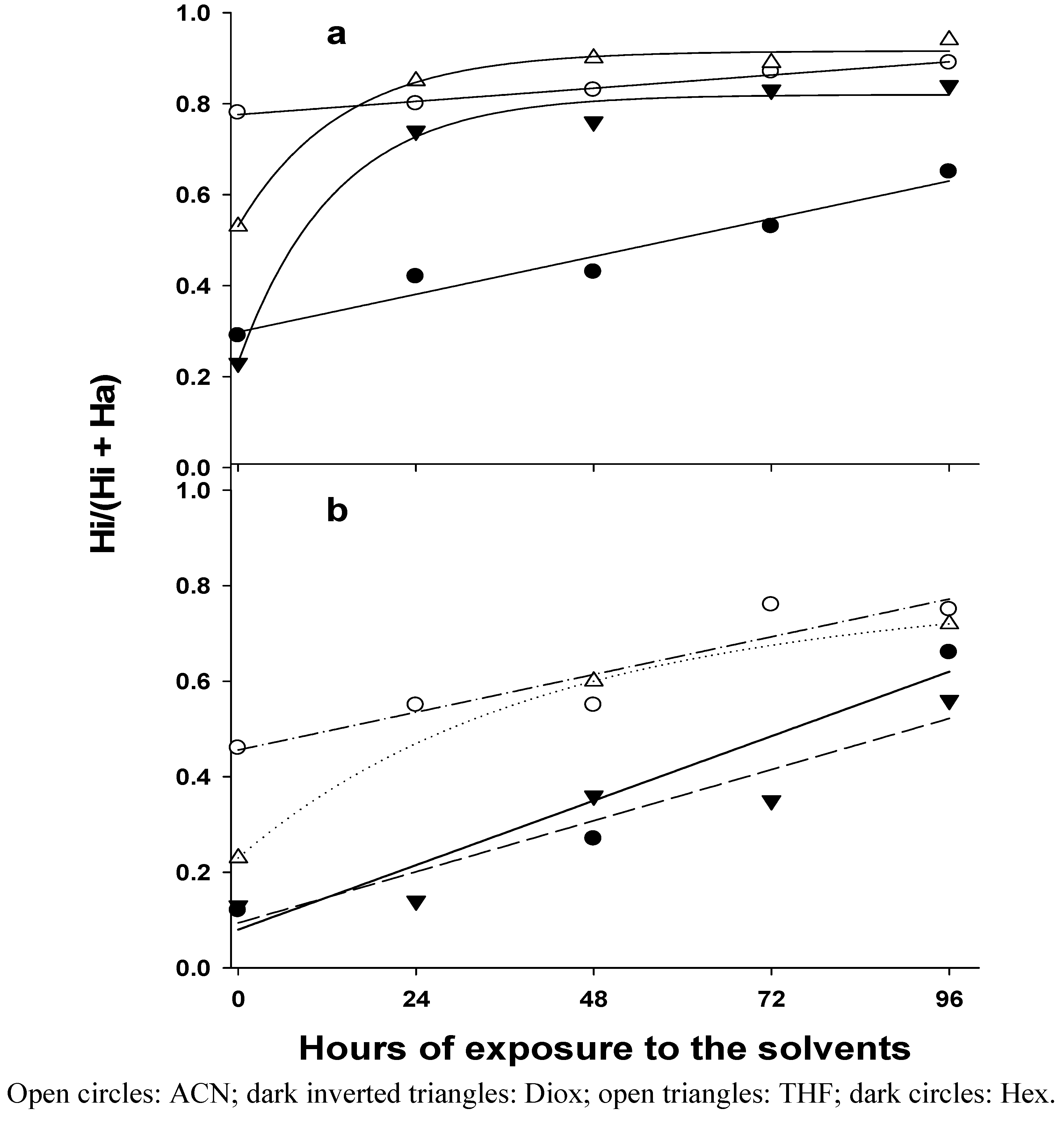
| Hi/(Hi/Ha) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hexane | Acetonitrile | 1,4-dioxane | THF | ||||||||
| Hours | PEG | Lyo | PEG | Lyo | PEG | Lyo | PEG | Lyo | |||
| 0 | 0.29 | 0.12 | 0.78 | 0.46 | 0.23 | 0.13 | 0.53 | 0.23 | |||
| 24 | 0.42 | 0.80 | 0.55 | 0.74 | 0.14 | 0.85 | |||||
| 48 | 0.43 | 0.27 | 0.83 | 0.55 | 0.76 | 0.36 | 0.90 | 0.60 | |||
| 72 | 0.53 | 0.87 | 0.76 | 0.83 | 0.35 | 0.89 | |||||
| 96 | 0.65 | 0.66 | 0.89 | 0.75 | 0.84 | 0.56 | 0.94 | 0.72 | |||

| Storage for |  | 0 h | 96 h | |||
|---|---|---|---|---|---|---|
| Vs (mmol/min.mg) | E | Vs (mmol/min.mg) | E | |||
| Hex | Lyo | 0.016 ± 0.007 | 12 ± 2 | 0.0018 ± 0.00006 | 10 ± 1 | |
| PEG | 0.47 ± 0.01 | 7.4 ± 0.1 | 0.25 ± 0.01 | 6.8 ± 0.1 | ||
| ACN | Lyo | 0.0035 ± 0.0009 | 4.6 ± 0.7 | 0.00015 ± 0.00007 | 4.7 ± 0.7 | |
| PEG | 0.077 ± 0.002 | 5.1 ± 0.1 | 0.011 ± 0.0001 | 5.2 ± 0.04 | ||
| THF | Lyo | 0.025 ± 0.001 | 34 ± 5 | 0.006 ± 0.001 | 21 ± 3 | |
| PEG | 0.62 ± 0.01 | 36.7 ± 0.4 | 0.095 ± 0.007 | 32.1 ± 0.2 | ||
| Diox | Lyo | 0.032 ± 0.002 | 30 ± 9 | 0.0027 ± 0.0001 | 17 ± 1 | |
| PEG | 0.6 ± 0.1 | 21.2 ± 0.3 | 0.15 ± 0.01 | 16 ± 1 | ||
3. Experimental
3.1. Materials
3.2. Enzyme Preparation (lyophilisation and PEG’s)
3.3. Spin Labeling
3.4. Active Site Spin Labeling (ASSL)
3.5. Enzyme Preparation and Kinetic Measurements
3.6. Enzyme Concentration
4. Conclusions
Acknowledgements
- Samples Availability: Samples of the TEMPO spin label are available from the authors.
References and Notes
- Effenberger, F.; Syed, J. Stereoselective synthesis of biologically active tetronic acids. Tetrahedron 1998, 9, 817–825. [Google Scholar] [CrossRef]
- Jungen, M.; Gais, H. Application of pig liver esterase catalyzed transesterification in organic media to the kinetic resolution of glycerol derivatives. Tetrahedron-Asymmetry 1999, 10, 3747–3758. [Google Scholar]
- Klibanov, A.M. Improving enzymes by using them in organic solvents. Nature 2001, 409, 241–246. [Google Scholar] [CrossRef]
- Lee, T.; Jones, J.B. Probing the abilities of synthetically useful serine proteases to discriminate between the configurations of remote stereocenters using chiral aldehyde inhibitors. J. Am. Chem. Soc. 1996, 118, 502–508. [Google Scholar]
- Macritchie, J.A.; Silcock, A.; Willis, C.L. Enantioselective synthesis of unsaturated α-hydroxy acids. Tetrahedron: Asymmetry 1997, 8, 3895–3902. [Google Scholar]
- Roberts, S.M.; Williamson, N.M. The use of enzymes for the preparation of biologically active natural products and analogues in optically active form. Curr. Org. Chem. 1997, 1, 1–20. [Google Scholar]
- Sanchez, V.M.; Rebolledo, F.; Gotor, V. Candida antarctica lipase-catalyzed doubly enantioselective aminolysis reactions. Chemoenzymatic synthesis of 3-hydroxypyrrolidines and 4-(silyloxy)-2-oxopyrrolidines with two stereogenic centers. J. Org. Chem. 1999, 64, 1464–1470. [Google Scholar]
- Zaks, A.; Klibanov, A.M. Enzymatic catalysis in nonaqueous solvents. J. Biol. Chem. 1988, 263, 3194–3201. [Google Scholar]
- Carrea, G.; Riva, S. Properties and synthetic applications of enzymes in organic solvents. Angew. Chem. Int. Edit. 2000, 39, 2226–2254. [Google Scholar] [CrossRef]
- DeSantis, G.; Davis, B.G. The expanding roles of biocatalysis and biotransformation. Curr. Opin. Chem. Biol. 2006, 10, 139–140. [Google Scholar] [CrossRef]
- Fitzpatrick, P.A.; Steinmetz, A.C.U.; Ringe, D.; Klibanov, A.M. Enzyme crystal structure in a neat organic solvent. Proc. Natl. Acad. Sci. USA 1993, 90, 8653–8657. [Google Scholar] [CrossRef]
- Griebenow, K.; Klibanov, A.M. Lyophilization-induced reversible changes in the secondary structure of proteins. Proc. Natl. Acad. Sci. USA 1995, 92, 10969–10976. [Google Scholar] [CrossRef]
- Martinez, S.G.; Alvira, E.; Cordero, L.V.; Ferrer, A.; Montanes-Clemente, I.; Barletta, G. High initial activity but low storage stability observed for several preparations of subtilisin carslberg suspended in organic solvents. Biotechnol. Progr. 2002, 18, 1462–1466. [Google Scholar] [CrossRef]
- Columbus, L.; Hubbell, W.L. Mapping backbone dynamics in solution with site-directed spin labeling: Gcn4-58 bzip free and bound to DNA. Biochemistry 2004, 43, 7273–7287. [Google Scholar] [CrossRef]
- Columbus, L.; Kálai, T.; Jekö, J.; Hideg, K.; Hubbell, W.L. Molecular motion of spin labeled side chains in α-helices: Analysis by variation of side chain structure. Biochemistry 2001, 40, 3828–3846. [Google Scholar]
- Clark, D.S. Characteristics of nearly dry enzymes in organic solvents: Implications for biocatalysis in the absence of water. Phil. Trans. R. Soc. Lond. B 2004, 359, 1299–1307. [Google Scholar] [CrossRef]
- Lietzow, M.A.; Hubbell, W.L. Motion of spin label side chains in cellular retinol-binding protein: Correlation with structure and nearest-neighbor interactions in an antiparallel β-sheet. Biochemistry 2004, 43, 3137–3151. [Google Scholar]
- Ueji, S.; Taniguchi, T.; Okamoto, T.; Watanabe, K.; Ebara, Y.; Ohta, H. Flexibility of lipase brought about by solvents controls its enantioselectivity in organic media. Bull. Chem. Soc. Jpn. 2003, 76, 399–403. [Google Scholar] [CrossRef]
- Watanabe, K.; Yoshida, T.; Ueji, S. The role of conformation flexibility on enzymes in the diecrimination between amino acid and ester substrates for the subtilisin-catalyzed reaction in organic solvents. Bioorg. Chem. 2004, 32, 504–515. [Google Scholar] [CrossRef]
- Castillo, B.; Bansal, V.; Ganesan, A.; Halling, P.; Secundo, F.; Ferrer, A.; Griebenow, K.; Barletta, G. On the activity loss of hydrolases in organic solvents: II. A mechanistic study of subtilisin carlsberg. BMC Biotechnol. 2006, 6. [Google Scholar] [CrossRef]
- Castillo, B.; Pacheco, Y.; Al-Azzam, W.; Griebenow, K.; Devi, M.; Ferrer, A.; Barletta, G. On the activity loss of hydrolases in organic solvents: I. Rapid loss of activity of a variety of enzymes and formulations in a range of organic solvents. J. Mol. Catal. B-Enzym. 2005, 35, 147–153. [Google Scholar]
- Castillo, B.; Sola, R.J.; Ferrer, A.; Barletta, G.; Griebenow, K. Effect of peg modification on subtilisin carlsberg activity, enantioselectivity, and structural dynamics in 1,4-dioxane. Biotechnol. Bioeng. 2008, 99, 9–17. [Google Scholar] [CrossRef]
- Fernandez, J.F.A.; Halling, P. Operational stability of high initial activity protease catalysts in organic solvents. Biotechnol. Progr. 2002, 18, 1455–1457. [Google Scholar] [CrossRef]
- Fasoli, E.; Ferrer, A.; Barletta, G.L. Hydrogen/deuterium exchange study of subtilisin carlsberg during prolonged exposure to organic solvents. Biotechnol. Bioeng. 2009, 102, 1025–1032. [Google Scholar] [CrossRef]
- Bansal, V.; Delgado, Y.; Fasoli, E.; Ferrer, A.; Griebenow, K.; Secundo, F.; Barletta, G.L. Effect of prolonged exposure to organic solvents on the active site environment of subtilisin carlsberg. J. Mol. Catal. B-Enzym. 2010, 64, 38–44. [Google Scholar]
- Watanabe, K.; Yoshida, T.; Ueji, S. Optimum conformational flexibility of subtilisin to maximixe the enantioselectivity for subtilisin-catalysed transesterification in an organic solvent with an addition of dimethyl sulfoxide. Chem. Commun. 2001, 1260–1261. [Google Scholar]
- Guo, Z.; Cascio, D.; Hideg, K.; Kálái, T.; Hubbell, W.L. Structural determinants of nitroxide motion in spin-labeled proteins: Tertiary contact and solvent-inaccessible sites in helix g of t4 lysozyme. Protein Sci. 2007, 16, 1069–1086. [Google Scholar] [CrossRef]
- Cruz, A.; Ramirez, E.; Santana, A.; Barletta, G.; Lopez, G. Molecular dynamic study of subtilisin carlsberg in aqueous and nonaqueous solvents. Mol. Simulat. 2009, 1–8. [Google Scholar]
- Habeeb, A.F.S.A.; Cassidy, H.G.; Singer, S.J. Molecular structural effects produced in proteins by reaction with succinic anhydride. Biochim. Biophys. Acta 1958, 29, 587–593. [Google Scholar] [CrossRef]
- Schonbaum, G.R.; Zerner, B.; Bender, M.L. The spectrophotometric determination of the operational normality of an alpha-chymotrypsin solution. J. Biol. Chem. 1961, 236, 2930–2935. [Google Scholar]
- Hubbell, W.L.; Cafiso, D.S.; Altenbach, C. Identifying conformational changes with site-directed spin labeling. Nat. Struct. Biol. 2000, 7, 735–739. [Google Scholar] [CrossRef]
- Hamilton, C.L.; McConney, H.M. Spin Labels. In Structural Chemistry and Molecular Biology, Rich, A., Davidson,N.R., Eds.; W.H. Freeman and Company: New York, NY, USA, 1968; p. 115. [Google Scholar]
- Griebenow, K.; Laureano, Y.; Santos, A.M.; Montanez Clemente, I.; Rodriguez, L.; Vidal, M.W.; Barletta, G. Improved enzyme activity and enantioselectivity in organic solvents by methyl-β-cyclodextrin. J. Am. Chem. Soc. 1999, 121, 8157–8163. [Google Scholar]
- Fersht, A. Enzyme Structure and Mechanism, 2nd ed; W.H. Freeman and Company: New York, NY, USA, 1985. [Google Scholar]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bansal, V.; Delgado, Y.; Legault, M.D.; Barletta, G. Low Operational Stability of Enzymes in Dry Organic Solvents: Changes in the Active Site Might Affect Catalysis. Molecules 2012, 17, 1870-1882. https://doi.org/10.3390/molecules17021870
Bansal V, Delgado Y, Legault MD, Barletta G. Low Operational Stability of Enzymes in Dry Organic Solvents: Changes in the Active Site Might Affect Catalysis. Molecules. 2012; 17(2):1870-1882. https://doi.org/10.3390/molecules17021870
Chicago/Turabian StyleBansal, Vibha, Yamixa Delgado, Marc D. Legault, and Gabriel Barletta. 2012. "Low Operational Stability of Enzymes in Dry Organic Solvents: Changes in the Active Site Might Affect Catalysis" Molecules 17, no. 2: 1870-1882. https://doi.org/10.3390/molecules17021870