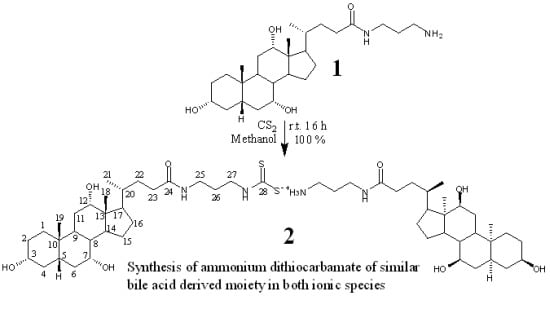
Synthesis of Both Ionic Species of Ammonium Dithiocarbamate Derived Cholic Acid Moieties
Abstract
:1. Introduction
2. Results and Discussion
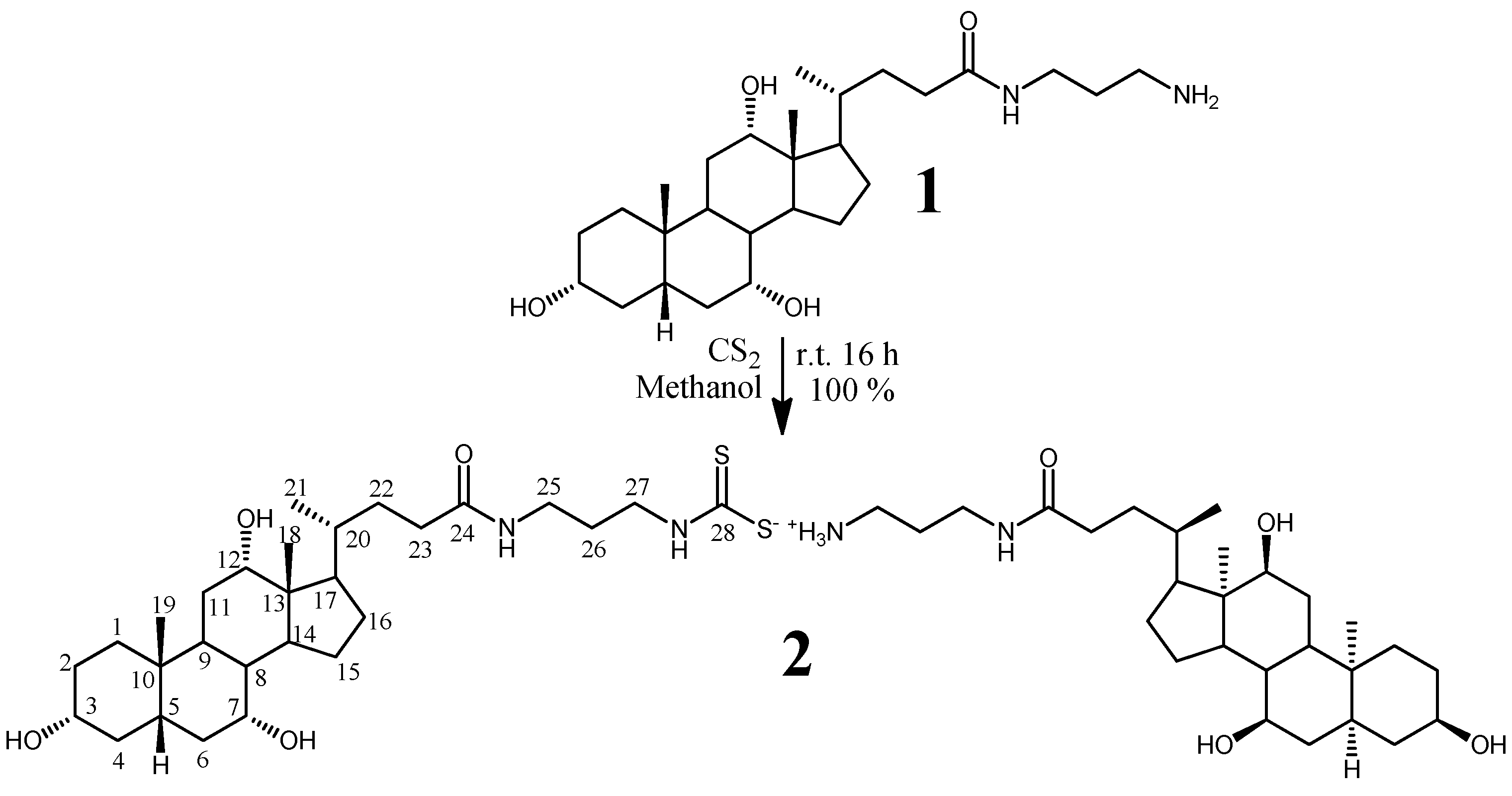
2.1. Chemistry
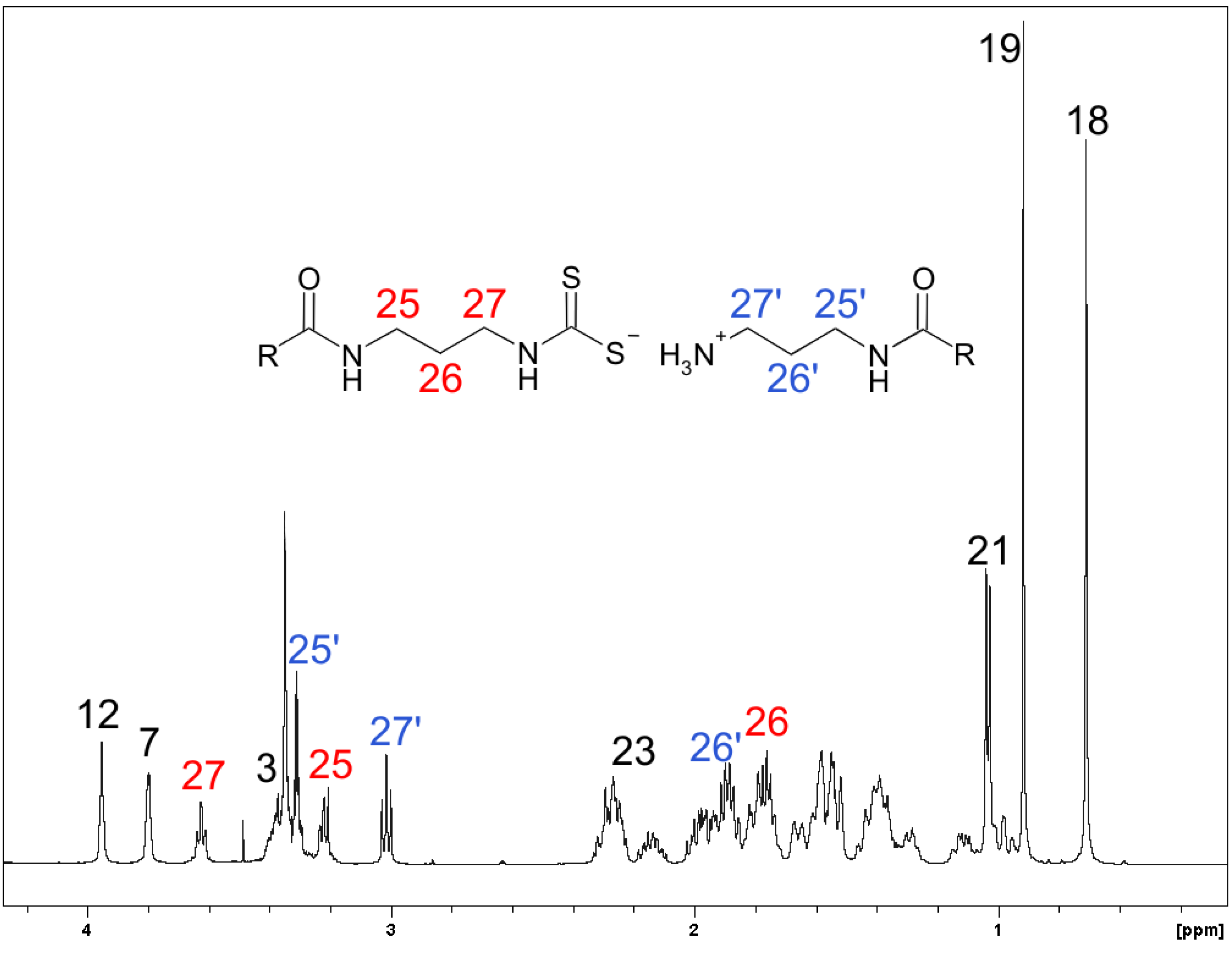
2.2. Spectroscopy
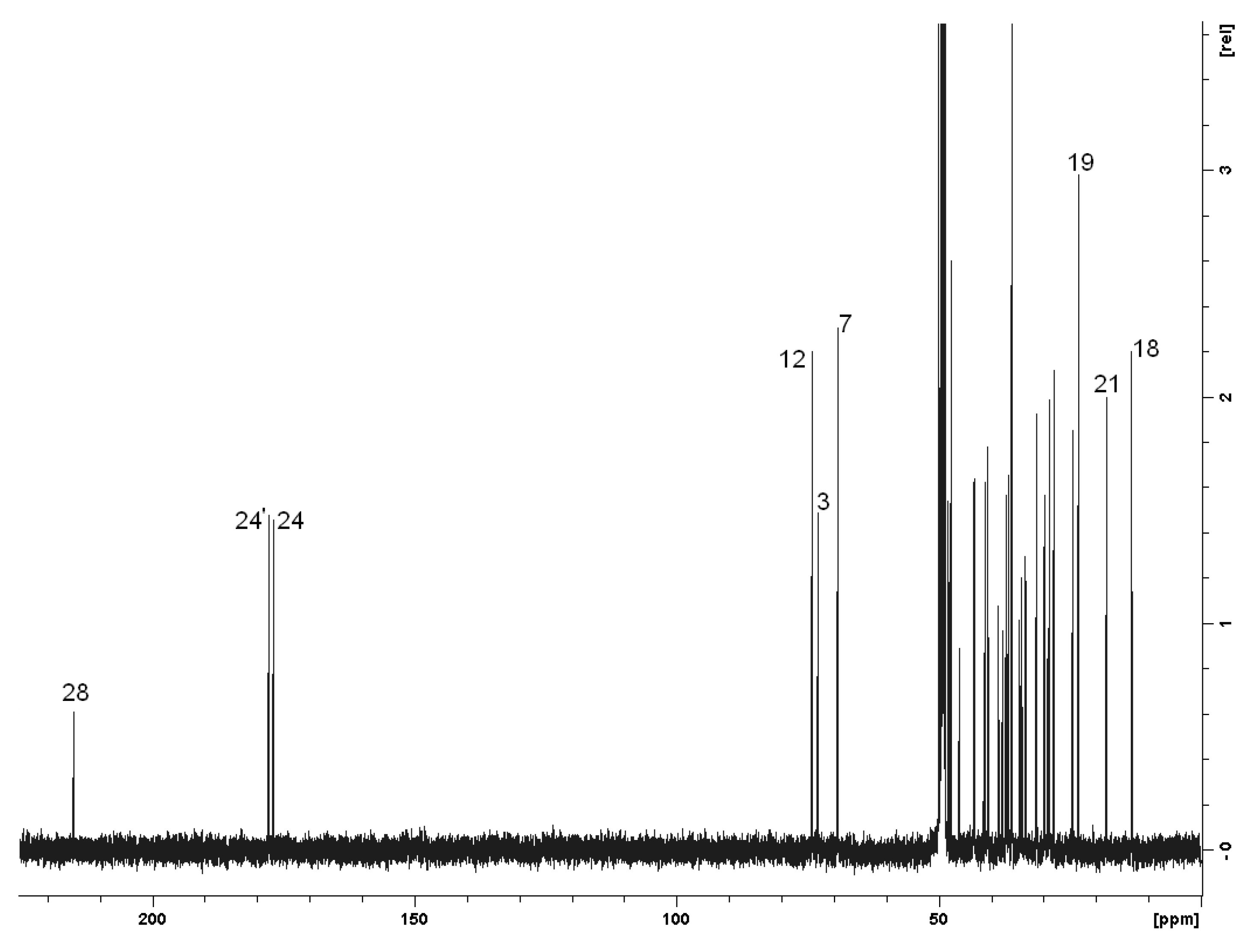
| Carbon | δ (ppm) | Carbon | δ (ppm) |
|---|---|---|---|
| 1,1’ | 36.6 | 15,15’ | 24.4 |
| 2,2’ | 31.3 | 16,16’ | 28.8 |
| 3,3’ | 73.0 | 17,17’ | 48.2,48.1 |
| 4,4’ | 40.6 | 18,18’ | 13.2 |
| 5,5’ | 43.3 | 19,19’ | 23.4 |
| 6,6’ | 37.0 | 20,20’ | 37.2 |
| 7,7’ | 69.1 | 21,21’ | 18.0 |
| 8,8’ | 41.1 | 22,22’ | 33.5,33.4 |
| 9,9’ | 28.0 | 23,23’ | 34.5,34.2 |
| 10,10’ | 36.0 | 24,24’ | 176.8,177.6 |
| 11,11’ | 29.9 | 25,25’ | 37.8,37.2 |
| 12,12’ | 74.0 | 26,26’ | 29.9,29.1 |
| 13,13’ | 47.6 | 27,27’ | 45.9,38.6 |
| 14,14’ | 43.1 | 28 | 214.7 |
3. Experimental
3.1. General
3.2. Synthesis of N-(3-aminopropyl)-3-α,7-α,12-α-trihydroxy-5-β-cholan-24-oyl ditihiocarbamate of N-(3-ammoniumpropyl)-3-α,7-α,12-α-trihydroxy-5-β-cholan-24-oic acid amide (2)
4. Conclusions
Acknowledgements
References
- Critchfield, F.E.; Johnson, J.B. Reaction of carbon disulfide with primary and secondary aliphatic amines as analytical tool. Anal. Chem. 1956, 28, 430–436. [Google Scholar] [CrossRef]
- Sassaman, M.B.; Giovanelli, J.; Sood, V.K.; Eckelman, W.C. Synthesis and screening of conformationally restricted and conformationally free N-(tertiary aminoalkyl)dithiocarbamic acids and esters as inhibitors of neuronal nitric oxide synthase. Bioorg. Med. Chem. 1998, 6, 1759–1766. [Google Scholar] [CrossRef]
- Sztanke, K.; Pasternak, K.; Sidor-Wojtowicz, A.; Truchlińska, J.; Jóźwiak, K. Synthesis of imidazoline and imidazo[2,1-c][1,2,4]triazole aryl derivatives containing the methylthio group as possible antibacterial agents. Bioorg. Med. Chem. 2006, 14, 3635–3642. [Google Scholar] [CrossRef]
- Wong, R.; Dolman, S.J. Isothiocyanates from tosyl chloride mediated decomposition of in situ generated dithiocarbamic acid salts. J. Org. Chem. 2007, 72, 3969–3971. [Google Scholar] [CrossRef]
- Pérez, R.; Reyes, O.; Suarez, M.; Garay, H.E.; Cruz, L.J.; Rodríguez, H.; Molero-Vilchez, M.D.; Ochoa, C. Solid phase synthesis of 3-(5′-carboxypentyl)-5-substituted tetrahydro-2H-1,3,5-thiadiazin-2-thione derivatives. Tetrahedron Lett. 2000, 41, 613–616. [Google Scholar]
- Azizi, N.; Ebrahimi, F.; Aakbari, E.; Aryanasab, F.; Saidi, M.R. Waste-free and environment-friendly uncatalyzed synthesis of dithiocarbamates under solvent-free conditions. Synlett. 2007, 2797–2800. [Google Scholar]
- Yavari, I.; Seyfi, S.; Hossaini, Z.; Sabbaghan, M.; Shirgahi-Talari, F. Efficient synthesis of 2-thioxo-1,3-thiazolanes from primary amines, CS2, and ethyl bromopyruvate. Monatsh. Chem. 2008, 139, 1479–1482. [Google Scholar] [CrossRef]
- Caldas, E.D.; Hosana Conceicűa, M.; Miranda, M.C.C.; Souza, L.; Lima, J.F. Determination of dithiocarbamate fungicide residues in food by a spectrophotometric method using a vertical disulfide reaction system. J. Agric. Food Chem. 2001, 49, 4521–4525. [Google Scholar] [CrossRef]
- Erian, A.W.; Sherif, S.M. The chemistry of thiocyanic esters. Tetrahedron 1999, 55, 7957–8024. [Google Scholar] [CrossRef]
- Beji, M.; Sbihi, H.; Baklouti, A.; Cambon, A. Synthesis of F-alkyl N-sulfonyl carbamates and thiocarbamates. J. Fluor. Chem. 1999, 99, 17–24. [Google Scholar] [CrossRef]
- Goel, A.; Mazur, S.J.; Fattah, R.J.; Hartman, T.L.; Turpin, J.A.; Huang, M.; Rice, W.G.; Appella, E.; Inman, J.K. Benzamide-based thiolcarbamates: a new class of HIV-1 NCp7 inhibitors. Bioorg. Med. Chem. Lett. 2002, 12, 767–770. [Google Scholar]
- Mizuno, T.; Nishiguchi, I.; Okushi, T.; Hirashima, T. Facile synthesis of S-alkyl thiocarbamates through reaction of carbamoyl lithium with elemental sulfur. Tetrahedron Lett. 1991, 32, 6867–6868. [Google Scholar] [CrossRef]
- Rafin, C.; Veignie, E.; Sancholle, M.; Postal, D.; Len, C.; Villa, P.; Ronco, G. Synthesis and antifungal activity of novel bisdithiocarbamate derivatives of carbohydrates against Fusarium oxysporum f. sp. lini. J. Agric. Food Chem. 2000, 48, 5283–5287. [Google Scholar] [CrossRef]
- Len, C.; Postal, D.; Ronco, G.; Villa, P.; Goubert, C.; Jeufrault, E.; Mathon, B.; Simon, H. Synthesis of carbamic esters derivatives of itols: Antifungal activity against various crop diseases. J. Agric. Food Chem. 1997, 45, 3–6. [Google Scholar] [CrossRef]
- Morf, P.; Raimondi, F.; Nothofer, H.G.; Schnyder, B.; Yasuda, A.; Wessels, J.M.; Jung, T.A. Dithiocarbamates: Functional and versatile linkers for the formation of self-assembled monolayers. Langmuir 2006, 22, 658–663. [Google Scholar]
- McClain, A.; Hsieh, Y.L. Synthesis of polystyrene-supported dithiocarbamates and their complexation with metal ions. J. Appl. Polym. Sci. 2004, 92, 218–225. [Google Scholar] [CrossRef]
- Dunn, A.D.; Rudorf, W.D. Carbon Disulphide in Organic Chemistry; Ellis Horwood: Chichester, UK, 1989; pp. 226–367. [Google Scholar]
- Ronconi, L.; Marzano, C.; Zanello, P.; Corsini, M.; Miolo, G.; Macca, C.; Trevisan, A.; Fregona, D. Gold(III) dithiocarbamate derivatives for the treatment of cancer: solution chemistry, DNA binding, and hemolytic properties. J. Med. Chem. 2006, 49, 1648–1657. [Google Scholar]
- Elgemeie, G.H.; Sayed, S.H. Synthesis and chemistry of dithioles. Synthesis 2001, 1747–1771. [Google Scholar] [CrossRef]
- Kalenius, E.; Koivukorpi, J.; Kolehmainen, E.; Vainiotalo, P. Noncovalent saccaride recognition by tetrakis(bile acid)-porphyrin conjugate: selectivity, co-operation and stability. Eur. J. Org. Chem. 2010, 1052–1058. [Google Scholar]
- Kralová, J.; Koivukorpi, J.; Kejik, Z.; Poucková, P.; Sievänen, E.; Kolehmainen, E.; Král, V. Porphyrin–bile acid conjugates: from saccharide recognition in the solution to the selective cancer cell fluorescence detection. Org. Biomol. Chem. 2008, 6, 1548–1552. [Google Scholar]
- Lee, S.; Lee, J.; Lee, D.Y.; Kim, S.K.; Lee, Y.; Byun, Y. A new drug carrier, Nalpha-deoxycholyl-L-lysyl-methylester, for enhancing insulin absorption in the intestine. Diabetologia 2005, 48, 405–411. [Google Scholar]
- Valkonen, A.; Lahtinen, M.; Virtanen, E.; Kaikkonen, S.; Kolehmainen, E. Bile acid amidoalcohols: Simple organogelators. Biosens. Bioelect. 2004, 20, 1233–1241. [Google Scholar] [CrossRef]
- Rance, M.; Sørensen, O.W.; Bodenhausen, G.; Wagner, G.; Ernst, R.R.; Wűthrich, K. Improved spectral resolution in COSY 1H NMR spectra of proteins via double quantum filtering. Biochem. Biophys. Res. Commun. 1984, 117, 479–485. [Google Scholar]
- Derome, A.; Williamson, M. Rapid-pulsing artefacts in double-quantum-filtered COSY. J. Magn. Reson. 1990, 88, 177–185. [Google Scholar]
- Bax, A.; Griffey, R.H.; Hawkins, B.L. Correlation of proton and nitrogen-15 chemical shifts by multiple quantum NMR. J. Magn. Reson. 1983, 55, 301–315. [Google Scholar]
- Bax, A.; Subramanian, S. Sensitivity-enhanced two-dimensional heteronuclear shift correlation NMR spectroscopy. J. Magn. Reson. 1986, 67, 565–570. [Google Scholar]
- Bax, A.; Summers, M.F. 1H and 13C assignments from sensitivity enhanced detection of heteronuclear multiple-bond connectivity by two-dimensional multiple quantum NMR. J. Am. Chem. Soc. 1986, 108, 2093–2094. [Google Scholar] [CrossRef]
- Dias, J.R.; Gao, H.; Kolehmainen, E. 13C nuclear magnetic resonance data of bile acid derivates. Spectrochim. Acta Part A 2000, 56, 53–77. [Google Scholar] [CrossRef]
- Messori, L.; Marcon, G.; Orioli, P. Gold(III) compounds as new family of anticancer drugs. Bioinorg. Chem. Appl. 2003, 1, 177–187. [Google Scholar] [CrossRef]
- Milacic, V.; Chen, D.; Ronconi, L.; Landis-Piwowar, L.R.; Fregona, D.; Dou, Q.P. A Novel anticancer gold(III) dithiocarbamate compound inhibits the activity of a purified 20S proteasome and 26S proteasome in human breast cancer cell cultures and xenografts. Cancer Res. 2006, 66, 10478–10486. [Google Scholar]
- Aldinucci, D.; Lorenzon, D.; Stefani, L.; Giovagnini, L.; Colombatti, A.; Fregona, D. Antiproliferative and apoptotic effects of two new gold(III) methylsarcosinedithiocarbamate derivatives on human acute myeloid leukemia cells in vitro. Anticancer Drugs 2007, 18, 323–332. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Koivukorpi, J.; Kolehmainen, E. Synthesis of Both Ionic Species of Ammonium Dithiocarbamate Derived Cholic Acid Moieties. Molecules 2011, 16, 6306-6312. https://doi.org/10.3390/molecules16086306
Koivukorpi J, Kolehmainen E. Synthesis of Both Ionic Species of Ammonium Dithiocarbamate Derived Cholic Acid Moieties. Molecules. 2011; 16(8):6306-6312. https://doi.org/10.3390/molecules16086306
Chicago/Turabian StyleKoivukorpi, Juha, and Erkki Kolehmainen. 2011. "Synthesis of Both Ionic Species of Ammonium Dithiocarbamate Derived Cholic Acid Moieties" Molecules 16, no. 8: 6306-6312. https://doi.org/10.3390/molecules16086306
APA StyleKoivukorpi, J., & Kolehmainen, E. (2011). Synthesis of Both Ionic Species of Ammonium Dithiocarbamate Derived Cholic Acid Moieties. Molecules, 16(8), 6306-6312. https://doi.org/10.3390/molecules16086306