Quantitative Structure Inter-Activity Relationship (QSInAR). Cytotoxicity Study of Some Hemisynthetic and Isolated Natural Steroids and Precursors on Human Fibrosarcoma Cells HT1080
Abstract
:1. Introduction
2. Chemistry and Cytotoxicity Assay



| No./G/NG * | Name (Hemisynthetic, if any) | Chemical Formula | Structure | IC50 (mM) (observed hours) [A = log10(1/IC50)] | QSAR properties | ||
|---|---|---|---|---|---|---|---|
| LogP | Pol [Å3] | −Etot (kcal/mol) | |||||
| 1/G1 | lup-20(29)-en-3β-ol (Lupeol) | C30H50O |  | 1 (48) [0.000] | 8.03 | 52.4 | 106582.9141 |
| 2/G2 | 3β-hydroxyolean-12-en-28-oic acid (Oleanolic acid) | C30H48O3 |  | 0.7 (48) [0.155] | 7.32 | 53.12 | 119358.2422 |
| 3/G3 | 3-O-acetyl-5,6 epoxystigmast-22-ene-3-ol (Hemisynthetic) | C31H50O3 |  | 0.54 (48) [0.268] | 6.30 | 54.96 | 122840.5859 |
| 4/G4 | Stigmasta-4,22-dien-3-one (Hemisynthetic) | C29H46O |  | 0.3 [0.523] | 8.19 | 50.60 | 102422.1563 |
| 5/G5 | Chlorhydrate of Aminocinnamyl (Hemisynthetic) | C9H12ClN |  | 0.1 (48) [1.000] | 2.82 | 19.03 | 39170.58984 |
| 6/G6 | Acovenosigenin A or1β,3β,14β-trihydroxycard-20(22)-enolide | C23H34O5 |  | 0.0012 [2.921] | 2.07 | 41.55 | 108835.2969 |
| 7/G7 | Periplogenin or 3β,5β,14β-trihydroxycard-20(22)-enolide | C23H34O5 |  | 0.00081 [4.092] | 1.89 | 41.55 | 108837.7656 |
| 8/G8 | Periplogenin or 3-O-β-D-glucopyranosyl-5β,14β-dihydroxycard-20(22)-enolide | C29H44O10 |  | 0.00016 [3.796] | 0.67 | 54.97 | 162652.2813 |
| 9/G9 | 17α-H-digitoxigenin or 3β,14β-dihydroxycard-20(22)-enolide | C23H34O4 |  | 0.0015 [2.823] | 3.04 | 40.91 | 102065.0234 |
| 10/G10 | 1α,2β-dihydroxyolean-12-en-28-oic acid (Maslinic acid) | C30H48O4 |  | 0.075 (48) [1.125] | 6.55 | 53.76 | 126133.4297 |
| 11/G11 | Aminocinnamyl-2,3,4,6 -O-tetraacetyl- α-D-glucopyranoside (Hemisynthetic) | C23H29NO9 |  | 0.88 (48) [0.056] | 1.13 | 45.54 | 137839.3281 |
| 12/NG1 | Ginsenoside 20(S)-protopanaxadiol or 3β,12β,20S-trihydroxydammar-24-ene | C30H52O3 |  | 0.07678 [1.115] | 6.29 | 54.45 | 120871.4531 |
| 13/NG2 | Acovenosigenin A digitoxoside or 3-O-β-D-digitoxopyranosyl-1β,14β-dihydroxycard-20(22)-enolide | C29H44O8 |  | 0.0011 [2.958] | 2.25 | 53.70 | 149114.3281 |
| 14/NG3 | Periplogenin Digitoxoside or 3-O-β-D-digitoxopyranosyl-5β,14β-dihydroxycard-20(22)-enolide | C29H44O8 |  | 0.000093 [4.032] | 2.07 | 53.70 | 149112.1719 |
| 15/NG4 | 17α-H-periplogenin-3-O-β-D-cymaroside or 3-O-β-D-cymarosyl-5β,14β-dihydroxycard-20(22)-enolide | C30H46O8 |  | 0.000096 [4.018] | 2.35 | 55.53 | 152546.9375 |
| 16 | Tormentic acid | C30H48O5 |  | Toxicity < 50% for max 100 µM dose | |||
| 17 | stigmasterol | C29H48O |  | Toxicity < 50% for max 500 µM dose | |||
| 18 | Stigmasta-4,22-dien-3,6-dione (Hemisynthetic) | C29H44O2 |  | Toxicity < 50% for max 10 µM dose | |||
| 19 | 3-O-β-D-glucopyranoside of sitosterol | C35H60O6 |  | Toxicity < 50% for max 100 µM dose | |||
3. Inter-Activity Models and Discussion
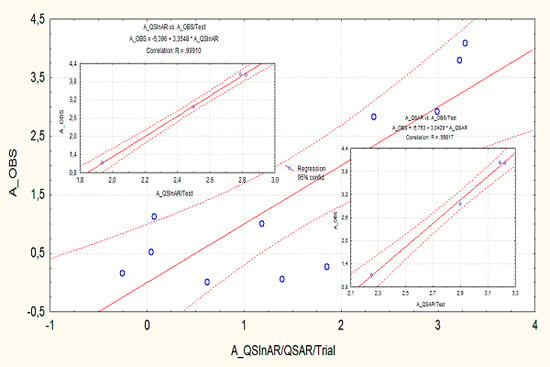
3.1. Calibration Quantitative Structure Inter-Activity Relationship (QSInAR)






- It does not reproduce Equation (4) in regards to the computed coefficients of the structurally-projected activities, while
- Displaying equal correlation respecting the direct structural-parameters-activity correlation of Equation (4).
| Index | A1QSAR | A2QSAR | A3QSAR |
|---|---|---|---|
| G1 | 0.191 | 1.372 | 1.473 |
| G2 | 0.449 | 1.355 | 1.583 |
| G3 | 0.820 | 1.310 | 1.613 |
| G4 | 0.133 | 1.416 | 1.438 |
| G5 | 2.085 | 2.188 | 0.90 |
| G6 | 2.358 | 1.638 | 1.493 |
| G7 | 2.423 | 1.638 | 1.493 |
| G8 | 2.866 | 1.310 | 1.954 |
| G9 | 2.005 | 1.653 | 1.435 |
| G10 | 0.729 | 1.339 | 1.641 |
| G11 | 2.699 | 1.540 | 1.741 |
3.2. Predictive QSInAR


| Index | A1QSAR | A2QSAR | A3QSAR | AQSAR | AQSInAR |
|---|---|---|---|---|---|
| NG1 | 0.824 | 1.324 | 1.648 | 2.251 | 1.938 |
| NG2 | 2.292 | 1.343 | 1.902 | 2.898 | 2.500 |
| NG3 | 2.358 | 1.343 | 1.902 | 3.187 | 2.787 |
| NG4 | 2.256 | 1.298 | 1.933 | 3.224 | 2.822 |

- The tested correlation performances are notably superior to those recorded for the calibration set for both QSAR and QSInAR frameworks; this way giving more confidence to the present models as reliable predictive activity tools for future designed steroids and triterpenes;
- Although the interactivity QSInAR model is equal with the direct QSAR one in the trial-correlation set, see Equations (4) and (5), it is slightly superior for the externally tested one, see Equations (6) and (7);
- The consecrated triplet of Hansch parameters (LogP, POL and Etot) is sufficiently powerful to provide significant and reliable correlations of the structure with the measured activities of steroids and triterpenes, yet their projected activities are even more efficient in prediction when considered together in the inter-activity correlations;
- For hemisynthetic compounds 4 one record in Table 2 shows that it reaches the minimum (LogP) projected computed (QSAR) activities in the Gaussian series; the extreme values are also seen for the hemisynthetic compound 5 for POL and Etot projected (QSAR) computed maximum and minimum activities, respectively; this means that these compounds are not predicted as active through the membrane transgression (compounds 4) and by the steric interaction (compound 5) but only by signal transduction (compound 5);
- Instead, the hemisynthetic compound 11, that was globally observed with the lowest cytotoxicity activity in Table 1, it is predicted as having moderate anti-cancer activity especially by membrane diffusion, but also through polarizability and steric effects on tumoral cells; this means that this compound is especially adapted for membrane transduction, while being afterwards somehow inhibited by other (by this approach not revealed) molecular mechanisms during its interaction with tumor cell; further studies may improve upon replacing the total energy indicator, that is strongly depending on the size of the molecule and the number of hydrogen-bonding partner it features, with a key energy information relaying on the binding-site models (atomistic, quasi-atomistic, virtual) which might serve to estimate the strength of the interaction;
- Polarizability has little influence on the recorded activity, see eq. (2), this way eliminating, in principle, the electrophilic mechanism specific to modeling genotoxic carcinogenity [24]; this is naturally since, in fact, the present study reveals the cytotoxic series that due to their induced cancer cell apoptosis acts contrarily to those producing cancer.
4. Experimental
4.1. General
4.2. Plant Material
4.3. Isolation
4.4. Cellular Viability
4.5. Compounds Chosen from the Literature
5. Conclusions
- The cytotoxicity of congeneric (both trial and test) series was considered and modeled as being projected on three fundamental Hansch parameters such as LogP, POL and Etot; it was found that the hydrophobicity (LogP) is the main mechanism of interaction, while the polarizability (POL) has little influence on anti-tumoral mechanism—Rejecting this way the eventual generalization of Miller’s electrophilic theory of carcinogenicity to its reversible evolution though cancer cells’ apoptosis; in fact, while LogP was found to have little influence on carcinogenity [17], it appears here as having the main influence; one may conclude therefore that indeed the carcinogenity (formation of cancer cells) and the cytotoxicity (death of cancer cells) are complementary and not symmetrical with respect to QSAR analysis;
- The presented inter-activity QSInAR correlation equations show overall slightly better statistical prediction with respect to the custom QSAR counterpart, while providing equal performance for our trial set of newly hemisynthesyzed and isolated natural steroids and precursors triterpenes; this behavior assures the present reliable QSInAR analysis and the conclusions that result from it, namely:
- ○ The newly hemisynthesized compounds are not all well suited to accounting for cytotoxicity of HT1080 cells, while their anti-cancer action is more relevant through the projection on some particular stages of molecular mechanism of ligand-receptor interaction, as following:
- ○ The compound stigmasta-4,22-dien-3-one (compound 4 in Table 1) is particularly not adapted for membrane transduction and therefore should be omitted from further clinical studies;
- ○ The compound aminocinnamyl chlorohydrate (compound 5 in Table 1) is less effective in tumoral apoptosis by steric signals but potentially active by polarization and therefore it should be further developed into another derivatives with improved structural projected activities;
- ○ Aminocinnamyl-2,3,4,6-O-tetraacetyl-α-D-glucopyranoside (compound 11 in Table 1) although it recorded the lowest observed overall molecular activity it is predicted to have moderately high cyt-activities as projected on all hydrophobic, polarizability and steric influences; such a behavior suggest that it undergoes successive molecular interaction within the cancer cells where some unrevealed inhibition interaction impedes its structurally-projected activities from being globally manifest towards tumor apoptosis.
Acknowledgements
References and Notes
- Ma, C.-M.; Cai, S.-Q.; Cui, J.-R.; Wang, R.-Q.; Tu, P.-F.; Hattori, M.; Daneshtalab, M. The cytotoxic activity of ursolic acid derivatives. Eur. J. Med. Chem. 2005, 40, 582–589. [Google Scholar] [CrossRef]
- Awad, A.B.; Fink, C.S. Phytosterols as anticancer dietary components: Evidence and mechanism of action. J. Nutr. 2000, 130, 2127–2130. [Google Scholar]
- Villaseñor, I.M.; Angelada, J.; Canlas, A.P.; Echegoyen, D. Bioactivity studies on b-Sitosterol and its glucoside. Phytother. Res. 2002, 16, 417–421. [Google Scholar] [CrossRef]
- Jamaluddin, F.; Mohamed, S.; Lajis, Md.N. Hypoglycaemic effect of Parkia speciosa seeds due to the synergistic action of b-sitosterol and stigmasterol. Food Chem. 1994, 49, 339–345. [Google Scholar] [CrossRef]
- Saez, J.; Cardona, W.; Espinal, D.; Blair, S.; Mesa, J.; Bocar, M.; Jossang, A. Five new steroids from Solanum nudum. Tetrahedron 1998, 54, 10771–10778. [Google Scholar] [CrossRef]
- Itoh, H.; Ito, H.; Hibasami, H. Blazein of a new steroid isolated from Agaricus blazei Murrill (himematsutake) induces cell death and morphological change indicative of apoptotic chromatin condensation in human lung cancer LU99 and stomach cancer KATO III cells. Oncol. Rep. 2008, 20, 1359–1361. [Google Scholar]
- Wang, Y.-S.; Yang, J.-H.; Luo, S.-D.; Zhang, H.-B.; Li, L. New cytotoxic steroid from Stachyurus himalaicus var. himalaicus. Molecules 2006, 11, 536–542. [Google Scholar]
- Samadi, A.K.; Tong, X.; Mukerji, R.; Zhang, H.; Timmermann, B.N.; Cohen, M.S. Withaferin A, a cytotoxic steroid from Vassobia breviflora, induces apoptosis in human head and neck squamous cell carcinoma. J. Nat. Prod. 2010, 73, 1476–1481. [Google Scholar] [CrossRef]
- Shang, X.-Y.; Li, J.-J.; Liu, M.-T.; Li, S.; Liu, Y.; Wang, Y.-F.; Huang, X.; Jin, Z.-L. Cytotoxic steroids from Monascus purpureus-fermented rice. Steroids 2011, 76, 1185–1189. [Google Scholar] [CrossRef]
- Amagata, T.; Doi, M.; Tohgo, M.; Minoura, K.; Numata, A. Dankasterone, a new class of cytotoxic steroid produced by a Gymnascella species from a marine sponge. Chem. Commun. 1999, 1321–1322. [Google Scholar]
- Zhang, H.-J.; Sun, J.-B.; Lin, H.-W.; Wang, Z.-L.; Tang, H.; Cheng, P.; Chen, W.-S.; Yi, Y.-H. A new cytotoxic cholesterol sulfate from marine sponge Halichondria rugosa. Nat. Prod. Res. 2007, 21, 953–958. [Google Scholar] [CrossRef]
- Brassart, B.; Gomez, D.; De Cian, A.; Paterski, R.; Montagnac, A.; Qui, K.-H.; Temime-Smaali, N.; Trentesaux, C.; Mergny, J.-L.; Gueritte, F.; et al. A new steroid derivative stabilizes G-quadruplexes and induces telomere uncapping in human tumor cells. Mol. Pharmacol. 2007, 72, 631–640. [Google Scholar] [CrossRef]
- Rickardson, L.; Fryknäs, M.; Dhar, S.; Lövborg, H.; Gullbo, J.; Rydåker, M.; Nygren, P.; Gustafsson, M.G.; Larsson, R.; Isaksson, A. Identification of molecular mechanisms for cellular drug resistance by combining drug activity and gene expression profiles. Br. J. Cancer 2005, 93, 483–492. [Google Scholar] [CrossRef]
- Caraglia, M.; Santini, D.; Marra, M.; Vincenzi, B.; Tonini, G.; Budillon, A. Emerging anti-cancer molecular mechanisms of aminobisphosphonates. Endocr. Relat. Cancer 2006, 13, 7–26. [Google Scholar] [CrossRef]
- Comprehensive Chemometrics: Chemical and Biochemical Data Analysis; Brown, S.D.; Tauler, R.; Walczak, B. (Eds.) Elsevier: Amsterdam, The Nederland, 2009.
- QSAR & SPECTRAL-SAR in Computational Ecotoxicology; Putz, M.V. (Ed.) Apple Academics: Ontario, Canada, 2011.
- Putz, M.V. Residual-QSAR. Implications for genotoxic carcinogenesis. Chem. Central J. 2011, 5, 29. [Google Scholar] [CrossRef]
- Hansch, C.; Kurup, A.; Garg, R.; Gao, H. Chem-bioinformatics and QSAR: A review of QSAR lacking positive hydrophobic terms. Chem. Rev. 2001, 101, 619–672. [Google Scholar] [CrossRef]
- Syamala, M.S.; Das, J.; Baskaran, S.; Chandrasekaran, S. A novel and highly b-selective epoxidation of Δ5-unsaturated steroids with permanganate ion. J. Org. Chem. 1992, 57, 1928–1930. [Google Scholar] [CrossRef]
- Li, S.-H.; Li, T.-S. Steroidal 5-en-3-ones, intermediates of the transformation of steroidal 5-en-3b-ols to steroidal 4-en-3,6-diones oxidized by pyridinium dichromate and pyridinium chlorochromate. Steroids 1998, 63, 76–79. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, X.; He, Z.; Jin, Y.; Gong, L.; Mi, A. Reduction of azides to amines or amides with zinc and ammonium chloride as reducing agent. Syn. Comm. 2002, 32, 3279–3284. [Google Scholar] [CrossRef]
- Chowdhury, B.T.; Bhat, G.K. Current concepts in apoptosis: The physiological suicide program revisited. Cell. Mol. Biol. Lett. 2006, 11, 506–525. [Google Scholar] [CrossRef]
- Program Package, HyperChem 7.01; Hypercube, Inc.: Gainesville, FL, USA, 2002.
- Miller, E.C.; Miller, J.A. Searches for ultimate chemical carcinogens and their reactions with cellular macromolecules. Cancer 1981, 47, 2327–2345. [Google Scholar] [CrossRef]
- Bras, M.; Queenan, B.; Susin, S.A. Programmed cell death via mitochondria: Different modes of dying. Biochemistry (Moscow) 2005, 2, 231–239. [Google Scholar]
- Galluzi, L.; Zamzami, N.; de La Motte-Rouge, T.; Lemaire, C.; Brenner, C.; Kroemer, G. Methods for the assessment of mitochondrial membrane permeabilization in apoptosis. Apoptosis 2007, 12, 803–813. [Google Scholar] [CrossRef]
- Darzynkiewicz, Z.; Juan, G.; Li, X.; Gorczyca, W.; Murakami, T.; Traganos, F. Cytometry in cell necrobiology: analysis of apoptosis and accidental cell death (necrosis). Cytometry 1997, 27, 1–20. [Google Scholar] [CrossRef]
- Li, G.; Wang, Z.; Sun, Y.; Liu, K.; Wang, Z. Ginsenoside 20(S)-protopanaxadiol inhibits the proliferation and invasion of human fibrosarcoma HT1080 cells. Basic Clin. Pharmacol. Toxicol. 2006, 98, 588–592. [Google Scholar] [CrossRef]
- Im, K.S.; Chang, E.H.; Je, N.G. A modified alkaline hydrolysis of total ginsenosides yielding genuine aglycones and prosapogenols. Arch. Pharm. Res. 1995, 18, 454–457. [Google Scholar] [CrossRef]
- Ueda, J.-Y.; Tezuka, Y.; Banskota, A.H.; Tran, Q.L.; Tran, Q.K.; Saiki, I.; Kadota, S. Constituents of the Vietnamese medicinal plant Streptocaulon juventas and their antiproliferative activity against the human HT-1080 fibrosarcoma cell line. J. Nat. Prod. 2003, 66, 1427–1433. [Google Scholar] [CrossRef]
- Karle, I.L.; Karle, J. The crystal structure of digitoxigenin, C23H34O4. Acta Cryst. 1969, 25, 434–442. [Google Scholar] [CrossRef]
- Khine, M.M. Isolation and characterization of phytoconstituents from myanmar medicinal plants. In Dissertation; Martin-Luther-Universität Halle-Wittenberg: Halle, Germany, 2006; pp. 114–119. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the author Louis P. Sandjo.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Putz, M.V.; Lazea, M.; Sandjo, L.P. Quantitative Structure Inter-Activity Relationship (QSInAR). Cytotoxicity Study of Some Hemisynthetic and Isolated Natural Steroids and Precursors on Human Fibrosarcoma Cells HT1080. Molecules 2011, 16, 6603-6620. https://doi.org/10.3390/molecules16086603
Putz MV, Lazea M, Sandjo LP. Quantitative Structure Inter-Activity Relationship (QSInAR). Cytotoxicity Study of Some Hemisynthetic and Isolated Natural Steroids and Precursors on Human Fibrosarcoma Cells HT1080. Molecules. 2011; 16(8):6603-6620. https://doi.org/10.3390/molecules16086603
Chicago/Turabian StylePutz, Mihai V., Marius Lazea, and Louis P. Sandjo. 2011. "Quantitative Structure Inter-Activity Relationship (QSInAR). Cytotoxicity Study of Some Hemisynthetic and Isolated Natural Steroids and Precursors on Human Fibrosarcoma Cells HT1080" Molecules 16, no. 8: 6603-6620. https://doi.org/10.3390/molecules16086603
APA StylePutz, M. V., Lazea, M., & Sandjo, L. P. (2011). Quantitative Structure Inter-Activity Relationship (QSInAR). Cytotoxicity Study of Some Hemisynthetic and Isolated Natural Steroids and Precursors on Human Fibrosarcoma Cells HT1080. Molecules, 16(8), 6603-6620. https://doi.org/10.3390/molecules16086603